Abstract
Most models of the primitive atmosphere around the time life originated suggest that the atmosphere was dominated by carbon dioxide, largely based on the notion that the atmosphere was derived via volcanic outgassing, and that those gases were similar to those found in modern volcanic effluent. These models tend to downplay the possibility of a strongly reducing atmosphere, which had been thought to be important for prebiotic synthesis and thus the origin of life. However, there is no definitive geologic evidence for the oxidation state of the early atmosphere and bioorganic compounds are not efficiently synthesized from CO2 atmospheres. In the present study, it was shown that a CO-CO2-N2-H2O atmosphere can give a variety of bioorganic compounds with yields comparable to those obtained from a strongly reducing atmosphere. Atmospheres containing carbon monoxide might therefore have been conducive to prebiotic synthesis and perhaps the origin of life. CO-dominant atmospheres could have existed if the production rate of CO from impacts of extraterrestrial materials were high or if the upper mantle had been more reduced than today.
Based on putative microfossil (1) and light carbon evidence in ancient sedimentary rocks (2) and the possible sterilizing consequences of the late heavy bombardment suggested by lunar records (3), the ancestor of modern life is thought to have originated about 3.8 billion years ago, although recent findings have questioned some of these data (4–7). The composition of the primitive atmosphere around this time remains uncertain, but volcanic outgassing could have been a major source of atmospheric gases. The oxidation state of volcanic gases would have depended on the oxidation state of the upper mantle (8). If the upper mantle had been in a reduced oxidation state, the gases would have been composed mainly of H2, H2O, CO, and N2. At more moderate temperatures, CO would have reacted with H2 to yield CH4 and H2O in the presence of catalysts, and N2 would have reacted with H2 to yield NH3 (9). The most reducing atmosphere possible would have been composed of CH4, NH3, H2, and H2O (herein referred to as a strongly reducing atmosphere), although it is difficult to reach this state because of factors such as photo-decomposition (10).
The oxidation state of the upper mantle 3.8 billion years ago is generally thought to have been near its current value (11, 12). Modern volcanic gases are composed mainly of H2O and CO2 (8). Walker suggested that the partial pressure of CO2 in the early atmosphere could have been as high as 10 bars (13). The dominant view in recent years has thus been that the atmosphere when life originated was composed of CO2, N2, and H2O combined with lesser amounts of CO, CH4, and H2 (11–14).
Strongly reducing atmospheres are more favorable for the synthesis of bioorganic compounds than CO2-N2-H2O atmospheres. Schlesinger and Miller (15) reported that carbon yields of amino acids from CH4-N2-H2O, and CO2-N2-H2O were 1% and 0.0006%, respectively. It was thus suggested that a strongly reducing atmosphere would have been required for the origin of life, which is contrary to the conclusions drawn from atmospheric modeling.
Recent research suggests that the continental crust and oceans could have formed by 4.3 billion years ago (16, 17). This notion suggests that CO2 precipitation as CaCO3 or MgCO3 could have started well before life originated (13). The partial pressure of CO2 in the primitive atmosphere when life originated may then have been much lower than previously thought, and a N2-dominant atmosphere may have existed. Sleep and Zahnle (18) suggested that the atmospheric mixing ratio of CO2 in the Hadean era would have been low because of vigorous mantle cycling of CO2 and the reaction of CO2 with impact ejecta. Geochemical data from Paleosols suggest that the atmospheric CO2 concentration at 2.8 billion years ago would have been a factor of 20 or more lower than those needed to keep the Earth's surface from freezing (19).
