Abstract
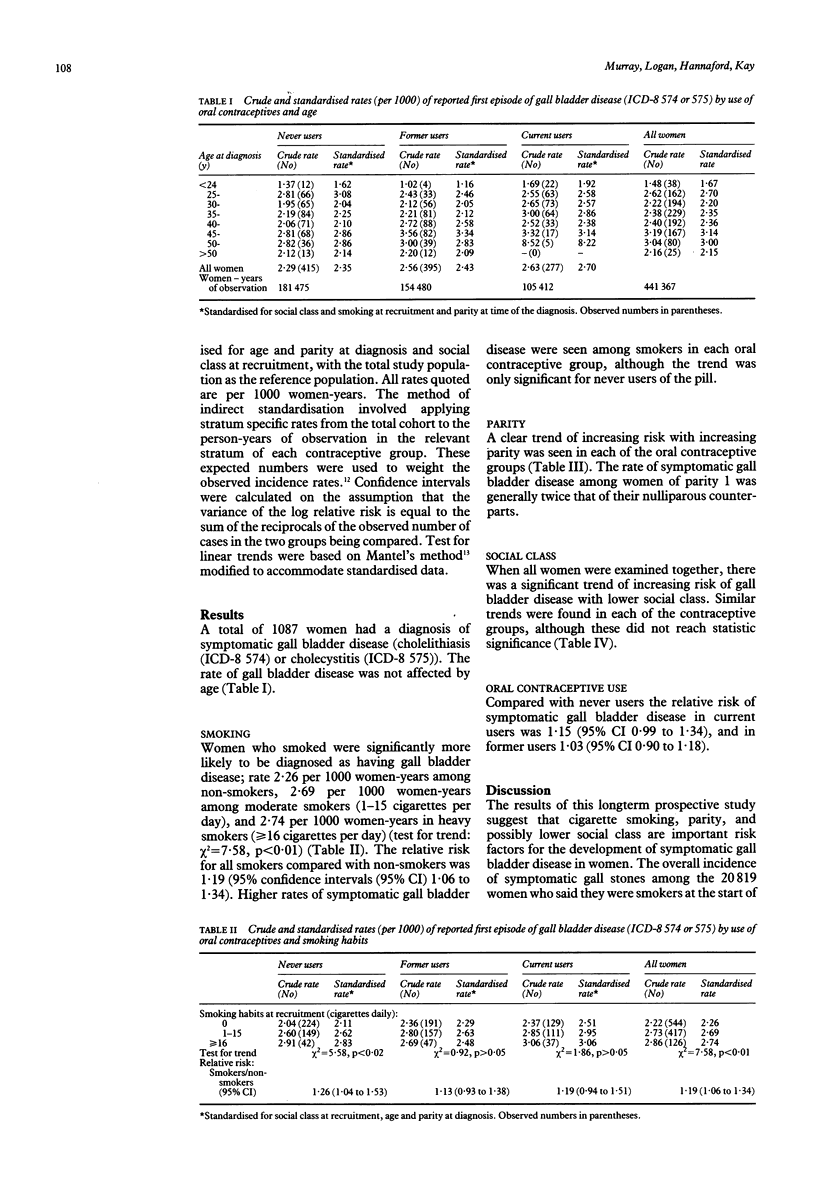
The effects of cigarette smoking and parity on the development of symptomatic gall bladder disease remain controversial. These relations have been examined in a cohort of 46,000 women followed for up to 19 years during the Royal College of General Practitioners' (RCGP) oral contraception study. During follow up, 1087 women were recorded as experiencing their first ever episode of symptomatic cholelithiasis (International Classification of Diseases, 8th revision (ICD-8) 574) or cholecystitis (ICD-8 575). Smokers were more likely to develop symptomatic gall bladder disease than non-smokers (relative risk 1.19; 95% confidence intervals (95% CI) 1.06 to 1.34) and there was a significant trend with the number of cigarettes smoked daily (test for trend chi 2 = 7.58, p < 0.01). This relation was most apparent among never users of oral contraceptives, although similar trends were found among current and former users. A significant direct relation between symptomatic gall bladder disease and parity was also found (test for trend chi 2 = 21.89, p < 0.001). When all were examined together a trend of increasing risk with lower social class was also found (test for trend chi 2 = 5.72, p = 0.02). Current users of oral contraceptives had a moderately increased risk of symptomatic gall bladder disease (relative risk 1.15; 95% CI 0.99 to 1.34), unlike former users (relative risk 1.03; 95% CI 0.90 to 1.18). These results suggest that smoking and parity are important risk factors for the development of symptomatic gall bladder disease in women.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Diehl A. K. Epidemiology and natural history of gallstone disease. Gastroenterol Clin North Am. 1991 Mar;20(1):1–19. [PubMed] [Google Scholar]
- Diehl A. K., Haffner S. M., Hazuda H. P., Stern M. P. Coronary risk factors and clinical gallbladder disease: an approach to the prevention of gallstones? Am J Public Health. 1987 Jul;77(7):841–845. doi: 10.2105/ajph.77.7.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen T. Gall stones in a Danish population. Relation to weight, physical activity, smoking, coffee consumption, and diabetes mellitus. Gut. 1989 Apr;30(4):528–534. doi: 10.1136/gut.30.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern F., Jr, Everson G. T. Contraceptive steroids increase cholesterol in bile: mechanisms of action. J Lipid Res. 1987 Jul;28(7):828–839. [PubMed] [Google Scholar]
- Layde P. M., Vessey M. P., Yeates D. Risk factors for gall-bladder disease: a cohort study of young women attending family planning clinics. J Epidemiol Community Health. 1982 Dec;36(4):274–278. doi: 10.1136/jech.36.4.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. P., Maher K., Nicholls J. F. Origin and fate of biliary sludge. Gastroenterology. 1988 Jan;94(1):170–176. doi: 10.1016/0016-5085(88)90626-9. [DOI] [PubMed] [Google Scholar]
- Lee S. P., Nicholls J. F. Nature and composition of biliary sludge. Gastroenterology. 1986 Mar;90(3):677–686. doi: 10.1016/0016-5085(86)91123-6. [DOI] [PubMed] [Google Scholar]
