Abstract
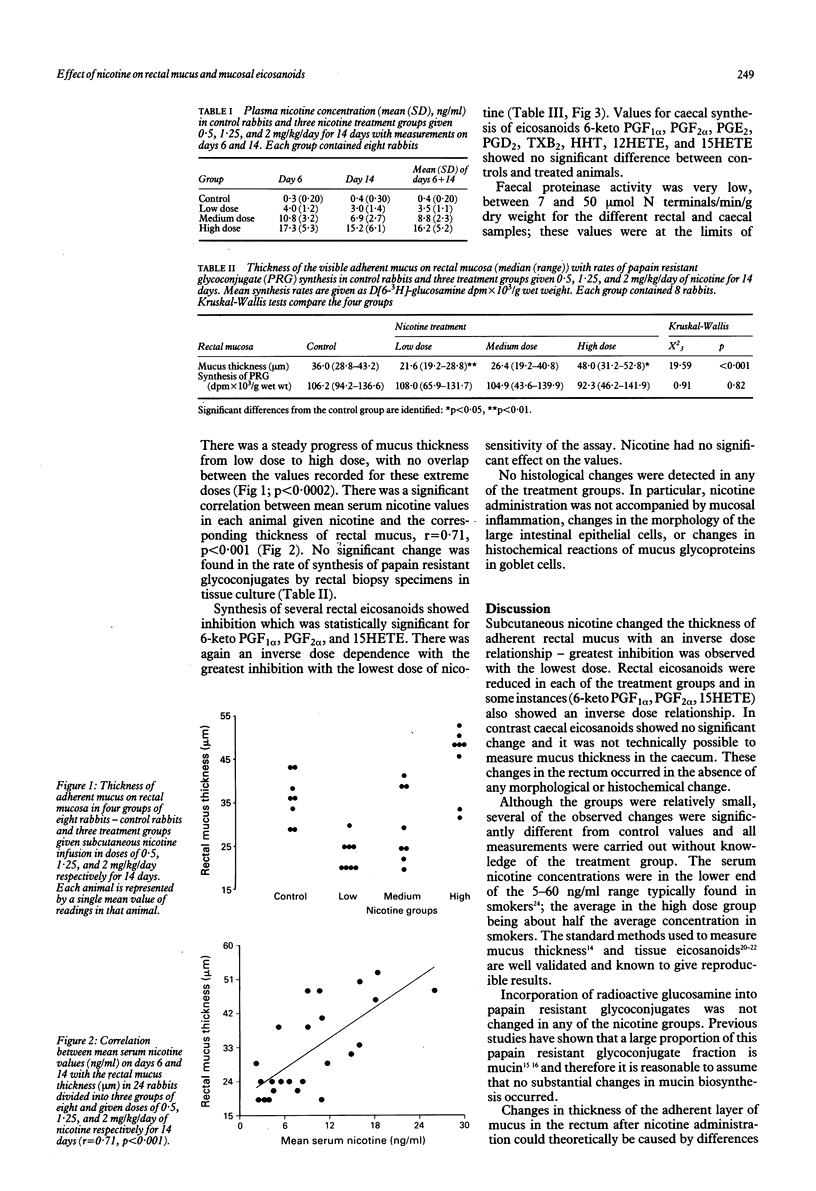
Because ulcerative colitis is largely a disease of non-smokers and nicotine may have a beneficial effect on the disease, the effect of nicotine on rectal mucosa in rabbits was examined. Nicotine was given subcutaneously by an Alzet mini-pump in doses of 0.5, 1.25, and 2 mg/kg/day for 14 days to three groups of eight animals and compared with eight controls. Mean (SD) serum nicotine concentrations (ng/ml) were 3.5 (1.1), 8.8 (2.3), and 16.2 (5.2) respectively in the treated groups. The thickness of adherent mucus on rectal mucosa in controls (median 36 microns) was significantly reduced by low dose (22 microns, p = 0.0011), and increased by high dose nicotine (48 microns, p = 0.035). Incorporation of radioactive glucosamine into papain resistant glycoconjugates was unchanged, indicating that mucin synthesis was unaltered. Prostaglandins (PG) were reduced, in some cases significantly (6-keto PGF1 alpha, PGF2 alpha, and hydroxy-eicosatetraenoic acid), by nicotine, which showed an inverse dose dependence--with greatest inhibition in relation to the lowest dose. Nicotine, and possibly smoking, may affect colitis by an action on mucosal eicosanoids and on adherent surface mucus secretion in the rectum and large bowel.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cope G. F., Heatley R. V., Kelleher J. K. Smoking and colonic mucus in ulcerative colitis. Br Med J (Clin Res Ed) 1986 Aug 23;293(6545):481–481. doi: 10.1136/bmj.293.6545.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfield A. P., Williams A. J., Clamp J. R., Wagner S. A., Mountford R. A. Degradation by bacterial enzymes of colonic mucus from normal subjects and patients with inflammatory bowel disease: the role of sialic acid metabolism and the detection of a novel O-acetylsialic acid esterase. Clin Sci (Lond) 1988 Jan;74(1):71–78. doi: 10.1042/cs0740071. [DOI] [PubMed] [Google Scholar]
- Feyerabend C., Russell M. A. A rapid gas-liquid chromatographic method for the determination of cotinine and nicotine in biological fluids. J Pharm Pharmacol. 1990 Jun;42(6):450–452. doi: 10.1111/j.2042-7158.1990.tb06592.x. [DOI] [PubMed] [Google Scholar]
- Harries A. D., Baird A., Rhodes J. Non-smoking: a feature of ulcerative colitis. Br Med J (Clin Res Ed) 1982 Mar 6;284(6317):706–706. doi: 10.1136/bmj.284.6317.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter A. C., Allen A., Garner A. Studies on mucus biosynthesis in the gastrointestinal tract. Symp Soc Exp Biol. 1989;43:27–36. [PubMed] [Google Scholar]
