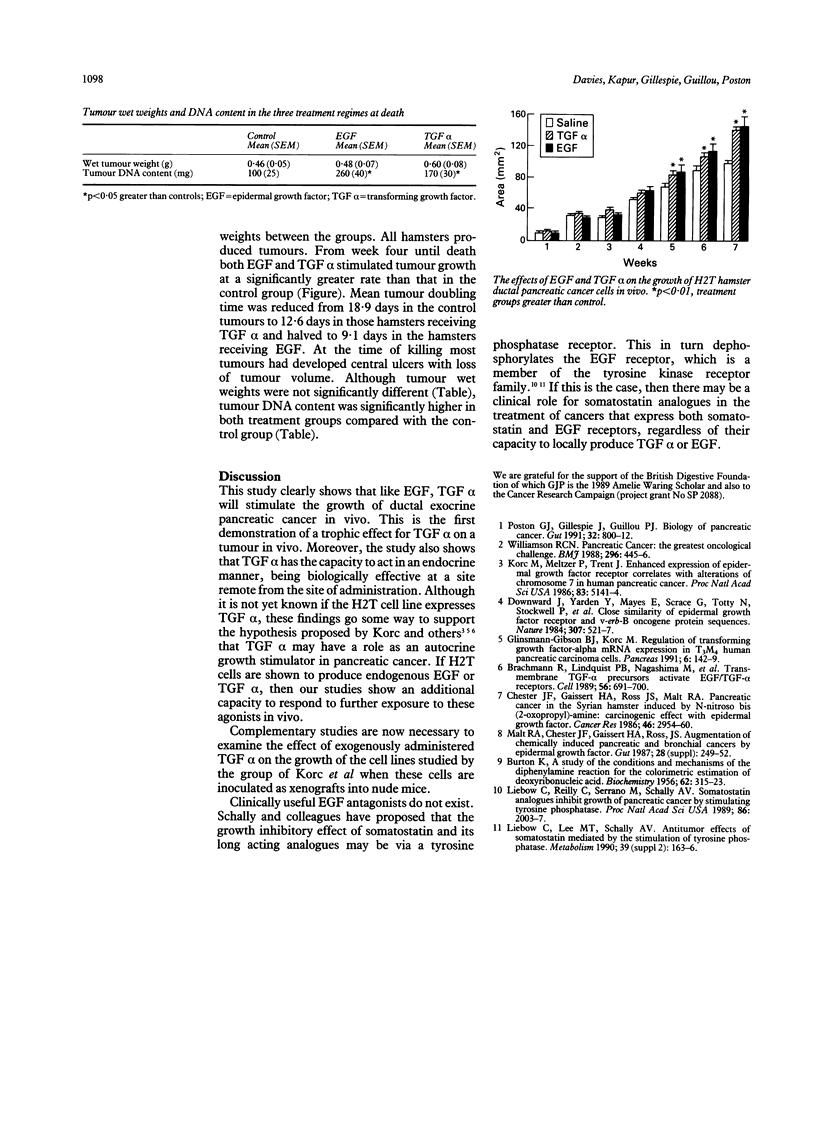
Abstract
Pancreatic cancer cell lines overexpress epidermal growth factor (EGF) receptors and also have the capacity to produce transforming growth factor alpha (TGF alpha), the alternate agonist of the EGF receptor. The purpose of this study was to determine if TGF alpha had a trophic effect on the growth of pancreatic cancer in vivo. Syrian golden hamsters were inoculated with 50,000 H2T hamster ductal pancreatic cancer cells. The hamsters were then randomised to three equal groups: the groups received either saline (control), EGF, or TGF alpha, each by intraperitoneal injection, three times a day. Treatment continued for seven weeks, and each week the hamsters were weighed and tumour areas were measured. The hamsters were then killed, and the tumours were excised, weighed, and extracted for assay of DNA content as a measure of cellularity. From week four onwards both EGF and TGF alpha significantly stimulated tumour growth. Although tumour weights were not significantly different, tumour DNA content had nearly doubled after exposure to both EGF and TGF alpha. It is concluded that like EGF, TGF alpha can stimulate pancreatic cancer growth in vivo, and this may in part explain the aggressive nature of these cancers in clinical practice.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachmann R., Lindquist P. B., Nagashima M., Kohr W., Lipari T., Napier M., Derynck R. Transmembrane TGF-alpha precursors activate EGF/TGF-alpha receptors. Cell. 1989 Feb 24;56(4):691–700. doi: 10.1016/0092-8674(89)90591-6. [DOI] [PubMed] [Google Scholar]
- Chester J. F., Gaissert H. A., Ross J. S., Malt R. A. Pancreatic cancer in the Syrian hamster induced by N-nitrosobis(2-oxopropyl)-amine: cocarcinogenic effect of epidermal growth factor. Cancer Res. 1986 Jun;46(6):2954–2957. [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Glinsmann-Gibson B. J., Korc M. Regulation of transforming growth factor-alpha mRNA expression in T3M4 human pancreatic carcinoma cells. Pancreas. 1991 Mar;6(2):142–149. doi: 10.1097/00006676-199103000-00003. [DOI] [PubMed] [Google Scholar]
- Korc M., Meltzer P., Trent J. Enhanced expression of epidermal growth factor receptor correlates with alterations of chromosome 7 in human pancreatic cancer. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5141–5144. doi: 10.1073/pnas.83.14.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebow C., Lee M. T., Schally A. Antitumor effects of somatostatin mediated by the stimulation of tyrosine phosphatase. Metabolism. 1990 Sep;39(9 Suppl 2):163–166. doi: 10.1016/0026-0495(90)90237-7. [DOI] [PubMed] [Google Scholar]
- Liebow C., Reilly C., Serrano M., Schally A. V. Somatostatin analogues inhibit growth of pancreatic cancer by stimulating tyrosine phosphatase. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2003–2007. doi: 10.1073/pnas.86.6.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malt R. A., Chester J. F., Gaissert H. A., Ross J. S. Augmentation of chemically induced pancreatic and bronchial cancers by epidermal growth factor. Gut. 1987;28 (Suppl):249–251. doi: 10.1136/gut.28.suppl.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston G. J., Gillespie J., Guillou P. J. Biology of pancreatic cancer. Gut. 1991 Jul;32(7):800–812. doi: 10.1136/gut.32.7.800. [DOI] [PMC free article] [PubMed] [Google Scholar]


