Abstract
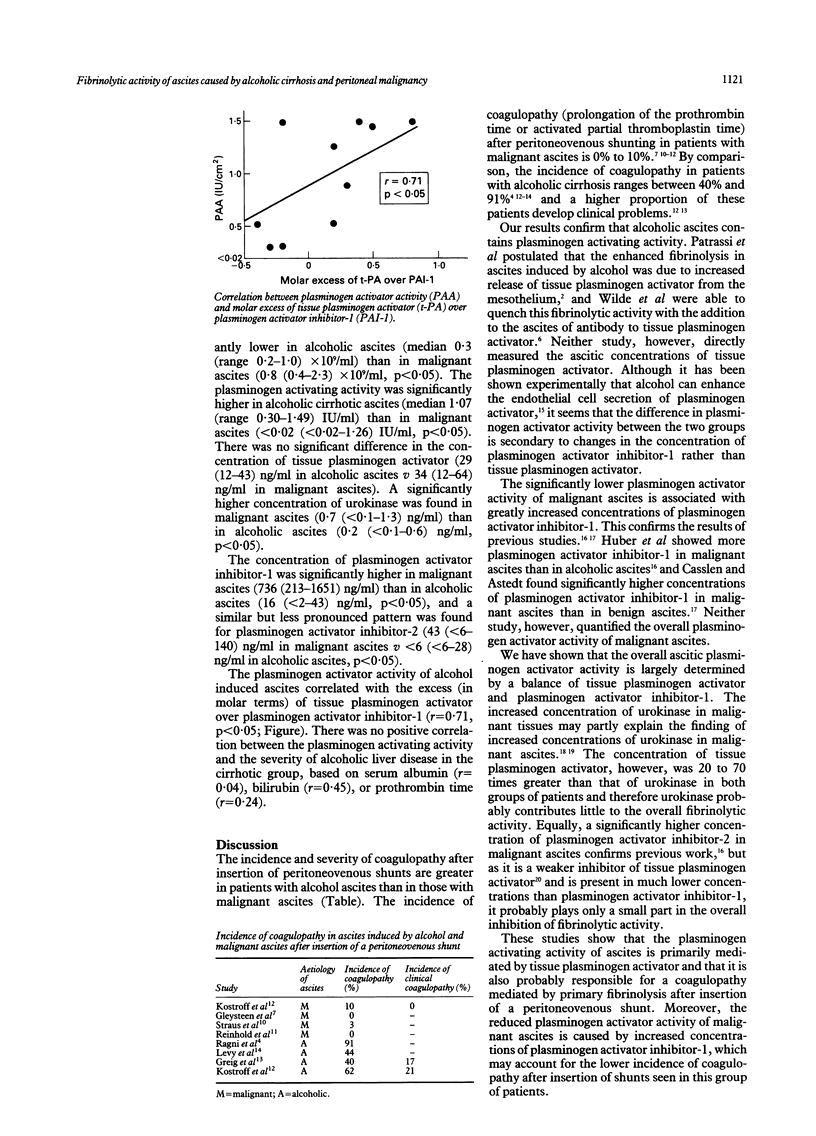
Coagulopathy is a well recognised complication of peritoneovenous shunting for ascites. The relative contributions of primary fibrinolysis and disseminated intravascular coagulation remain controversial. Plasminogen activating activity was significantly lower in malignant ascites (n = 10, median < 0.02 (range < 0.02-1.26) IU/ml) than in alcoholic ascites (n = 10, 1.07 (0.30-1.49) IU/ml) (p < 0.05). Fibrinolytic activity was determined by a balance between tissue plasminogen activator and plasminogen activator inhibitor-1. There was no significant difference between the two groups in the concentration of tissue plasminogen activator (34 (12-64) ng/ml in malignant ascites v 29 (12-43) ng/ml in alcoholic ascites), but the concentration of plasminogen activator inhibitor-1 was significantly higher in malignant ascites (736 (213-1651) ng/ml) than in alcohol ascites (29 (12-43) ng/ml) (p < 0.05). Malignant ascites contained significantly higher concentrations of urokinase (0.7 (< 0.1-1.3) ng/ml v 0.2 (< 0.1-0.6) ng/ml in alcoholic ascites) and plasminogen activator inhibitor-2 (33 (< 6-140) ng/ml v 9 (< 6-28) ng/ml alcoholic ascites). The plasminogen activating activity of alcohol ascites may lead to primary fibrinolysis after peritoneovenous shunting. The considerably lower activity found in malignant ascites may explain why coagulopathy after shunting is less pronounced in this group of patients.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baele G., Rasquin K., Barbier F. Coagulant, fibrinolytic, and aggregating activity in ascitic fluid. Am J Gastroenterol. 1986 Jun;81(6):440–443. [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Gleysteen J. J., Hussey C. V., Heckman M. G. The cause of coagulopathy after peritoneovenous shunt for malignant ascites. Arch Surg. 1990 Apr;125(4):474–477. doi: 10.1001/archsurg.1990.01410160060013. [DOI] [PubMed] [Google Scholar]
- Greig P. D., Langer B., Blendis L. M., Taylor B. R., Glynn M. F. Complications after peritoneovenous shunting for ascites. Am J Surg. 1980 Jan;139(1):125–131. doi: 10.1016/0002-9610(80)90241-x. [DOI] [PubMed] [Google Scholar]
- Henderson J. M., Stein S. F., Kutner M., Wiles M. B., Ansley J. D., Rudman D. Analysis of Twenty-three plasma proteins in ascites. The depletion of fibrinogen and plasminogen. Ann Surg. 1980 Dec;192(6):738–742. doi: 10.1097/00000658-198012000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber K., Wojta J., Kirchheimer J. C., Ermler D., Binder B. R. Plasminogen activators and plasminogen activator inhibitor in malignant and non-malignant ascitic fluid. Eur J Clin Invest. 1988 Dec;18(6):595–599. doi: 10.1111/j.1365-2362.1988.tb01273.x. [DOI] [PubMed] [Google Scholar]
