Abstract
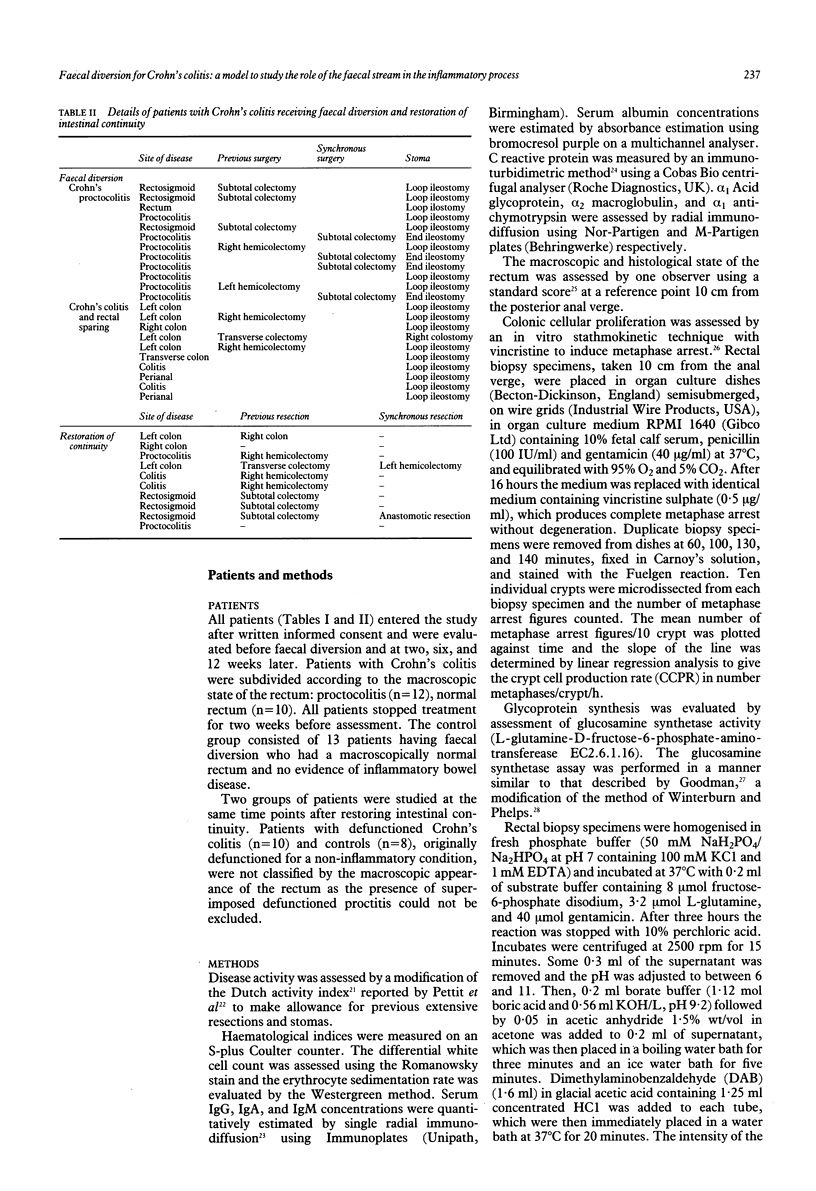
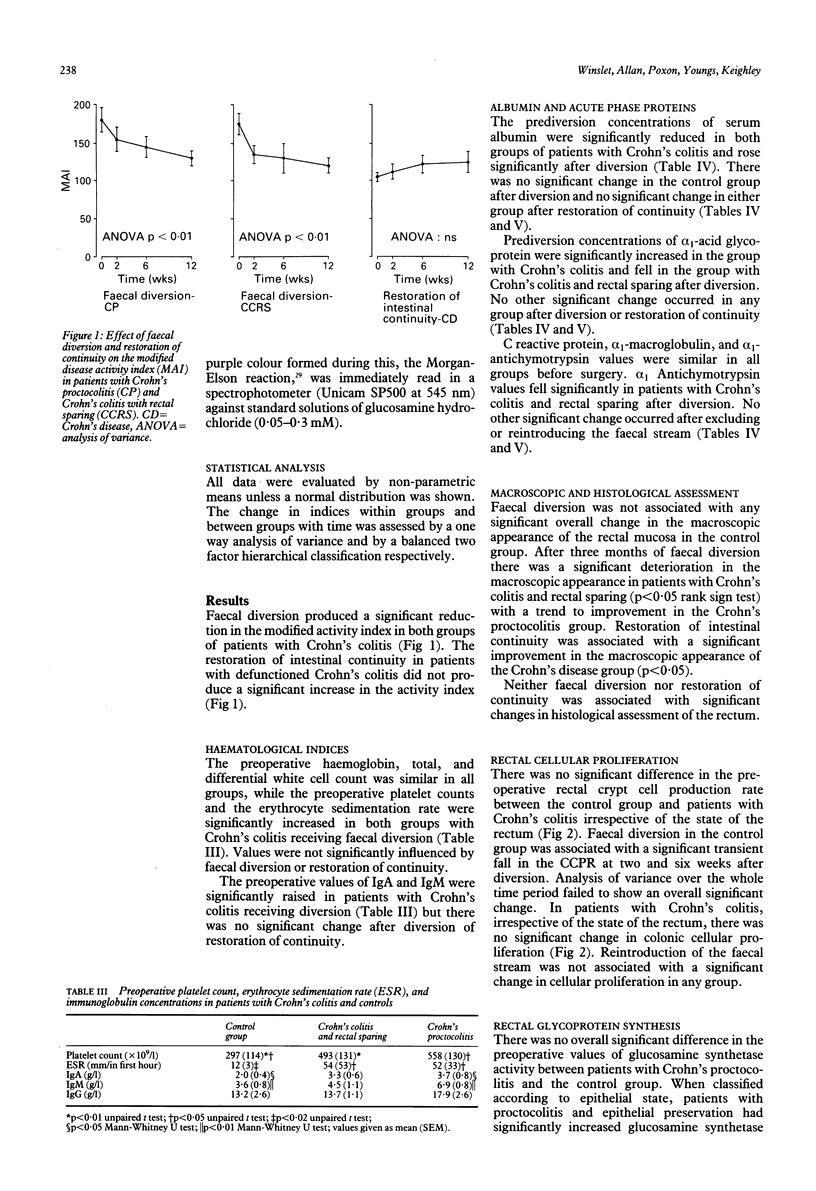
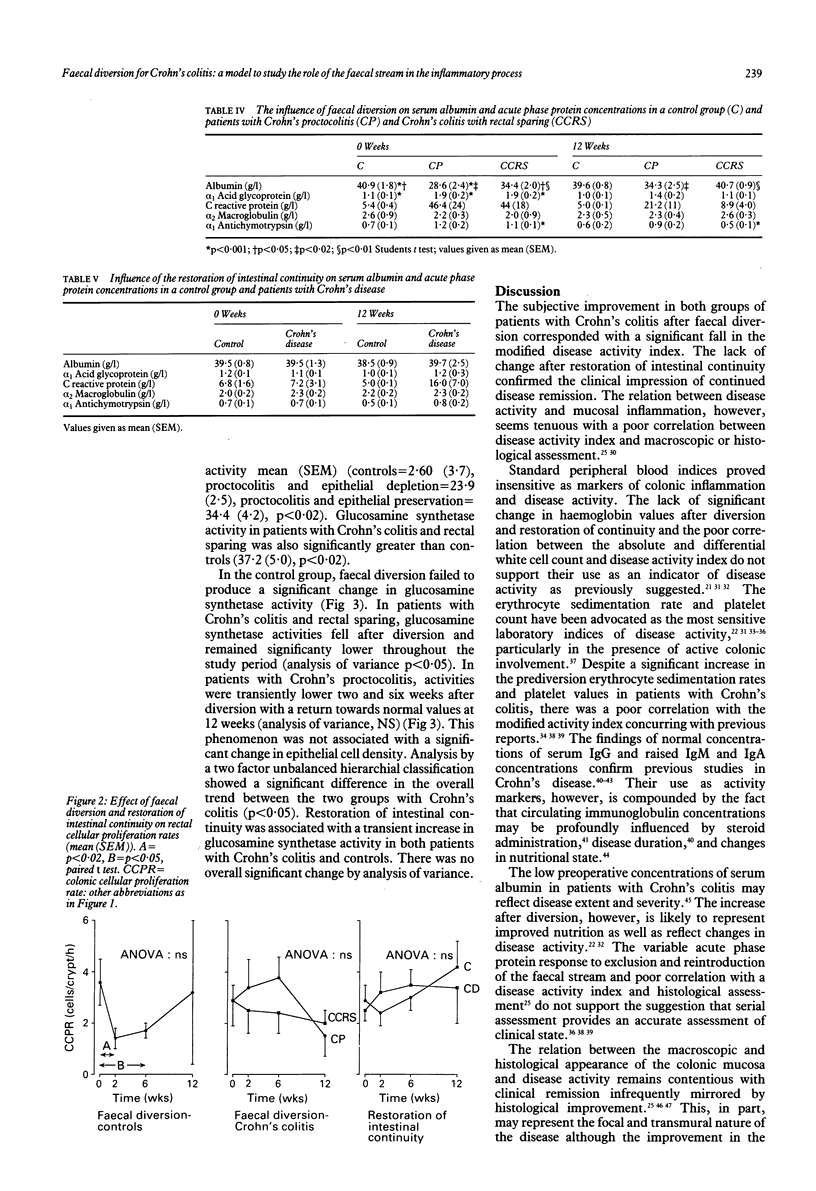
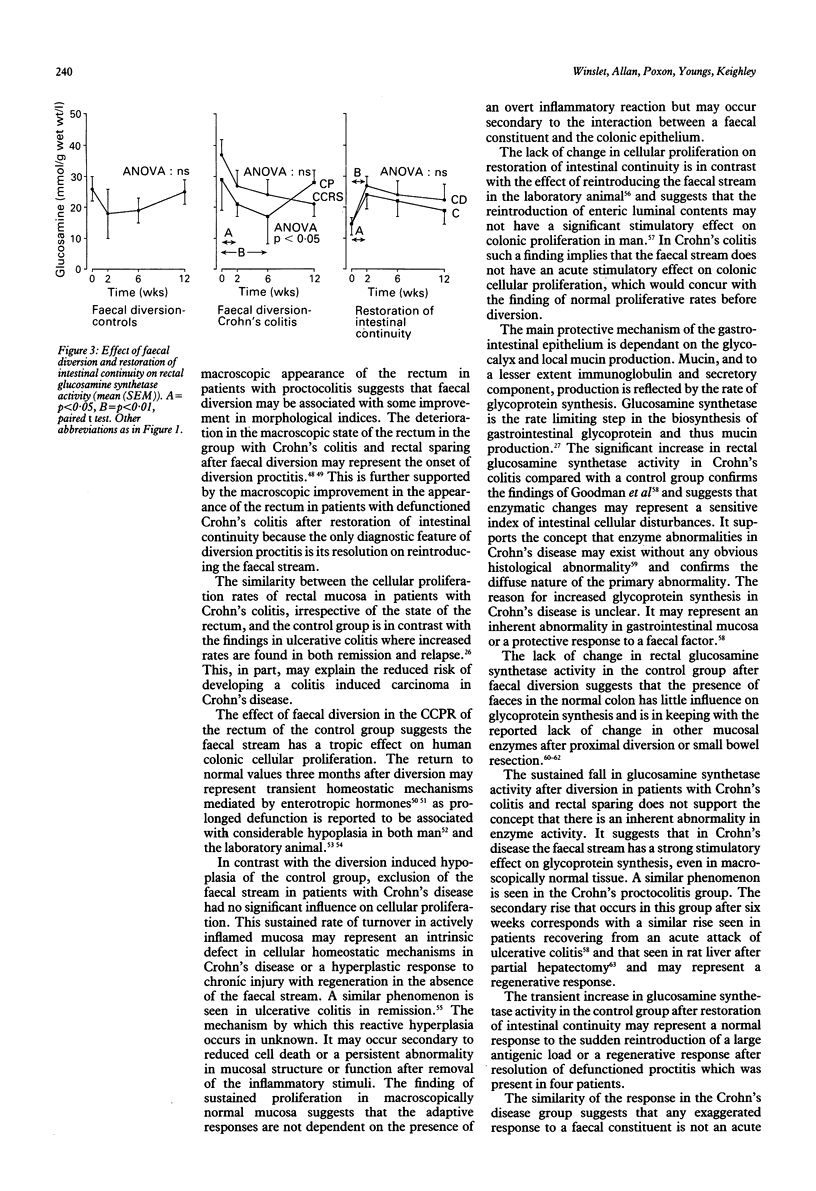
The high incidence of clinical remission after faecal diversion for Crohn's colitis suggests the faecal stream may play a part in the inflammatory mechanism. The effect of faecal diversion (n = 22) and restoration of intestinal continuity (n = 10) was assessed in patients with Crohn's colitis and compared with controls. Faecal diversion produced significant improvement in the disease activity index mean (SEM) (before 176 (9); after 114 (9), p < 0.01) and serum albumin concentrations (before 33 (3.0); after 38 (3.0), p < 0.05) in all patients with Crohn's colitis. The crypt cell production rate (CCPR) was maintained after faecal diversion for Crohn's colitis but fell in the control group (before = 3.6 (0.8)), at two (1.4 (0.4), p < 0.02), and six weeks (1.6 (0.4), p < 0.05). Mucosal glucosamine synthetase activity, reflecting glycoprotein synthesis, was significantly lower in patients with Crohn's colitis (analysis of variance p < 0.05) after diversion but was maintained in the control group. Restoration of intestinal continuity failed to produce reciprocal changes. The sustained cellular proliferation and fall in glycoprotein synthesis in Crohn's colitis after faecal diversion may represent the end of an exaggerated protective response and regenerative hyperplasia after exclusion of the faecal stream. This study suggests the faecal stream may participate in the inflammatory process in Crohn's colitis. The underlying mechanism is unknown.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan A., Bristol J. B., Williamson R. C. Crypt cell production rate in ulcerative proctocolitis: differential increments in remission and relapse. Gut. 1985 Oct;26(10):999–1003. doi: 10.1136/gut.26.10.