Human populations within technologically advanced countries are enjoying ever-increasing life spans. The average life span in the United States now extends well into the seventh decade of life. This average is in striking contrast to that of ≈50 years in 1900. Unfortunately, the benefit of a longer life span for many individuals is accompanied by an increased incidence of age-related diseases. The example that is addressed in this commentary is age-related macular degeneration (AMD), which presents itself in the retina and choroidal layers of the eye. The retina, an extension of the brain, forms the inner lining of the posterior eyeball. The choroid is the pigmented, vascular layer exterior to the retina and just underneath the “white” layer of the eye called the sclera. AMD is now the major cause of untreatable loss of vision in technologically advanced countries (1–3).
AMD affects a region of the human retina called the macula, which lies in the central axis of vision. The macula is a region ≈6 mm in diameter; at its center, there lies a depression called the fovea, which contains a population of photoreceptor cells of sufficient density to resolve individual letters in fine print. Therefore, it is this region of our retina that is used for reading and, because of the prevalence of cone photoreceptor cells, it is the region that initiates color vision. Reading is often the primary recreational activity during the “golden years” of life and sadly, this activity is severely impaired or lost in an expanding component of our population over the age of 65. Because the life span of humans continues to increase as a function of improved nutrition and increased awareness of environmental factors, AMD is expected to nearly double in the next 25 years (4). To place this in perspective, ≈35% of the human population of 75 years or older has some degree of AMD (1). Projections by the National Institute of Aging suggest that one in five people in the USA will be 65 or older by 2030. Individuals 85 and older could exceed 10 million at that time. There are considerable data to support the hypothesis that AMD has a significant genetic basis (4). Therefore, the identification of the underlying genes and their products is now a high priority. The search for disease-causing genes, although currently intense, has not yet met with significant success. Nonetheless, it is gratifying to note from the paper by Crabb et al. (5) in this issue of PNAS that proteome analysis has now been added to the armamentarium in the quest for insights into this important ocular disease. Crabb et al. used liquid chromatography tandem mass spectrometry in an analysis of a putative biomarker of AMD, termed “druse” (German for nodule or crystal). The strategy is analogous to analysis of protein plaques in Alzheimer's disease or atherosclerosis.
Proteome analysis has now been added to the armamentarium in the quest for insights into this important ocular disease.
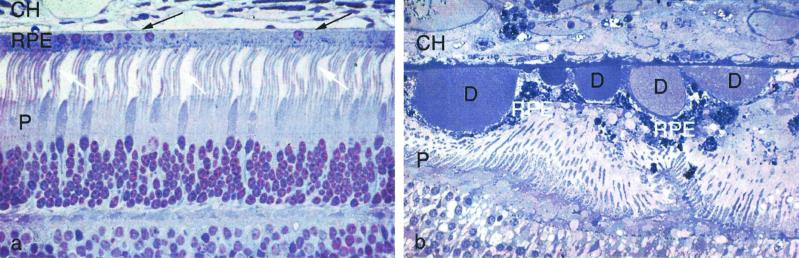
The clinical condition that is now known as AMD is diagnosed by viewing the macula of the retina with an ophthalmoscope. One of the early descriptions of AMD was given in 1884 by Nettleship (6), who described it as “central senile areolar choroidal atrophy.” Shortly thereafter, Haab (7) dubbed it “senile macular degeneration.” This unfortunate moniker was used for nearly 100 years until social sensibility finally eliminated the suggestion that the condition includes impairment of cognition. There is wide agreement among ophthalmologists that numerous large drusen, when present in both eyes, represent a significant risk factor for the evolution of early AMD into more advanced AMD, with loss of central vision (8). Drusen (Fig. 1b) are located between the retinal pigment epithelium (RPE) and Brüch's membrane, a multilayered extracellular matrix that lies between the RPE and the capillaries of the choroidal layer (Fig. 1a). These capillaries, called the choriocapillaris, supply the RPE and underlying photoreceptor cells with oxygen and nutrients. Clinicians divide drusen into two categories: “hard” drusen, which are in fact nodular, and “soft” drusen, which have a more diffuse appearance in histological sections. Drusen that reach a critical size can be viewed by the ophthalmologist. They are believed by many to be the first clinical indication of impending AMD. Hard drusen are more frequently associated with the “dry” form of AMD, which is not accompanied by the growth of new blood vessels from the choroid into the retina. Soft drusen are often associated with new vessel ingrowth (9). The wet form, although representing only ≈10% of AMD, is responsible for ≈90% of serious visual loss in AMD (10). Ophthalmologists have debated the relationship between the hard and soft forms of drusen and whether the hard form evolves into the soft form. Given the advantage provided by proteomic analysis of drusen according to morphological type, perception can now give way to hard scientific evidence. The paper by Crabb et al. (5) paves the way for this advance.
