Abstract
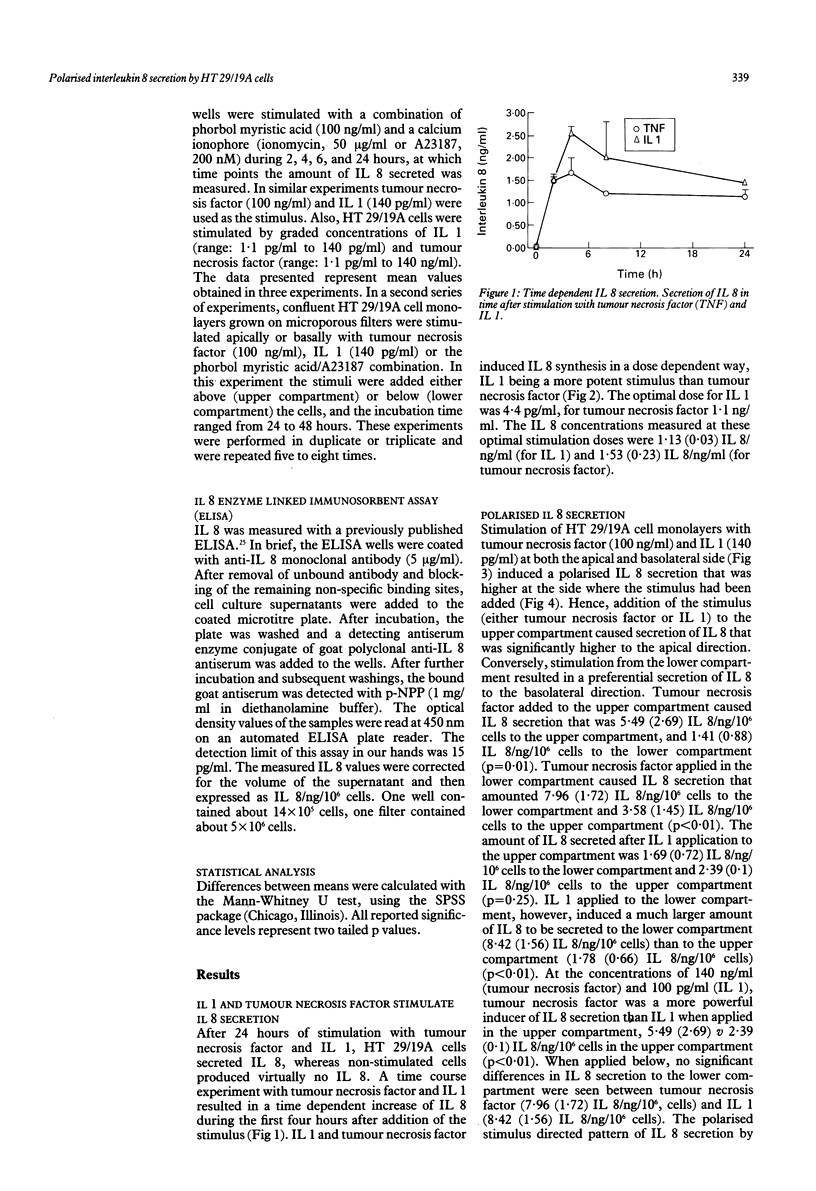
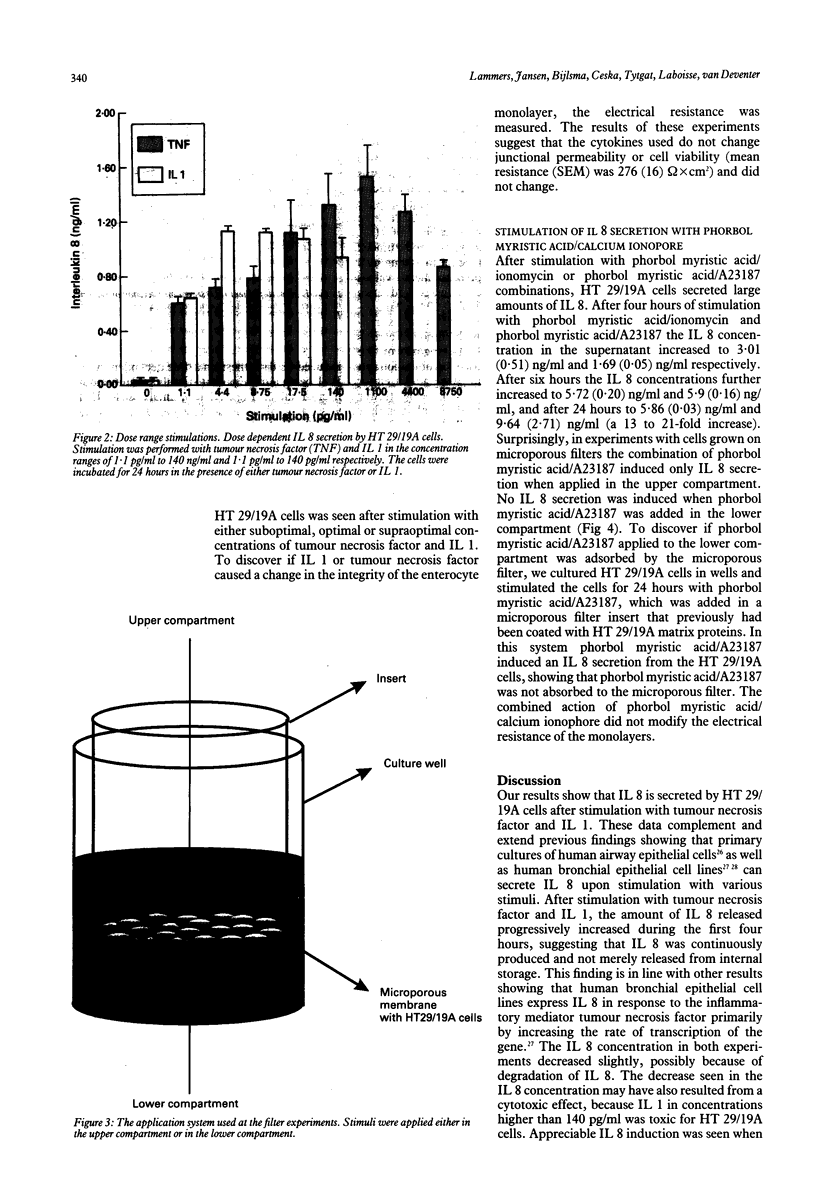
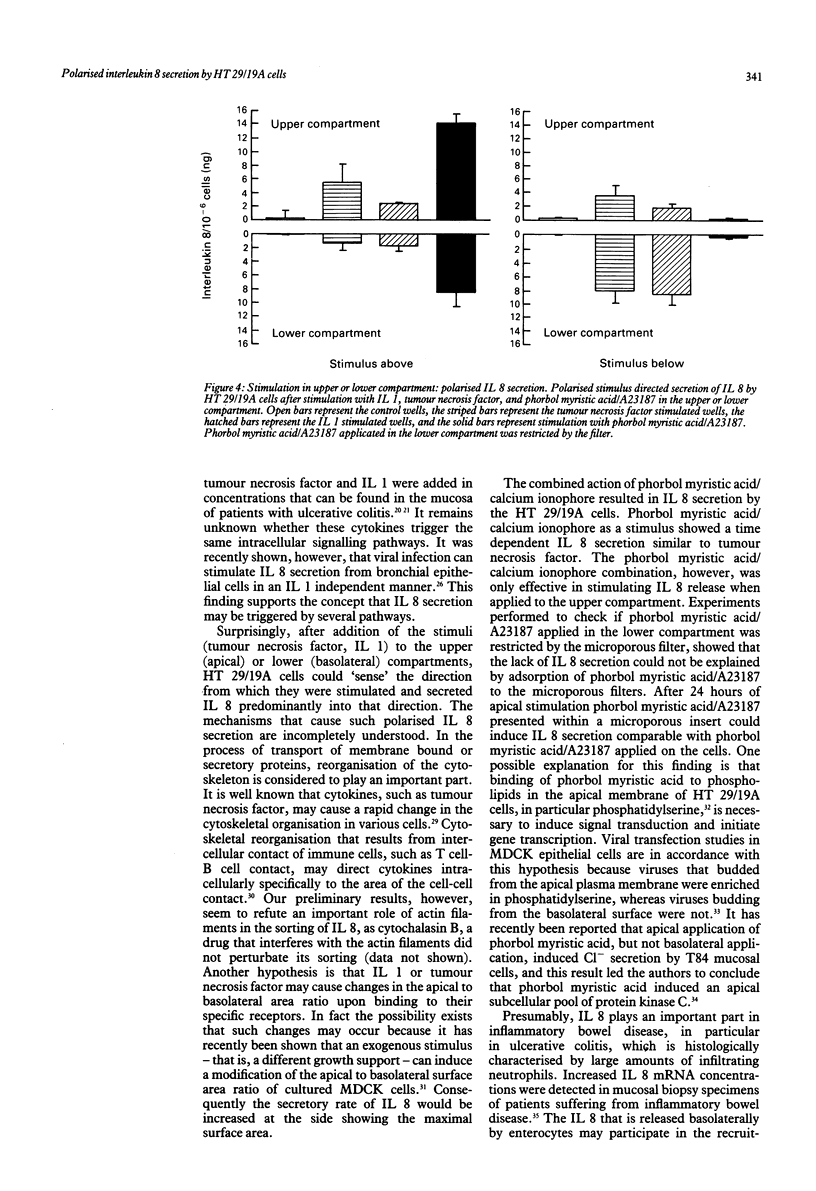
Interleukin 8 is a neutrophil chemotactic and stimulating cytokine induced by various inflammatory stimuli, including tumour necrosis factor, interleukin 1, and endotoxin. The ability of HT 29/19A enterocytes to synthesise interleukin 8 was studied. The results show that interleukin 1 is an important stimulus for interleukin 8 synthesis and secretion by HT 29/19A cells, being more potent than tumour necrosis factor. The tumour necrosis factor and interleukin 1 induced interleukin 8 secretion by HT 29/19A cells was seen to be polarised according to the direction of stimulation. These results support the concept that mucosal cells (enterocytes) may play an important part in initiating mucosal inflammation. Furthermore, it is proposed that HT 29/19A cells constitute a tool to study stimulus directed polarised cytokine secretion.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augeron C., Laboisse C. L. Emergence of permanently differentiated cell clones in a human colonic cancer cell line in culture after treatment with sodium butyrate. Cancer Res. 1984 Sep;44(9):3961–3969. [PubMed] [Google Scholar]
- Braegger C. P., Nicholls S., Murch S. H., Stephens S., MacDonald T. T. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet. 1992 Jan 11;339(8785):89–91. doi: 10.1016/0140-6736(92)90999-j. [DOI] [PubMed] [Google Scholar]
- Brynskov J., Tvede N., Andersen C. B., Vilien M. Increased concentrations of interleukin 1 beta, interleukin-2, and soluble interleukin-2 receptors in endoscopical mucosal biopsy specimens with active inflammatory bowel disease. Gut. 1992 Jan;33(1):55–58. doi: 10.1136/gut.33.