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre C., Descos L., Landais P., Fermanian J. Assessment of appropriate laboratory measurements to supplement the Crohn's disease activity index. Gut. 1981 Jul;22(7):571–574. doi: 10.1136/gut.22.7.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton G. V., Williamson R. C. Hypoplasia of defunctioned rectum. Br J Surg. 1989 Aug;76(8):787–789. doi: 10.1002/bjs.1800760807. [DOI] [PubMed] [Google Scholar]
- Bley R. L., Okubo H., Chandler A. M. Regulation of glucosamine synthesis in injury and partial hepatectomy. Proc Soc Exp Biol Med. 1973 Oct 1;144(1):134–140. doi: 10.3181/00379727-144-37542. [DOI] [PubMed] [Google Scholar]
- Blichfeldt P., Blomhoff J. P., Myhre E., Gjone E. Metronidazole in Crohn's disease. A double blind cross-over clinical trial. Scand J Gastroenterol. 1978;13(1):123–127. doi: 10.3109/00365527809179816. [DOI] [PubMed] [Google Scholar]
- Bolton P. M., James S. L., Newcombe R. G., Whitehead R. H., Hughes L. E. The immune competence of patients with inflammatory bowel disease. Gut. 1974 Mar;15(3):213–219. doi: 10.1136/gut.15.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignola C., Lanfranchi G. A., Campieri M., Bazzocchi G., Devoto M., Boni P., Farruggia P., Veggetti S., Tragnone A. Importance of laboratory parameters in the evaluation of Crohn's disease activity. J Clin Gastroenterol. 1986 Jun;8(3 Pt 1):245–248. doi: 10.1097/00004836-198606000-00008. [DOI] [PubMed] [Google Scholar]
- Bristol J. B., Ghatei M. A., Smith J. H., Bloom S. R., Williamson R. C. Elevated plasma enteroglucagon alone fails to alter distal colonic carcinogenesis in rats. Gastroenterology. 1987 Mar;92(3):617–624. doi: 10.1016/0016-5085(87)90009-6. [DOI] [PubMed] [Google Scholar]