- Hutton D. A., Pearson J. P., Allen A., Foster S. N. Mucolysis of the colonic mucus barrier by faecal proteinases: inhibition by interacting polyacrylate. Clin Sci (Lond) 1990 Mar;78(3):265–271. doi: 10.1042/cs0780265. [DOI] [PubMed] [Google Scholar]
- Jentjens T., Smits H. L., Strous G. J. 16,16-Dimethyl prostaglandin E2 stimulates galactose and glucosamine but not serine incorporation in rat gastric mucous cells. Gastroenterology. 1984 Aug;87(2):409–416. [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kerss S., Allen A., Garner A. A simple method for measuring thickness of the mucus gel layer adherent to rat, frog and human gastric mucosa: influence of feeding, prostaglandin, N-acetylcysteine and other agents. Clin Sci (Lond) 1982 Aug;63(2):187–195. doi: 10.1042/cs0630187. [DOI] [PubMed] [Google Scholar]
- Motley R. J., Rhodes J., Kay S., Morris T. J. Late presentation of ulcerative colitis in ex-smokers. Int J Colorectal Dis. 1988 Aug;3(3):171–175. doi: 10.1007/BF01648362. [DOI] [PubMed] [Google Scholar]
- Motley R. J., Rhodes J., Williams G., Tavares I. A., Bennett A. Smoking, eicosanoids and ulcerative colitis. J Pharm Pharmacol. 1990 Apr;42(4):288–289. doi: 10.1111/j.2042-7158.1990.tb05411.x. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Isselbacher K. J. Glycoprotein composition of colonic mucosa. Specific alterations in ulcerative colitis. Gastroenterology. 1984 Nov;87(5):991–998. [PubMed] [Google Scholar]
- Psaila J. V., Myers B., Jones I. R., Rhodes J. Effect of prostaglandin PGE2 on alcohol-induced ulceration in the rat colon. Digestion. 1986;35(4):224–228. doi: 10.1159/000199372. [DOI] [PubMed] [Google Scholar]
- Quimby G. F., Bonnice C. A., Burstein S. H., Eastwood G. L. Active smoking depresses prostaglandin synthesis in human gastric mucosa. Ann Intern Med. 1986 May;104(5):616–619. doi: 10.7326/0003-4819-104-5-616. [DOI] [PubMed] [Google Scholar]
- Rhodes J. M., Black R. R., Gallimore R., Savage A. Histochemical demonstration of desialation and desulphation of normal and inflammatory bowel disease rectal mucus by faecal extracts. Gut. 1985 Dec;26(12):1312–1318. doi: 10.1136/gut.26.12.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. M. Colonic mucus and mucosal glycoproteins: the key to colitis and cancer? Gut. 1989 Dec;30(12):1660–1666. doi: 10.1136/gut.30.12.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. M., Gallimore R., Elias E., Allan R. N., Kennedy J. F. Faecal mucus degrading glycosidases in ulcerative colitis and Crohn's disease. Gut. 1985 Aug;26(8):761–765. doi: 10.1136/gut.26.8.