Abstract
Understanding of the Pleistocene megafaunal extinctions has been advanced recently by the application of simulation models and new developments in geochronological dating. Together these have been used to posit a rapid demise of megafauna due to over-hunting by invading humans. However, we demonstrate that the results of these extinction models are highly sensitive to implicit assumptions concerning the degree of prey naivety to human hunters. In addition, we show that in Greater Australia, where the extinctions occurred well before the end of the last Ice Age (unlike the North American situation), estimates of the duration of coexistence between humans and megafauna remain imprecise. Contrary to recent claims, the existing data do not prove the “blitzkrieg” model of overkill.
The cause of the extinction of giant birds, reptiles, and mammals in the late Pleistocene is, for palaeobiology, what Fermat's last theorem was for mathematics (1): a long-standing scientific puzzle that has captured the imagination of specialists and nonspecialists alike (2). Debate about the possible cause of the extinction has continued for over 150 yr (3–5), stimulated by new fossil finds, dating techniques, and modes of analysis. The debate is not strictly scientific, however, because it impacts on the broader understanding of the evolutionary theater of early human cultures, the fate of contemporary global biodiversity, and the rights of indigenous hunters (4, 6, 7). Indeed, the demise of the megafauna is often packaged as an environmental morality tale (8, 9). No matter why the debate interests people, there is no doubt that it is of great significance.
A great variety of competing scenarios have been proposed to explain the extinction of the megafauna such as climate change, disease, altered habitat condition (particularly due to the effects of landscape burning by humans), and the breakdown of food webs (3, 10, 11), but the presently ascendant idea is the so-called overkill hypothesis. This theory posits that extinctions were exclusively a result of human hunting (12), with a popular variant being the most restrictive case (termed “blitzkrieg”), where the extinction phase occurred within a few thousand years of human colonization (9, 13). In North America, human colonization coincided with the end of the last Ice Age, stymieing the disentanglement of human from environmental causes (12, 14). Nonetheless, proponents of the overkill hypothesis point out that the megafauna had survived previous glacial cycles where there was no human predation. In Greater Australia (Australia and the islands of Tasmania and New Guinea) and New Zealand, by contrast, extinction of the megafauna (which were generally more diminutive than their extra-Australian counterparts) apparently occurred independent of climate change. For this reason, the situation in Greater Australia and New Zealand are seen as important “independent tests” of the impact of human colonization on megafauna (3).
Although a residual extant megafauna did survive the Pleistocene extinction event (e.g., red kangaroo, bison, Asian elephant, llama, etc.), the only continent on Earth where a diverse assemblage of megafauna remains is Africa, which is also where modern humans arose. The African “anomaly” is typically explained by long-term coevolution of megafauna with humans such that the prey and predator are matched evenly, thereby creating trophic equilibrium. By contrast, the extra-African megafauna are characterized as completely naive to the human predator and therefore vulnerable to overkill and the disintegration of food webs (8, 15).
A recent series of articles, widely reported in the international media, have been heralded as a major breakthrough in this debate. First, a mathematical simulation model (16) was interpreted as showing the North American megafauna most likely succumbed to over-hunting after human colonization at the end of the last Ice Age. This article built on an earlier modeling exercise concerning New Zealand extinctions (17). Second, a metaanalysis of megafaunal remains (18) found a coincidence between megafaunal extinction and human colonization of Greater Australia (19, 20) ≈40,000–50,000 calendar years before present (yr B.P.). Given the significance and impact of these articles, we chose to examine their assumptions by undertaking some additional simulations and statistical analyses and reflect on possible future avenues that may advance this complicated problem.
Modeling Overkill
Recent computer modeling has been used to “prove” that megafaunal extinction was due solely to over-hunting by humans (16, 17). However, in both these models the unstated assumption was complete and unchanging prey naivety. Recent field research has shown that both contemporary marsupial and eutherian prey populations can develop vigilance toward novel predators quite rapidly (21, 22), which raises the question: What would happen to the predictions of these models if this assumption was relaxed?
Sensitivity to the Assumption of Prey Naivety.