Fig 1.
Histological sections of normal (a) and AMD (b) retina and choroid at the level of the choriocapillaris (CH), retinal pigment epithelium (RPE), and photoreceptor (P) cells. In the normal retina, the photoreceptor cells are highly organized, and their light-sensitive outer segments (white arrows) are in close contact with the RPE. Brüch's membrane, a thin layer of extracellular matrix between the RPE and choriocapillaris (dark arrows), is barely visible at this magnification. The AMD photoreceptors are highly disorganized because of the presence of drusen (D), which also distorts the retinal pigment epithelium and Brüch's membrane.
The cellular source(s) of drusen has also been a subject of considerable interest and debate. The source is a critical issue because an answer to this question will provide insight into cellular targets for nutritional, drug, or gene-based therapy. Fundamentally, there are two hypotheses for the origin or drusen (8). One claims a strong vascular association and, therefore, a vascular origin. The second suggests that the RPE is responsible for drusen formation. The hypothesis regarding a vascular origin of drusen is based upon their association with the choriocapillaris (11). Most recently, in the context of current information concerning the etiology of atherosclerotic plaques, Curcio et al. (12) have drawn an analogy between drusen formation in Brüch's membrane and the formation of plaques in the intima of the arterial wall.
The hypothesis that drusen are formed by the RPE has its origin in the ultrastructural observations of Farkas et al. (13), who based some of their interpretations on one of the unique functions of the RPE. This cell monolayer is endowed with the responsibility for eating and digesting the distal ends of rod and cone photoreceptor cell outer segments (the light sensitive limbs of these cells) on a daily basis throughout the lifetime of the individual (14). Farkas et al. (13) suggested that drusen represented disposal sites for RPE cells. They proposed that RPE expels incompletely digested outer segment material into the extracellular matrix between the RPE and Brüch's membrane. Others provided evidence that RPE cells shed portions of their basal plasma membrane and its included cytoplasm into this matrix (15, 16), thereby contributing further to the process. The notion that the RPE might encounter problems with digestion of its considerable phagocytic burden is a reasonable one. Photoreceptor outer segments live in a potentially toxic environment that includes high oxygen and high photon flux. These conditions are conducive to photooxidative damage and the production of byproducts of lipid peroxidation such as malondialdehyde, which can cross link proteins (17). Of course it is possible, and even likely, that drusen originate from multiple sources, namely cells of the choroidal vasculature, the RPE, and blood components. Indeed, the paper by Crabb et al. (5) identifies components that could potentially arise from all of these sources.
Hageman and collaborators (18) have provided a provocative hypothesis for drusen biogenesis. Through a combination of drusen immunocytochemistry and analysis of mRNA in neighboring cells, they have shown that the RPE expresses many of the proteins that have been identified in drusen. A significant number of these proteins are cytosolic and, therefore, not secreted. Others include components of the complement cascade or other aspects of the immune response such as HLA antigens, which are intrinsic membrane proteins. Hageman et al., therefore, suggest that inflammation after RPE injury (brought on by gene mutations, oxidative damage, or lipofuscin accumulation) may play a role in drusen formation. This scenario proposes the release of cytokines by injured RPE, which attract monocytes from the neighboring choroid. Monocytes then mature in situ into dendritic cells, which interact with the necrotic RPE cells and ultimately retract, leaving components of their own processes along with the detritis from the dead RPE cells (19). Interestingly, Crabb et al. (5) have verified several of the proteins identified through immunocytochemistry by Hageman et al. (18). The list generated by Crabb et al. is substantially more extensive and enhanced further by the power of mass spectroscopy. Also included were carboxyethylpyrrole (CEP) and carboxymethyl lysine protein adducts. The CEP adducts are produced by the oxidation of docosahexaneoic acid-rich lipids. Cross-linked versions of TIMP-3 (a metalloproteinase inhibitor) and vitronectin, proteins that are made and secreted by the RPE, were also found. Drusen are found in normal aged eyes as well as clinically documented AMD eyes. In their groundbreaking paper, Crabb et al. (5) were able to distinguish the differences between drusen from normal and AMD eyes. Interestingly, crystallins, which are nonsecreted heat shock proteins (20) known to be synthesized by both the neurosensory retina and RPE, are among the most common proteins in drusen from AMD eyes. Again, this observation suggests a cellular origin for some of the drusen components as well as an oxidative stress component in drusen formation.