- Levy P. F., Smith B. F., LaMont J. T. Human gallbladder mucin accelerates nucleation of cholesterol in artificial bile. Gastroenterology. 1984 Aug;87(2):270–275. [PubMed] [Google Scholar]
- Maclure K. M., Hayes K. C., Colditz G. A., Stampfer M. J., Speizer F. E., Willett W. C. Weight, diet, and the risk of symptomatic gallstones in middle-aged women. N Engl J Med. 1989 Aug 31;321(9):563–569. doi: 10.1056/NEJM198908313210902. [DOI] [PubMed] [Google Scholar]
- Maringhini A., Ciambra M., Baccelliere P., Raimondo M., Pagliaro L. Sludge, stones, and pregnancy. Gastroenterology. 1988 Oct;95(4):1160–1161. doi: 10.1016/0016-5085(88)90218-1. [DOI] [PubMed] [Google Scholar]
- Murray F. E., Stinchcombe S. J., Hawkey C. J. Effect of indomethacin and misoprostol on fasted gallbladder volume and meal-induced gallbladder contractility in humans. Dig Dis Sci. 1992 Aug;37(8):1228–1231. doi: 10.1007/BF01296564. [DOI] [PubMed] [Google Scholar]
- Myers S. I. Effect of estrogen on rabbit gallbladder prostaglandin biosynthesis. J Surg Res. 1985 Jun;38(6):630–634. doi: 10.1016/0022-4804(85)90085-x. [DOI] [PubMed] [Google Scholar]
- O'Donnell L. J., Wilson P., Guest P., Catnach S. M., McLean A., Wickham J. E., Fairclough P. D. Indomethacin and postprandial gallbladder emptying. Lancet. 1992 Feb 1;339(8788):269–271. doi: 10.1016/0140-6736(92)91333-4. [DOI] [PubMed] [Google Scholar]
- Oral contraceptives and gallbladder disease. Royal College of General Practitioners' oral contraception study. Lancet. 1982 Oct 30;2(8305):957–959. [PubMed] [Google Scholar]
- Pastides H., Tzonou A., Trichopoulos D., Katsouyanni K., Trichopoulou A., Kefalogiannis N., Manousos O. A case-control study of the relationship between smoking, diet, and gallbladder disease. Arch Intern Med. 1990 Jul;150(7):1409–1412. [PubMed] [Google Scholar]
- Petitti D. B., Friedman G. D., Klatsky A. L. Association of a history of gallbladder disease with a reduced concentration of high-density-lipoprotein cholesterol. N Engl J Med. 1981 Jun 4;304(23):1396–1398. doi: 10.1056/NEJM198106043042305. [DOI] [PubMed] [Google Scholar]
- Rhodes M., Venables C. W. Symptomatic gallstones--a disease of non-smokers? Digestion. 1991;49(4):221–226. doi: 10.1159/000200725. [DOI] [PubMed] [Google Scholar]
- Ryan J. P., Pellecchia D. Effect of progesterone pretreatment on guinea pig gallbladder motility in vitro. Gastroenterology. 1982 Jul;83(1 Pt 1):81–83. [PubMed] [Google Scholar]
- Scragg R. K., McMichael A. J., Seamark R. F. Oral contraceptives, pregnancy, and endogenous oestrogen in gall stone disease--a case-control study. Br Med J (Clin Res Ed) 1984 Jun 16;288(6433):1795–1799. doi: 10.1136/bmj.288.6433.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijs C., Knipschild P., Leffers P. Pregnancy and gallstone disease: an empiric demonstration of the importance of specification of risk periods. Am J Epidemiol. 1991 Jul 15;134(2):186–195. doi: 10.1093/oxfordjournals.aje.a116071. [DOI] [PubMed] [Google Scholar]
- Vessey M., Doll R., Peto R., Johnson B., Wiggins P. A long-term follow-up study of women using different methods of contraception--an interim report. J Biosoc Sci. 1976 Oct;8(4):373–427. doi: 10.1017/s0021932000010890. [DOI] [PubMed] [Google Scholar]