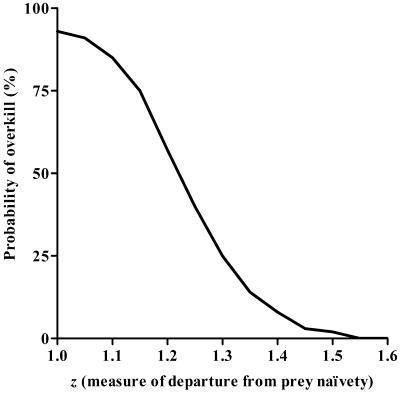
We ran a simplified (single-species) version of Alroy's (16) overkill model to evaluate the sensitivity of its results to axiomatic prey naivety. This was done by systematically varying the term z in the equation that describes predation efficiency (see Supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org, and Fig. 1 for details). To date, all overkill models assume prey off-take is described by a “type II functional response,” where z = 1, implying complete naivety of prey (e.g., refs. 10 and 16); or else take the extremely unrealistic view that off-take is entirely independent of prey density (e.g., ref. 17).
Fig 1.
Declining probability of simulated overkill as the cause of the megafaunal extinctions in relation to the departure from maximal predation efficiency (when z = 1 in the equation of functional response, where predation rate = FPz/[G + Pz]; see ref. 47). The parameter z can be interpreted as a proxy of relative megafauna naivety and has been fixed at 1 in all previous megafaunal extinction models, implying complete prey naivety.
When a functional response consistent with “predator-hardened” prey is used, the probability of overkill rapidly decays (Fig. 1). Yet when we set z = 1 (Alroy's assumption), our prediction of the probability of overkill (93%) actually exceeded Alroy's results (81%, based on the 37 scenarios in which he treated megafauna as an undifferentiated ecological unit, as we have done). Our higher predicted probability of overkill when z = 1 reflects our conservative assumption that human population dynamics were independent of prey density, in contrast to Alroy's coupling of these terms.
Alroy (16) conceded that his model failed to explain the differential survival of “geographically overlapping, morphologically similar and phylogenetically related extinct and extant species” such as the North American extinct and extant bison. He speculated that this may be related to the secondary importance of ecological factors that were not included in his model. Yet an alternative and equally valid explanation of this failure to discriminate extinct and extant species may be because of his invariant assumption of a type II functional response. These anomalies, and the acknowledgment of “gaps” in the model's realism, underline the critical importance of considering whether all of the extinct megafauna were naive and incapable of adapting to a new predator.
“Calibration” of Model Assumptions.
Contemporary studies provide the only realistic opportunity to test assumptions about how megafauna adapt to human predation (10). For instance, Berger et al. (21) studied how the relationship between moose (megafaunal prey) and wolves (predator) changed in response to the time period over which they had coexisted. Compared with populations in which wolves and moose have always coexisted, moose exposed to reintroduced wolves showed an initially elevated level of predation and lack of response to threatening stimuli but also rapidly lost this naive vulnerability (although the z parameter cannot be calculated from their data). Berger et al. (21) concluded that the megafauna that survived the end-Pleistocene extinctions in North America may have been better able to adapt to novel predation than the species that went extinct.
In northern Australia, experience gained from management programs designed to control feral animals such as the introduced Asian water buffalo has the potential to provide invaluable insights into the reaction of megaherbivores to intense human hunting pressure. Ridpath and Waithman (23) and Bayliss and Yeomans (24) demonstrated that the cost of exterminating buffalo rises in a negative exponential fashion as populations decline to low densities. This is due to both a stimulation of the reproductive and natural survival rates to approach maximal levels (24, 25) and reductions in search efficiency. The latter response reflects, in part, changed behavior of the animals such as increased wariness of humans (23) and a shift from diurnal to nocturnal grazing (26).
An objection to inferences made from such studies is that extant eutherian megafauna such as buffalo and moose might remain genetically “hard-wired” to be wary of predators with which they have coevolved (e.g., humans and wolves, respectively). However, some elegant recent studies have demonstrated that prey from a diverse range of phylogenies can rapidly develop vigilance to completely novel predators (see refs. 22, 27, and 28), substantially weakening the force of this criticism.
The Limits of Modeling.