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butor C., Davoust J. Apical to basolateral surface area ratio and polarity of MDCK cells grown on different supports. Exp Cell Res. 1992 Nov;203(1):115–127. doi: 10.1016/0014-4827(92)90046-b. [DOI] [PubMed] [Google Scholar]
- Camussi G., Turello E., Tetta C., Bussolino F., Baglioni C. Tumor necrosis factor induces contraction of mesangial cells and alters their cytoskeletons. Kidney Int. 1990 Nov;38(5):795–802. doi: 10.1038/ki.1990.273. [DOI] [PubMed] [Google Scholar]
- Carlier M. F. Actin: protein structure and filament dynamics. J Biol Chem. 1991 Jan 5;266(1):1–4. [PubMed] [Google Scholar]
- Choi A. M., Jacoby D. B. Influenza virus A infection induces interleukin-8 gene expression in human airway epithelial cells. FEBS Lett. 1992 Sep 14;309(3):327–329. doi: 10.1016/0014-5793(92)80799-m. [DOI] [PubMed] [Google Scholar]
- Colditz I., Zwahlen R., Dewald B., Baggiolini M. In vivo inflammatory activity of neutrophil-activating factor, a novel chemotactic peptide derived from human monocytes. Am J Pathol. 1989 Apr;134(4):755–760. [PMC free article] [PubMed] [Google Scholar]
- Elner V. M., Strieter R. M., Elner S. G., Baggiolini M., Lindley I., Kunkel S. L. Neutrophil chemotactic factor (IL-8) gene expression by cytokine-treated retinal pigment epithelial cells. Am J Pathol. 1990 Apr;136(4):745–750. [PMC free article] [PubMed] [Google Scholar]
- Gregory H., Young J., Schröder J. M., Mrowietz U., Christophers E. Structure determination of a human lymphocyte derived neutrophil activating peptide (LYNAP). Biochem Biophys Res Commun. 1988 Mar 15;151(2):883–890. doi: 10.1016/s0006-291x(88)80364-4. [DOI] [PubMed] [Google Scholar]