- Burman J. H., Thompson H., Cooke W. T., Williams J. A. The effects of diversion of intestinal contents on the progress of Crohn's disease of the large bowel. Gut. 1971 Jan;12(1):11–15. doi: 10.1136/gut.12.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrjalsen I., Ingwersen S. H. Immunoturbidimetry of serum C-reactive protein in low concentration of polyethylene glycol. Ann Clin Biochem. 1985 May;22(Pt 3):269–272. doi: 10.1177/000456328502200309. [DOI] [PubMed] [Google Scholar]
- Cooke W. T., Prior P. Determining disease activity in inflammatory bowel disease. J Clin Gastroenterol. 1984 Feb;6(1):17–25. [PubMed] [Google Scholar]
- Elson C. O., Layden T. J., Nemchausky B. A., Rosenberg J. L., Rosenberg I. H. An evaluation of total parenteral nutrition in the management of inflammatory bowel disease. Dig Dis Sci. 1980 Jan;25(1):42–48. doi: 10.1007/BF01312731. [DOI] [PubMed] [Google Scholar]
- Fagan E. A., Dyck R. F., Maton P. N., Hodgson H. J., Chadwick V. S., Petrie A., Pepys M. B. Serum levels of C-reactive protein in Crohn's disease and ulcerative colitis. Eur J Clin Invest. 1982 Aug;12(4):351–359. doi: 10.1111/j.1365-2362.1982.tb02244.x. [DOI] [PubMed] [Google Scholar]
- Gleeson M. H., Cullen J., Dowling R. H. Intestinal structure and function after small bowel by-pass in the rat. Clin Sci. 1972 Dec;43(6):731–742. doi: 10.1042/cs0430731. [DOI] [PubMed] [Google Scholar]
- Gleeson M. H., Dowling R. H., Peters T. J. Biochemical changes in intestinal mucosa after experimental small bowel by-pass in the rat. Clin Sci. 1972 Dec;43(6):743–757. doi: 10.1042/cs0430743. [DOI] [PubMed] [Google Scholar]
- Glotzer D. J., Glick M. E., Goldman H. Proctitis and colitis following diversion of the fecal stream. Gastroenterology. 1981 Mar;80(3):438–441. [PubMed] [Google Scholar]