The uncertainty of human–megafauna interactions is a great impediment to developing realistic overkill models. For instance, if hunting of megafauna was not primarily for subsistence but for motives such as prestige or sexual selection (e.g., ref. 29), then hunting pressure would be far higher than has been assumed in many models, and the probability of overkill would be increased greatly (10). Conversely, extinction may be an indirect consequence of human activities such as habitat modifications caused by landscape burning. For instance, the destruction of woody vegetation by burning has been postulated to explain the extinction of the Pleistocene Australian giant flightless bird, Genyornis newtoni, and all other megafauna ≈48,000 yr B.P. (11). This theory is consistent with the absence of evidence of human predation of the Australian megafauna such as kill sites or specialized tools (ref. 7 and S. Wroe, J. Field, R. Fullagar, and L. S. Jermiin, unpublished data) with the carbon-isotope signature of Genyornis fossil egg shells that indicates that it ate woody (C3 photosynthetic pathway) plant species, in contrast to fossil egg shells of the surviving ratite, the Emu (Dromaius noveahollandiae), which also eats tropical grass species (C4 photosynthetic pathway), and the apparent destruction of tropical rainforest in the Australian humid tropics ≈47,000 yr B.P. (30).
We conclude that models alone cannot resolve the question of whether the megafauna were hunted to extinction because of the critical importance of numerous assumptions, many of which may never be amenable to validation. However, the demonstration of a long period of coexistence would indicate some stabilization of the ecological relationship between megafauna and human hunters, whereas a short period of overlap could imply, inter alia, a blitzkrieg leading to a complete breakdown of the predator–prey system, or some other rapid anthropogenic impact such as massive landscape burning. An accurate determination of the degree of overlap between the extinct megafauna and human colonization is therefore clearly of great importance. This leads us next to consider recent advances in this field.
Dating the Greater Australian Record of Humans and Megafauna
No metaanalyses have tackled explicitly the question of the degree of overlap between megafauna and humans. This issue remains highly contentious, and resolution is frustrated by the scarcity of suitable fossil and archaeological sites and the difficulty in accurately dating them (18–20, 31, 32). However, a number of recent attempts to resolve the individual issues of the earliest colonization of Greater Australia by humans (19, 20) and the last occurrences of the Greater Australian megafauna (18) represent important steps in tidying up this messy field of inquiry. Here we consider the statistical robustness of the data for these datings (expressed in calendar years after appropriate calibrations).
When Did the Greater Australian Megafauna Become Extinct?
Recently, Roberts et al. (18) undertook a metaanalysis of the existing “reliable” data from 19 sites in Greater Australia and concluded that the megafauna went extinct sometime between 51,200 and 39,800 yr B.P., with a most likely date of 46,400 yr B.P. For their numerical analysis, they rejected all existing radiocarbon dates as too problematic, accepted only those skeletal remains that showed no evidence of anatomical disarticulation, and excluded any dates older than 55,000 yr B.P., leaving a sample size of seven sites with articulated remains. They believed these seven sites provided a statistically robust sample of the terminal extinction event.
A significant feature of the Roberts et al. (18) article is that they provided a 95% confidence interval around their estimate of the extinction event based on a compilation of dates rather than just using the measurement error associated with the laboratory estimate of a single youngest date, as has become customary in Australia (31). However, the assumptions driving their data pruning and the small sample size that results have been criticized (refs. 32 and 33 and S. Wroe, J. Field, R. Fullagar, and L. S. Jermiin, unpublished data), prompting the question: How sensitive are their conclusions to these assumptions?
What Is the Consequence of Data Pruning?
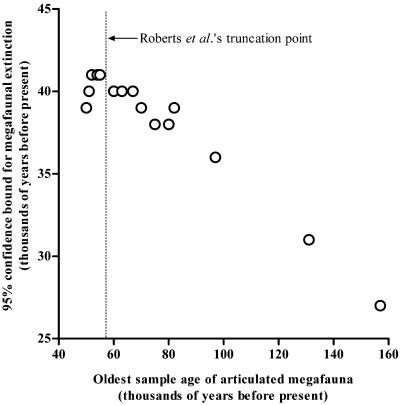
We used a simulation method akin to bootstrapping (see Supporting Text) to determine the lower age bound that resulted in a <5% probability of obtaining a sample of size n containing no date younger than 46,000 yr old. An important feature of this approach is that it seeks to identify the probable age of the oldest remains by analyzing the age distribution of all existing fossil finds, thereby obviating the futile quest to locate and date the very oldest site. However, these predictions cannot overcome the inherent laboratory measurement error associated with geochronology.