- Handler J. S. Overview of epithelial polarity. Annu Rev Physiol. 1989;51:729–740. doi: 10.1146/annurev.ph.51.030189.003501. [DOI] [PubMed] [Google Scholar]
- Heyman M., Crain-Denoyelle A. M., Nath S. K., Desjeux J. F. Quantification of protein transcytosis in the human colon carcinoma cell line CaCo-2. J Cell Physiol. 1990 May;143(2):391–395. doi: 10.1002/jcp.1041430225. [DOI] [PubMed] [Google Scholar]
- Isaacs K. L., Sartor R. B., Haskill S. Cytokine messenger RNA profiles in inflammatory bowel disease mucosa detected by polymerase chain reaction amplification. Gastroenterology. 1992 Nov;103(5):1587–1595. doi: 10.1016/0016-5085(92)91182-4. [DOI] [PubMed] [Google Scholar]
- Kupfer A., Mosmann T. R., Kupfer H. Polarized expression of cytokines in cell conjugates of helper T cells and splenic B cells. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):775–779. doi: 10.1073/pnas.88.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Oppenheim J. J., Matsushima K. Production of interleukin-8 by human dermal fibroblasts and keratinocytes in response to interleukin-1 or tumour necrosis factor. Immunology. 1989 Sep;68(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- Ligumsky M., Simon P. L., Karmeli F., Rachmilewitz D. Role of interleukin 1 in inflammatory bowel disease--enhanced production during active disease. Gut. 1990 Jun;31(6):686–689. doi: 10.1136/gut.31.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeman R. P., Chase H. S., Jr Protein kinase C does not participate in carbachol's secretory action in T84 cells. Am J Physiol. 1992 Jul;263(1 Pt 1):C140–C146. doi: 10.1152/ajpcell.1992.263.1.C140. [DOI] [PubMed] [Google Scholar]