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes M., Allen A., Dowling R. H., Murphy G., Lennard T. W. Inhibition of human gall bladder mucus synthesis in patients undergoing cholecystectomy. Gut. 1992 Aug;33(8):1113–1117. doi: 10.1136/gut.33.8.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert A., Nezamis J. E., Lancaster C., Hanchar A. J. Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology. 1979 Sep;77(3):433–443. [PubMed] [Google Scholar]
- Rozee K. R., Cooper D., Lam K., Costerton J. W. Microbial flora of the mouse ileum mucous layer and epithelial surface. Appl Environ Microbiol. 1982 Jun;43(6):1451–1463. doi: 10.1128/aem.43.6.1451-1463.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudra T., Motley R., Rhodes J. Does smoking improve colitis? Scand J Gastroenterol Suppl. 1989;170:61–68. doi: 10.3109/00365528909091354. [DOI] [PubMed] [Google Scholar]
- Russell M. A., Jarvis M. J., Feyerabend C., Saloojee Y. Reduction of tar, nicotine and carbon monoxide intake in low tar smokers. J Epidemiol Community Health. 1986 Mar;40(1):80–85. doi: 10.1136/jech.40.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata T., von Engelhardt W. Luminal mucin in the large intestine of mice, rats and guinea pigs. Cell Tissue Res. 1981;219(3):629–635. doi: 10.1007/BF00210000. [DOI] [PubMed] [Google Scholar]
- Zijlstra F. J., Vincent J. E., Mol W. M., Hoogsteden H. C., Van Hal P. T., Jongejan R. C. Eicosanoid levels in bronchoalveolar lavage fluid of young female smokers and non-smokers. Eur J Clin Invest. 1992 May;22(5):301–306. doi: 10.1111/j.1365-2362.1992.tb01466.x. [DOI] [PubMed] [Google Scholar]
- Zijlstra F. J., Wilson J. H. 15-HETE is the main eicosanoid present in mucus of ulcerative proctocolitis. Prostaglandins Leukot Essent Fatty Acids. 1991 May;43(1):55–59. doi: 10.1016/0952-3278(91)90133-p. [DOI] [PubMed] [Google Scholar]
- Zijlstra F. J., van Dijk A. P., Wilson J. H., van Riemsdijk-Overbeeke I. C., Vincent J. E., Ouwendijk R. J. 15-HETE is the main eicosanoid formed by human colonic mucosa. Agents Actions. 1992;Spec No:C53–C59. [PubMed] [Google Scholar]
- de Castella H. Non-smoking: a feature of ulcerative colitis. Br Med J (Clin Res Ed) 1982 Jun 5;284(6330):1706–1706. doi: 10.1136/bmj.284.6330.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ham A. C., Kort W. J., Bijma A. M., Zijlstra F. J., Vermeer M. A., Jeekel J. Eicosanoid profile of healing colon anastomosis and peritoneal macrophages in the rat. Gut. 1990 Jul;31(7):807–811. doi: 10.1136/gut.31.7.807. [DOI] [PMC free article] [PubMed] [Google Scholar]