Our analysis shows that the results of Roberts et al. (18) are extremely robust irrespective of their data pruning (Fig. 2). Only when the three oldest sites are included in the sample does the 95% confidence interval differ substantially from their estimate. Indeed, our results show that the estimates derived from the existing data already fall within the bounds of the inherent laboratory measurement errors, and thus additional dating of new fossil deposits will not be able to reduce this uncertainty further. We conclude that it is reasonable to state that the megafauna became extinct sometime before 38,000–41,000 yr B.P. (± the estimated laboratory measurement error). However, this conclusion depends critically on the assumption that articulated megafaunal skeletons provide the only practical way of reliably dating extinctions, because reliable and routine direct dating of bones is currently outside the reach of the available suite of geochronological techniques (although dating of tooth enamel has shown potential; see ref. 34). Is this a reasonable stricture?
Fig 2.
The sensitivity of the lower 95% confidence bound for the timing of the Australian megafaunal extinction to the arbitrary truncation of the maximum age of articulated megafaunal skeletons included in the analyses, assuming a uniform distribution through time. The two youngest sites had an identical age, necessitating a minimum inclusion of the three youngest sites. Roberts et al. (18) used a truncation age of 55,000 yr B.P. (n = 7 sites).
Are Disarticulated Remains Necessarily Suspect?
A serious point of contention with the Roberts et al. (18) article was their decision to treat all sites with disarticulated remains as suspect, thereby excluding them from their analysis (34). What are the consequences of rejecting all the disarticulated specimens?
Our statistical analysis of the total data set compiled by Roberts et al. (18) shows clearly that the sample of most recent ages from sites that contain disarticulated remains (n = 8) are substantially younger than those that contain articulated skeletons (n = 19); the median optically stimulated luminescence age (disarticulated) = 37,500 yr B.P. versus articulated = 63,000 yr B.P. A randomization test (35) shows this difference to be highly significant (P < 0.001). Further, the ratio of articulated to disarticulated bones older than 46,000 yr B.P. is ≈10:1, whereas none of the younger sites contain articulated material. It should be noted, however, that the true pre-46,000-yr-old ratio may be close to or much greater than unity because of the decision by Roberts et al. (18) to focus preferentially on articulated skeletons.
There are at least two explanations for the observed absence of articulated remains more recent than 46,000 yr old. First, it could be, as postulated by Roberts et al. (18), that the supply of articulated megafauna skeletons ceased sometime after 46,000 yr B.P. because the animals had become extinct. Alternatively, the megafauna may have actually persisted long after this date, but the probability of a skeleton being preserved in an articulated state substantially declined over time, possibly because humans were dismembering megafauna (e.g., by butchering or scavenging); however, anthropological observations of modern indigenous hunters report a positive relationship between prey body size and the likelihood that at least some skeletal elements will be left in an articulated state (36, 37). Equally, humans may have coincided with or caused environmental changes that reduced the likelihood of at least partially articulated skeletons being preserved. This could be due to increasing aridity (ref. 35 and S. Wroe, J. Field, R. Fullagar, and L. S. Jermiin, unpublished data) or some other factor that changed geomorphological processes such as accelerated soil erosion after sustained landscape burning.
Which of these alternative explanations is more plausible? Bootstrap resampling simulations (38) show that depending on assumptions one makes about how the ratio of articulated to disarticulated remains have changed over time (e.g., constant at 10:1 or changing to either 1:1 or 1:10), the probability of failing to uncover an articulated skeleton from a sample of six sites ranges from 0 to 2 to 43%. This uncertainty makes it presently impossible to discriminate confidently between alternative hypotheses.
A possible test of the assumption of Roberts et al. (18) could be to determine whether the likelihood of articulated fossils of extant species (e.g., kangaroos, emus, etc.) being preserved remained relatively uniform through time for fossil deposits in a variety of environmental settings (e.g., humid and arid zones). If there is no substantial change in the ratio after the arrival of humans, then this would support the contentious decision (32) to exclude disarticulated remains from sites in which only the surrounding sediments have been dated (e.g., Cuddie Springs) and remove the question mark over the timing of the megafaunal extinction event. If, however, a substantial change in the ratio did occur subsequent to human colonization, then the precise dating of the megafaunal extinction becomes highly problematic and intractable until direct dating of fossil remains becomes practical and routine.
When Did Humans First Arrive in Greater Australia?