- Martich G. D., Danner R. L., Ceska M., Suffredini A. F. Detection of interleukin 8 and tumor necrosis factor in normal humans after intravenous endotoxin: the effect of antiinflammatory agents. J Exp Med. 1991 Apr 1;173(4):1021–1024. doi: 10.1084/jem.173.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima K., Baldwin E. T., Mukaida N. Interleukin-8 and MCAF: novel leukocyte recruitment and activating cytokines. Chem Immunol. 1992;51:236–265. [PubMed] [Google Scholar]
- Nakamura H., Yoshimura K., McElvaney N. G., Crystal R. G. Neutrophil elastase in respiratory epithelial lining fluid of individuals with cystic fibrosis induces interleukin-8 gene expression in a human bronchial epithelial cell line. J Clin Invest. 1992 May;89(5):1478–1484. doi: 10.1172/JCI115738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Porat R., Clark B. D., Wolff S. M., Dinarello C. A. Enhancement of growth of virulent strains of Escherichia coli by interleukin-1. Science. 1991 Oct 18;254(5030):430–432. doi: 10.1126/science.1833820. [DOI] [PubMed] [Google Scholar]
- Radema S. A., van Deventer S. J., Cerami A. Interleukin 1 beta is expressed predominantly by enterocytes in experimental colitis. Gastroenterology. 1991 May;100(5 Pt 1):1180–1186. [PubMed] [Google Scholar]
- Schröder J. M., Mrowietz U., Morita E., Christophers E. Purification and partial biochemical characterization of a human monocyte-derived, neutrophil-activating peptide that lacks interleukin 1 activity. J Immunol. 1987 Nov 15;139(10):3474–3483. [PubMed] [Google Scholar]
- Schröder J. M., Sticherling M., Henneicke H. H., Preissner W. C., Christophers E. IL-1 alpha or tumor necrosis factor-alpha stimulate release of three NAP-1/IL-8-related neutrophil chemotactic proteins in human dermal fibroblasts. J Immunol. 1990 Mar 15;144(6):2223–2232. [PubMed] [Google Scholar]