- Gomes P., du Boulay C., Smith C. L., Holdstock G. Relationship between disease activity indices and colonoscopic findings in patients with colonic inflammatory bowel disease. Gut. 1986 Jan;27(1):92–95. doi: 10.1136/gut.27.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlad R. A., Lenton W., Ghatei M. A., Adrian T. E., Bloom S. R., Wright N. A. Effects of an elemental diet, inert bulk and different types of dietary fibre on the response of the intestinal epithelium to refeeding in the rat and relationship to plasma gastrin, enteroglucagon, and PYY concentrations. Gut. 1987 Feb;28(2):171–180. doi: 10.1136/gut.28.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M. J., Kent P. W., Truelove S. C. Glucosamine synthetase activity of the colonic mucosa in ulcerative colitis and Crohn's disease. Gut. 1977 Mar;18(3):219–228. doi: 10.1136/gut.18.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper P. H., Lee E. C., Kettlewell M. G., Bennett M. K., Jewell D. P. Role of the faecal stream in the maintenance of Crohn's colitis. Gut. 1985 Mar;26(3):279–284. doi: 10.1136/gut.26.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper P. H., Truelove S. C., Lee E. C., Kettlewell M. G., Jewell D. P. Split ileostomy and ileocolostomy for Crohn's disease of the colon and ulcerative colitis: a 20 year survey. Gut. 1983 Feb;24(2):106–113. doi: 10.1136/gut.24.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries A. D., Fitzsimons E., Fifield R., Dew M. J., Rhoades J. Platelet count: a simple measure of activity in Crohn's disease. Br Med J (Clin Res Ed) 1983 May 7;286(6376):1476–1476. doi: 10.1136/bmj.286.6376.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson H. J., Jewell D. P. The humoral immune system in inflammatory bowel disease. II. Immunologlobulin levels. Am J Dig Dis. 1978 Feb;23(2):123–128. doi: 10.1007/BF01073186. [DOI] [PubMed] [Google Scholar]
- Johansson S. G., Högman C. F., Killander J. Quantitative immunoglobulin determination. Comparison of two methods. Estimation of normal levels and levels in persons lacking IgA and IgD. Acta Pathol Microbiol Scand. 1968;74(4):519–530. [PubMed] [Google Scholar]