Hopefully, the application of powerful proteomic, molecular genetic, and cell biological methods will eventually resolve the role of drusen in the etiology of AMD and suggest therapeutic targets for this ocular disease. AMD will take an increasing emotional and economic toll if it remains a mystery to modern medicine. A recent report by Schutt et al. (21) reports a mass spectroscopic analysis of lipofuscin isolated from the RPE. Lipofuscin is a byproduct of intracellular digestion that is most likely harmful when present in sufficient quantity within the RPE cell (22). Thus, for both drusen and for lipofuscin, methods complementary to genomics have been applied in a creative manner in the quest for an understanding of the biogenesis of drusen and lipofuscin and their relationship to AMD.
See companion article on page 14682.
References
- 1.Klein R., Klein, B. E. & Linton, K. L. (1992) Ophthalmology 99, 933-943. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P., Smith, W., Attebo, K. & Wang, J. J. (1995) Ophthalmology 102, 1450-1460. [DOI] [PubMed] [Google Scholar]
- 3.Vingerling J. R., Dielemans, I., Hofman, A., Grobee, E. E., Hijmering, M., Kramer, C. F. & de Jong, P. T. (1995) Ophthalmology 102, 205-210. [DOI] [PubMed] [Google Scholar]
- 4.Stone E. M., Sheffield, V. C. & Hageman, G. S. (2001) Hum. Mol. Genet. 10, 2285-2292. [DOI] [PubMed] [Google Scholar]
- 5.Crabb J. W., Miyagi, M., Gu, X., Shadrach, K., West, K. A., Sakaguchi, H., Kamei, M., Hasan, A., Yan, L., Rayborn, M. E., Salomon, R. G. & Hollyfield, J. G. (2002) Proc. Natl. Acad. Sci. USA 99, 14682-14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nettleship D. (1884) Trans. Ophthalmol. Soc. U.K. 4, 165-166. [Google Scholar]
- 7.Haab O. (1885) Centralblat Augenheilkd 9, 384-391. [Google Scholar]
- 8.Penfold P. L., Madigan, M. C., Gillies, M. C. & Provis, J. M. (2001) Prog. Ret. Eye Res. 20, 385-414. [DOI] [PubMed] [Google Scholar]
- 9.Fine S. L., Berger, J. W., Maguire, M. G. & Ho, A. D. (2000) N. Engl. J. Med. 342, 483-492. [DOI] [PubMed] [Google Scholar]
- 10.Ferris F. L., Fine, S. L. & Hyman, L. (1984) Arch. Ophthalmol. 102, 1640-1642. [DOI] [PubMed] [Google Scholar]
- 11.Oosterhuis J. A. (1978) Excerpta Med. XXIII, 242-248. [Google Scholar]
- 12.Curcio C. A., Millican, C. L., Bailey, T. & Kruth, H. S. (2001) Invest. Ophthalmol. Visual Sci. 42, 265-274. [PubMed] [Google Scholar]
- 13.Farkas T. G., Sylvester, V. & Archer, D. (1971) Am. J. Ophthalmol. 71, 1196-1205. [DOI] [PubMed] [Google Scholar]
- 14.Young R. W. & Bok, D. (1969) J. Cell Biol. 42, 392-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burns R. P. & Feeney-Burns, L. (1980) Trans. Am. Ophthalmol. Soc. 116, 206-225. [PMC free article] [PubMed] [Google Scholar]
- 16.Isibashi R., Miller, J., Orr, G., Sorgente, N. & Ryan, S. J. (1987) Invest. Ophthalmol. Visual Sci. 28, 1116-1130. [PubMed] [Google Scholar]
- 17.Katz M. L. (1989) Adv. Exp. Med. Biol. 266, 109-116. [PubMed] [Google Scholar]
- 18.Hageman G. S., Luthert, P. J., Chong, N. H., Johnson, L. V., Anderson, D. H. & Mullins, R. F. (2001) Prog. Ret. Eye Res. 20, 705-732. [DOI] [PubMed] [Google Scholar]
- 19.Anderson D. H., Mullins, R. F., Hageman, G. S. & Johnson, L. V. (2002) Am. J. Ophthalmol. 134, 411-432. [DOI] [PubMed] [Google Scholar]
- 20.Horwitz J. (1993) Invest. Ophthalmol. Visual Sci. 34, 10-22. [PubMed] [Google Scholar]
- 21.Schutt F., Ueberle, B., Schnölzer, M., Holz, F. G. & Koplitz, J. (2002) FEBS Lett. 528, 217-221. [DOI] [PubMed] [Google Scholar]
- 22.Holz F. G., Schuett, F., Kopitz, J., Eldred, G. E., Kruse, F. E., Völcker, J. E. & Cantz, M. (1999) Invest. Ophthalmol. Visual Sci. 40, 737-743. [PubMed] [Google Scholar]