- Seitz M., Dewald B., Gerber N., Baggiolini M. Enhanced production of neutrophil-activating peptide-1/interleukin-8 in rheumatoid arthritis. J Clin Invest. 1991 Feb;87(2):463–469. doi: 10.1172/JCI115018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A., Matsushima K., Van Damme J., Wang J. M., Polentarutti N., Dejana E., Colotta F., Mantovani A. IL-1 transcriptionally activates the neutrophil chemotactic factor/IL-8 gene in endothelial cells. Immunology. 1990 Apr;69(4):548–553. [PMC free article] [PubMed] [Google Scholar]
- Strieter R. M., Kunkel S. L., Showell H. J., Remick D. G., Phan S. H., Ward P. A., Marks R. M. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1 beta. Science. 1989 Mar 17;243(4897):1467–1469. doi: 10.1126/science.2648570. [DOI] [PubMed] [Google Scholar]
- Thornton A. J., Strieter R. M., Lindley I., Baggiolini M., Kunkel S. L. Cytokine-induced gene expression of a neutrophil chemotactic factor/IL-8 in human hepatocytes. J Immunol. 1990 Apr 1;144(7):2609–2613. [PubMed] [Google Scholar]
- Van Damme J. Granulocyte and monocyte chemotactic factors: stimuli and producer cells. Adv Exp Med Biol. 1991;305:1–9. doi: 10.1007/978-1-4684-6009-4_1. [DOI] [PubMed] [Google Scholar]
- Walz A., Peveri P., Aschauer H., Baggiolini M. Purification and amino acid sequencing of NAF, a novel neutrophil-activating factor produced by monocytes. Biochem Biophys Res Commun. 1987 Dec 16;149(2):755–761. doi: 10.1016/0006-291x(87)90432-3. [DOI] [PubMed] [Google Scholar]
- Westwick J., Li S. W., Camp R. D. Novel neutrophil-stimulating peptides. Immunol Today. 1989 May;10(5):146–147. doi: 10.1016/0167-5699(89)90164-3. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Matsushima K., Tanaka S., Robinson E. A., Appella E., Oppenheim J. J., Leonard E. J. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]