- Kivel R. M., Taylor K. B., Oberhelman H., Jr Response to bypass ileostomy in ulcerative colitis and Crohn's disease of the colon. Lancet. 1967 Sep 23;2(7517):632–636. doi: 10.1016/s0140-6736(67)90682-4. [DOI] [PubMed] [Google Scholar]
- Korelitz B. I., Cheskin L. J., Sohn N., Sommers S. C. Proctitis after fecal diversion in Crohn's disease and its elimination with reanastomosis: implications for surgical management. Report of four cases. Gastroenterology. 1984 Sep;87(3):710–713. [PubMed] [Google Scholar]
- Korelitz B. I., Sommers S. C. Response to drug therapy in Crohn's disease: evaluation by rectal biopsy and mucosal cell counts. J Clin Gastroenterol. 1984 Apr;6(2):123–127. doi: 10.1097/00004836-198404000-00005. [DOI] [PubMed] [Google Scholar]
- Lee E. Split ileostomy in the treatment of Crohn's disease of the colon. Ann R Coll Surg Engl. 1975 Feb;56(2):94–102. [PMC free article] [PubMed] [Google Scholar]
- McIlrath D. C. Diverting ileostomy or colostomy in the management of Crohn's disease of the colon. Arch Surg. 1971 Aug;103(2):308–310. doi: 10.1001/archsurg.1971.01350080224035. [DOI] [PubMed] [Google Scholar]
- Morgan W. T., Elson L. A. A colorimetric method for the determination of N-acetylglucosamine and N-acetylchrondrosamine. Biochem J. 1934;28(3):988–995. doi: 10.1042/bj0280988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann C. G., Lawlor G. J., Jr, Stiehm E. R., Swenseid M. E., Newton C., Herbert J., Ammann A. J., Jacob M. Immunologic responses in malnourished children. Am J Clin Nutr. 1975 Feb;28(2):89–104. doi: 10.1093/ajcn/28.2.89. [DOI] [PubMed] [Google Scholar]
- Pettit S. H., Holbrook I. B., Irving M. H. Comparison of clinical scores and acute phase proteins in the assessment of acute Crohn's disease. Br J Surg. 1985 Dec;72(12):1013–1016. doi: 10.1002/bjs.1800721226. [DOI] [PubMed] [Google Scholar]