Since the lunar impact record suggests a high bolide flux on the Earth until 3.8 billion years ago (3), a large amount of CO may have been supplied by the impact of comets and asteroids. There would have been three possible extraterrestrial sources of CO. First, comets, which contain large amounts of CO (20), could have delivered CO directly. Second, organic carbon in comets or carbonaceous asteroids may have been oxidized by oxygen derived from silicates to form CO in impact plumes (21). Third, hot metallic iron produced by impacts of ordinary chondrites could have reduced atmospheric CO2 to CO (21). Thus, a CO-dominant atmosphere could have been built up from impacts. A CO-dominant atmosphere, however, could not have existed long, since CO is relatively unstable. One sink for CO could have been reaction to produce organic molecules. Another important sink is oxidation by OH radicals produced from water vapor photolysis to produce CO2 and hydration to produce formic acid (22).
A CO-dominant atmosphere might have existed intermittently around the time life originated and might have contributed to formation of bioorganic compounds. Abelson (23) pointed out that a CO atmosphere might have played an important role in the formation of bioorganic compounds and demonstrated the synthesis of HCN from such an atmosphere.
The syntheses of bioorganic compounds from a CO atmosphere is difficult because of the strong triple bond of CO. Bioorganic compounds are not effectively synthesized from a spark discharge in CO-N2-H2O, possibly because the CO is not efficiently dissociated by a spark discharge (24). High energy sources, such as cosmic rays and postimpact plumes, may be required to form bioorganic compounds.
We show here that with a high energy source CO-dominant atmospheres can give a variety of biologically important molecules with yields comparable to those obtained from a strongly reducing atmosphere. Protons of 2.5–3.0 MeV were used as the high energy source in this study. High energy protons are a major component of cosmic rays, and there was likely significant flux of cosmic rays on the early Earth.
Methods
An equimolar gas mixture of carbon monoxide (350 Torr; UHP grade, Toho Sanso, Yokohama, Japan) and 15N-enriched nitrogen (350 Torr; 99.8% 15N, Shoko, Tokyo) was enclosed in a glass tube (400 ml) containing liquid water (5 ml). 15N2 was used to identify possible contamination. The gas mixture was irradiated with protons generated by a van de Graaff accelerator (Tokyo Institute of Technology, Tokyo) at 297 K for 3 h. Each proton has an energy of 2.5–3.0 MeV, which is much higher than the bond dissociation energy of CO (11 eV) and N2 (9.8 eV). The total deposited energy was 13 kJ. The flux was 1 mA⋅cm−2. A sample of the aqueous solution of the irradiated mixture was withdrawn and acid hydrolyzed with 6 M HCl at 100°C for 24 h. Acid hydrolysis was performed because the bioorganic compounds are expected to be formed as their precursors. Most amino acids synthesized with this method are detectable only after acid hydrolysis (25). The samples were separated into three fractions on Dowex 50 (H+) with 1.5, 2.5, and 6 M HCl as eluant. Uracil, orotic acid, and 5-hydroxyuracil eluted in the 1.5 M HCl fraction. Nicotinic acid and 4,5-dihydroxypyrimidine eluted in the 2.5 M HCl fraction. Adenine and guanine eluted in the 6 M HCl fraction. These fractions were then rechromatographed by reversed-phase HPLC (Beckman 110B pump, 6.0 × 250 mm YMC ODS-AQ column, Kratos UV spectrophotometer set at 260 nm, 0.1 M, pH 4.5, sodium phosphate buffer) for final purification. The peak corresponding to each compound was collected and rechromatographed by using 0.1 M, pH 3, sodium phosphate buffer, then again by using 0.02 M, pH 5, ammonium acetate buffer. Compounds were identified by UV absorbance spectrum and GC/MS analysis of their trimethylsilyl derivatives. The trimethylsilyl derivatives were obtained by heating the compounds in a solution of N,O-bis(trimethylsilyl)trifluoroacetamide containing 1% trimethylchlorosilane and pyridine at 150°C for 30 min. The mass spectra can be seen in Figs. 2–6, which are published as supporting information on the PNAS web site, www.pnas.org. The quantitative data were obtained from the HPLC chromatograms with the ammonium acetate buffer by comparison with standard samples.