The timing of human colonization is disputed vigorously (31). O'Connell and Allen (19) argued that human colonization happened no earlier than 43,000 yr B.P. based on established radiocarbon-dating procedures (conventional and accelerator mass spectrometry methods with no advanced sample preparation) of archaeological material, a figure that also happens to be a limit of the technique (39). However, based on new advances in the removal of contaminants of radiocarbon-dated samples, Gillespie (20) disputes this conclusion and suggests that colonization occurred somewhere between 5,000 and 10,000 yr earlier than the previously accepted radiocarbon-date event horizon. Other new geochronological techniques such as luminescence dating of quartz and uranium/thorium-series dating have apparently pushed the date of human colonization to somewhere between 53,000 and 61,000 yr B.P. (40, 41), although the veracity of these dates is still debated (e.g., refs. 19, 42, and 43).
The uncertainty surrounding the validity of each of these advanced techniques makes it impossible to decide which samples should be retained or discarded. Indeed our resampling analyses bracket the date of human colonization of Greater Australia at between 44,200 and 71,500 yr B.P. (see Supporting Text). Obviously, such ambiguity has serious consequences for determining the degree of overlap between humans and megafauna.
How Long Did Humans and Megafauna Coexist in Greater Australia?
Inherent measurement errors associated with all geochronological techniques mean that the uncertainty surrounding each individual date makes it presently impossible to determine whether the overlap was less than the “radiometrically instantaneous interval” (for which their mean dated ages must be separated by at least three times the sum of their standard deviations; see ref. 31) regardless of the number of reliably dated samples. This precludes direct dating of “geologically instantaneous” events such as a human-induced rapid extinction, such as is required to demonstrate a blitzkrieg or massive ecological disruption associated with anthropogenic landscape burning. However, taking the extremes of the archaeological estimates for human colonization, it is at least possible to discriminate between two mutually exclusive alternatives: either there was no overlap, or there was a long period of coexistence.
If we take one extreme assumption, that all the dates derived from the advanced geochronological methods (including acid/base/dichromate oxidation with stepped combustion radiocarbon dates) are invalid, then simulations show that there is a less than 5% probability of any overlap between humans and megafauna. Nevertheless, to take this view is nonsensical. On the one hand there has been no dispute of the use of advanced geochronological techniques for dating the megafaunal extinction, but on the other hand the application of these same techniques for archaeological materials is considered unreliable. If we more rationally accept the radiocarbon dating based on advanced sample preparation as valid, then coexistence is absolutely certain, and the period of overlap falls between 2,000 and 10,000 yr. However, this time interval may change as more archaeological sites are reanalyzed after improved sample preparation, as has been advocated by Jones (44) and Roberts et al. (45). Yet even acid/base/dichromate oxidation with stepped combustion radiocarbon dating has an upper temporal limit (of ≈50,000–55,000 yr B.P.; see refs. 20 and 34), thus constraining the utility of the method in this situation. Finally, if the oldest archaeological dates based on the more controversial nonradiocarbon methodologies are indeed true, we predict a 5% probability that coexistence was as long as 25,500 yr (see Supporting Text for details). In sum, until the reliability of methods used to date the oldest archaeologist sites can be agreed on, it is difficult to conceive how this window of overlap can be determined more precisely.
Conclusions
Inferring robustly the cause of the extinction of the Pleistocene megafauna is a remarkably complicated problem that is very sensitive to assumptions concerning the analysis and interpretation of existing data. Although great progress has been made, it is premature to suggest (12, 16) that the problem has been cracked. On the balance of current evidence, it is most unlikely that there was no overlap between humans and megafauna, because for this to be true, all advanced geochronological techniques must be rejected as erroneous. In addition, field and/or laboratory tests are required to validate the assumption that the inferred age of all disarticulated fossils are unreliable. Although an improved chronology of the Greater Australian archaeological record will eliminate some possible explanations, dating alone cannot solve the problem of the cause of the extinctions, as evidenced by the ongoing debate concerning the interpretation of the superbly dated North American record (e.g., refs. 4 and 14). Stable isotope analyses of well dated fossil remains such as those used by Miller et al. (11) should yield important insights into the ecology and ecophysiology of Pleistocene fauna, thereby permitting the testing and development of more refined hypotheses about the extinction event.
Quantitative modeling can investigate various scenarios by integrating existing knowledge in a logical, structured, and transparent way, but it must be accepted that the output of these models is a product of explicit and implicit assumptions. Further, some explanations for extinction such as habitat modification, cultural motives for hunting large game other than for subsistence, climate change, and their interactions with hunting pressure are probably too complex to falsify with simulation modeling. These caveats should be borne in mind when making claims about the causes of past extinctions, particularly given the way such debates can influence current environmental management policy (see refs. 4 and 46).