- Rijke R. P., Gart R., Langendoen N. J. Epithelial cell kinetics in the descending colon of the rat. II. The effect of experimental bypass. Virchows Arch B Cell Pathol Incl Mol Pathol. 1979;31(1):23–30. doi: 10.1007/BF02889919. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P., Goboes K., Peeters M., Hiele M., Penninckx F., Aerts R., Kerremans R., Vantrappen G. Effect of faecal stream diversion on recurrence of Crohn's disease in the neoterminal ileum. Lancet. 1991 Sep 28;338(8770):771–774. doi: 10.1016/0140-6736(91)90663-a. [DOI] [PubMed] [Google Scholar]
- Sachar D. B., Smith H., Chan S., Cohen L. B., Lichtiger S., Messer J. Erythrocytic sedimentation rate as a measure of clinical activity in inflammatory bowel disease. J Clin Gastroenterol. 1986 Dec;8(6):647–650. doi: 10.1097/00004836-198612000-00011. [DOI] [PubMed] [Google Scholar]
- Sadikali F. Dipeptidase deficiency and malabsorption of glycylglycine in disease states. Gut. 1971 Apr;12(4):276–283. doi: 10.1136/gut.12.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagor G. R., Ghatei M. A., O'Shaughnessy D. J., Al-Mukhtar M. Y., Wright N. A., Bloom S. R. Influence of somatostatin and bombesin on plasma enteroglucagon and cell proliferation after intestinal resection in the rat. Gut. 1985 Jan;26(1):89–94. doi: 10.1136/gut.26.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini E. P., Kirk A. P., Chambers T. J. Rate and pattern of epithelial cell proliferation in ulcerative colitis. Gut. 1981 Aug;22(8):648–652. doi: 10.1136/gut.22.8.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater G., Kreel I., Aufses A. H., Jr Temporary loop ileostomy in the treatment of Crohn's disease. Ann Surg. 1978 Nov;188(5):706–709. doi: 10.1097/00000658-197811000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talstad I., Gjone E. The disease activity of ulcerative colitis and Crohn's disease. Scand J Gastroenterol. 1976;11(4):403–408. [PubMed] [Google Scholar]