- Kostroff K. M., Ross D. W., Davis J. M. Peritoneovenous shunting for cirrhotic versus malignant ascites. Surg Gynecol Obstet. 1985 Sep;161(3):204–208. [PubMed] [Google Scholar]
- Laug W. E. Ethyl alcohol enhances plasminogen activator secretion by endothelial cells. JAMA. 1983 Aug 12;250(6):772–776. [PubMed] [Google Scholar]
- Laug W. E., Jones P. A., Benedict W. F. Relationship between fibrinolysis of cultured cells and malignancy. J Natl Cancer Inst. 1975 Jan;54(1):173–179. doi: 10.1093/jnci/54.1.173. [DOI] [PubMed] [Google Scholar]
- LeVeen H. H., Ip M., Ahmed N., Hutto R. B., LeVeen E. G. Coagulopathy post peritoneovenous shunt. Ann Surg. 1987 Mar;205(3):305–311. doi: 10.1097/00000658-198703000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévy V. G., Opolon P., Pauleau N., Caroli J. Treatment of ascites by reinfusion of concentrated peritoneal fluid--review of 318 procedures in 210 patients. Postgrad Med J. 1975 Aug;51(598):564–566. doi: 10.1136/pgmj.51.598.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrassi G. M., Martinelli S., Sturniolo G. C., Cappellato M. G., Vicariotto M., Girolami A. Fibrinolytic study in plasma and ascitic fluid of cirrhotic patients before and after ascites concentration; reinfusion technique. Eur J Clin Invest. 1985 Aug;15(4):161–165. doi: 10.1111/j.1365-2362.1985.tb00162.x. [DOI] [PubMed] [Google Scholar]
- Ragni M. V., Lewis J. H., Spero J. A. Ascites-induced LeVeen shunt coagulopathy. Ann Surg. 1983 Jul;198(1):91–95. doi: 10.1097/00000658-198307000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold R. B., Lokich J. J., Tomashefski J., Costello P. Management of malignant ascites with peritoneovenous shunting. Am J Surg. 1983 Apr;145(4):455–457. doi: 10.1016/0002-9610(83)90039-9. [DOI] [PubMed] [Google Scholar]
- Straus A. K., Roseman D. L., Shapiro T. M. Peritoneovenous shunting in the management of malignant ascites. Arch Surg. 1979 Apr;114(4):489–491. doi: 10.1001/archsurg.1979.01370280143022. [DOI] [PubMed] [Google Scholar]
- Svanberg L., Astedt B. Coagulative and fibrinolytic properties of ascitic fluid associated with ovarian tumors. Cancer. 1975 May;35(5):1382–1387. doi: 10.1002/1097-0142(197505)35:5<1382::aid-cncr2820350522>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Thompson J. N., Paterson-Brown S., Harbourne T., Whawell S. A., Kalodiki E., Dudley H. A. Reduced human peritoneal plasminogen activating activity: possible mechanism of adhesion formation. Br J Surg. 1989 Apr;76(4):382–384. doi: 10.1002/bjs.1800760422. [DOI] [PubMed] [Google Scholar]
- Wilde J. T., Cooper P., Kennedy H. J., Triger D. R., Preston F. E. Coagulation disturbances following ascites recirculation. J Hepatol. 1990 Mar;10(2):217–222. doi: 10.1016/0168-8278(90)90055-v. [DOI] [PubMed] [Google Scholar]