Supplementary Material
Acknowledgments
This article builds on the constructive discussions raised during a specialist workshop D.M.J.S.B. attended on Australian megafaunal extinctions convened by Michael Smith of the National Museum of Australia in September 2001. We thank Richard Roberts and Richard Gillespie for generously sharing their knowledge and unpublished background materials. The article was improved greatly by the comments of Peter Bayliss, Graeme Brook, David Choquenot, Judith Field, Richard Gillespie, Geoff Hope, Jim O'Connell, Richard Roberts, Peter Whitehead, and Stephen Wroe. This work was undertaken by the Key Centre for Tropical Wildlife Management, Northern Territory University, under funding from the Australian Research Council.
References
- 1.Singh S., (1998) Fermat's Last Theorem: The Story of a Riddle That Confounded the World's Greatest Minds for 358 Years (Fourth Estate, London).
- 2.Diamond J. M. (2001) Nature 411, 755-757. [DOI] [PubMed] [Google Scholar]
- 3.Martin P. S. & Klein, R. G., (1984) Quaternary Extinctions: A Prehistoric Revolution (Univ. of Arizona Press, Tucson).
- 4.Grayson, D. K. (2002) Bull. Fla. Mus. Nat. Hist., in press.
- 5.Martin P. S. & Steadman, D. W. (1999) in Extinctions in Near Time, ed. MacPhee, R. D. E. (Kluwer Academic, New York), pp. 17–52.
- 6.Bowman D. (2001) J. Biogeogr. 28, 549-564. [Google Scholar]
- 7.Wroe S., Field, J. & Fullagar, R. (2002) Nat. Aust. 27, 54-61. [Google Scholar]
- 8.Flannery T. F., (2001) The Eternal Frontier: An Ecological History of North America and Its People (Atlantic Monthly, New York).
- 9.Flannery T. F., (1994) The Future Eaters: An Ecological History of the Australasian Lands and People (Reed Books, Sydney).
- 10.Choquenot D. & Bowman, D. M. J. S. (1998) Global Ecol. Biogeogr. Lett. 7, 167-180. [Google Scholar]
- 11.Miller G. H., Magee, J. W., Johnson, B. J., Fogel, M. L., Spooner, N. A., McCulloch, M. T. & Ayliffe, L. K. (1999) Science 283, 205-208. [DOI] [PubMed] [Google Scholar]
- 12.Flannery T. F. (1999) Science 283, 182-183. [Google Scholar]
- 13.Martin P. S. (1973) Science 179, 969-974. [DOI] [PubMed] [Google Scholar]
- 14.Beck M. W. (1996) Paleobiology 22, 91-103. [Google Scholar]
- 15.Martin P. S. (1967) in Pleistocene Extinctions: The Search for a Cause, eds. Martin, P. S. & Wright, H. E. (Yale Univ. Press, New Haven, CT), pp. 75–120.
- 16.Alroy J. (2001) Science 292, 1893-1896. [DOI] [PubMed] [Google Scholar]
- 17.Holdaway R. N. & Jacomb, C. (2000) Science 287, 2250-2254. [DOI] [PubMed] [Google Scholar]
- 18.Roberts R. G., Flannery, T. F., Ayliffe, L. K., Yoshida, H., Olley, J. M., Prideaux, G. J., Laslett, G. M., Baynes, A., Smith, M. A., Jones, R. & Smith, B. L. (2001) Science 292, 1888-1892. [DOI] [PubMed] [Google Scholar]
- 19.O'Connell J. F. & Allen, J. (1998) Evol. Anthropol. 6, 132-146. [Google Scholar]
- 20.Gillespie, R. (2002) Radiocarbon, in press.