- Terpstra O. T., Dahl E. P., Williamson R. C., Ross J. S., Malt R. A. Colostomy closure promotes cell proliferation and dimethylhydrazine-induced carcinogenesis in rat distal colon. Gastroenterology. 1981 Sep;81(3):475–480. [PubMed] [Google Scholar]
- Ursing B., Alm T., Bárány F., Bergelin I., Ganrot-Norlin K., Hoevels J., Huitfeldt B., Järnerot G., Krause U., Krook A. A comparative study of metronidazole and sulfasalazine for active Crohn's disease: the cooperative Crohn's disease study in Sweden. II. Result. Gastroenterology. 1982 Sep;83(3):550–562. [PubMed] [Google Scholar]
- Ursing B., Kamme C. Metronidazole for Crohn's disease. Lancet. 1975 Apr 5;1(7910):775–777. doi: 10.1016/s0140-6736(75)92438-1. [DOI] [PubMed] [Google Scholar]
- Weeke B., Jarnum S. Serum concentration of 19 serum proteins in Crohn's disease and ulcerative colitis. Gut. 1971 Apr;12(4):297–302. doi: 10.1136/gut.12.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R. C., Bauer F. L., Ross J. S., Malt R. A. Contributions of bile and pancreatic juice to cell proliferation in ileal mucosa. Surgery. 1978 May;83(5):570–576. [PubMed] [Google Scholar]
- Winterburn P. J., Phelps C. F. Purification and some kinetic properties of rat liver glucosamine synthetase. Biochem J. 1971 Feb;121(4):701–709. doi: 10.1042/bj1210701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelas P., Jagelman D. G. Loop illeostomy in the management of Crohn's colitis in the debilitated patient. Ann Surg. 1980 Feb;191(2):164–168. doi: 10.1097/00000658-198002000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hees P. A., van Elteren P. H., van Lier H. J., van Tongeren J. H. An index of inflammatory activity in patients with Crohn's disease. Gut. 1980 Apr;21(4):279–286. doi: 10.1136/gut.21.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]