- 21.Berger J., Swenson, J. E. & Persson, I. (2001) Science 291, 1036-1039. [DOI] [PubMed] [Google Scholar]
- 22.McLean I. G., Lundie-Jenkins, G. & Jarman, P. J. (1996) Biol. Conserv. 75, 51-62. [Google Scholar]
- 23.Ridpath M. G. & Waithman, J. (1988) Wildl. Soc. Bull. 16, 385-390. [Google Scholar]
- 24.Bayliss P. & Yeomans, K. M. (1989) Aust. Wildl. Res. 16, 651-676. [Google Scholar]
- 25.Boulton W. J. & Freeland, W. J. (1991) Wildl. Res. 18, 63-73. [Google Scholar]
- 26.Freeland W. J. & Bolton, W. J. (1990) Aust. Wildl. Res. 17, 411-420. [Google Scholar]
- 27.Griffin A. S., Blumstein, D. T. & Evans, C. (2000) Conserv. Biol. 14, 1317-1326. [Google Scholar]
- 28.Blumstein D. T., Daniel, J. C., Griffin, A. S. & Evans, C. S. (2000) Behav. Ecol. 11, 528-535. [Google Scholar]
- 29.Hawkes K., O'Connell, J. F. & Rogers, L. (1997) Trends Ecol. Evol. 12, 29-32. [DOI] [PubMed] [Google Scholar]
- 30.Turney C. S. M., Kershaw, A. P., Moss, P., Bird, M. I., Fifield, L. K., Cresswell, R. G., Santos, G. M., Di Tada, M. L., Hausladen, P. A. & Zhou, Y. (2001) J. Quaternary Sci. 16, 767-771. [Google Scholar]
- 31.Webb R. E. (1998) Radiocarbon 40, 749-758. [Google Scholar]
- 32.Field J., Fullager, R., Roberts, R. G., Yoshida, H., Flannery, T. F., Ayliffe, L. K., Olley, J., Prideaux, G. J., Laslett, G., Baynes, A., et al. (2001) Science 294, 7. [DOI] [PubMed] [Google Scholar]
- 33.Wroe S. & Field, J. (2001) Australasian Sci. 22, 21-25. [Google Scholar]
- 34.Turney C. S. M., Bird, M. I., Fifield, L. K., Roberts, R. G., Smith, M., Dortch, C. E., Grun, R., Lawson, E., Ayliffe, L. K., Miller, G. H., Dortch, J. & Cresswell, R. G. (2001) Quaternary Res. 55, 3-13. [Google Scholar]
- 35.Manly B. F. J., (1991) Randomization and Monte Carlo Methods in Biology (Chapman and Hall, London).
- 36.Bunn H. T., Bartram, L. & Kroll, E. (1988) J. Anthropol. Archaeol. 7, 412-457. [Google Scholar]
- 37.O'Connell J. F., Hawkes, K. & Blurton-Jones, N. G. (1992) J. Archaeol. Sci. 19, 319-345. [Google Scholar]
- 38.Efron B. & Tibshirani, R. J., (1993) An Introduction to the Bootstrap (Chapman & Hall, New York).
- 39.Chappell J., Head, J. & Magee, J. (1996) Antiquity 70, 543-552. [Google Scholar]
- 40.Roberts R. G., Jones, R., Spooner, N. A., Head, M. J., Murray, A. S. & Smith, M. A. (1994) Quaternary Sci. Rev. 13, 575-583. [Google Scholar]
- 41.Thorne A., Grun, R., Mortimer, G., Spooner, N. A., Simpson, J. J., McCulloch, M., Taylor, L. & Curnoe, D. (1999) J. Hum. Evol. 36, 591-612. [DOI] [PubMed] [Google Scholar]
- 42.Allen J. & Holdaway, S. (1995) Antiquity 69, 101-112. [Google Scholar]
- 43.Bowler J. M. & Magee, J. W. (2000) J. Hum. Evol. 38, 719-726. [DOI] [PubMed] [Google Scholar]
- 44.Jones R. (1982) in Archaeometry: An Australasian Perspective, eds. Ambrose, W. & Duerden, P. (Dept. of Prehist., Res. Sch. Pac. Stud., Australian Natl. Univ., Occas. Pap. Prehist. 12, Canberra).
- 45.Roberts R. G., Jones, R. & Smith, M. A. (1994) Antiquity 68, 611-616. [Google Scholar]
- 46.Jackson J. B. C., Kirby, M. X., Berger, W. H., Bjorndal, K. A., Botsford, L. W., Bourque, B. J., Bradbury, R. H., Cooke, R., Erlandson, J., Estes, J. A., et al. (2001) Science 293, 629-638. [DOI] [PubMed] [Google Scholar]
- 47.Real L. A. (1977) Am. Nat. 111, 289-300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.