For calculation of production rates from cosmic rays and corona discharges, the value of 0.046 J⋅cm−2⋅yr−1 (26) and 0.105 J⋅cm−2⋅yr−1 (27) were taken as the energy flux, respectively.
Results and Discussion
The compounds detected are shown in Table 1. Uracil, 5-hydroxyuracil, orotic acid, 4,5-dihydroxypyrimidine, and nicotinic acid were detected. Adenine and guanine were tentatively identified by HPLC retention time, but their mass spectra could not be obtained because the yields were too low. Since CO is oxidized by proton irradiation to form CO2, energy yields of bioorganic compounds decrease with irradiation time. Under these experimental conditions, about half of the CO should have been converted into CO2 (25).
Table 1.
Carbon and energy yields of biologically important compounds synthesized from CO-N2-H2O
| C yields, % | E yields, mol⋅J−1 | Source | Ref. | |
|---|---|---|---|---|
| Ura | 1.1 × 10−3 | 1.7 × 10−12 | PI | This work |
| Oro | 1.6 × 10−3 | 2.0 × 10−12 | PI | This work |
| HUra | 5.5 × 10−4 | 8.6 × 10−13 | PI | This work |
| 45DHPy | 3.6 × 10−5 | 5.6 × 10−14 | PI | This work |
| Nic | 4.3 × 10−4 | 4.5 × 10−13 | PI | This work |
| Gua | <1.4 × 10−5 | <1.8 × 10−14 | PI | This work |
| Ade | <1.4 × 10−5 | <1.8 × 10−14 | PI | This work |
| Ura | 8 × 10−2 | 2 × 10−13 | HTPD | 57 |
| Cyt | 1 × 10−2 | 6 × 10−14 | HTPD | 57 |
| Gua | 1 × 10−3 | 4 × 10−15 | HTPD | 42 |
| Ade | <1 × 10−4 | <4 × 10−16 | HTPD | 42 |
| Oro | 4 × 10−3 | — | ED | 58 |
| Im | — | 1.0 × 10−11 | PI | 25 |
| Gly | 0.22 | 2.1 × 10−9 | PI | |
| Gly | 0.1 | 1 × 10−12 | HTPD | 24 |
| Gly | 5.6 | — | ED | 58 |
| Asp | 1.3 × 10−2 | 6.0 × 10−11 | PI | |
| Asp | 7 × 10−3 | 2 × 10−13 | HTPD | 24 |
| Ser | 3.9 × 10−3 | 2.5 × 10−11 | PI | |
| Ala | 3.4 × 10−2 | 2.1 × 10−10 | PI | |
| Ala | 3 × 10−2 | 6 × 10−14 | HTPD | 24 |
| β-Ala | 2.0 × 10−2 | 1.3 × 10−10 | PI | |
| β-Ala | 1 × 10−2 | 1 × 10−13 | HTPD | 24 |
| Sar | — | — | PI | |
| Glygly | 0.64 | — | ED | 58 |
C yields, carbon yields; E yields, energy yields; PI, proton irradiation; HTPD, high-temperature plasma discharge; ED, electric discharge; Ura, uracil; Cyt, cytosine; Gua, guanine; Ade, adenine; Oro, orotic acid; HUra, 5-hydroxyuracil; 45DHPy, 4,5-dihydroxypyrimidine; Nic, nicotinic acid; Im, imidazole; Gly, glycine; Asp, aspartic acid; Ser, serine; Ala, alanine; β-Ala, β-alanine; Sar, sarcosine; Glygly, glycylglycine.
The gas mixture had been irradiated for 3 h.
The carbon yields were calculated from the percentage of carbon in products based on carbon in synthesized polymers.
CO2 produced from a reaction of CO and H2O was continuously removed with Ca(OH)2 during discharge.
A CO-N2-H2O gas mixture was irradiated with 40 MeV protons generated from an SF cyclotron (Institute for Nuclear Study, University of Tokyo). Total deposited energy was 3.9 kJ. The amino acids were identified by GC/MS.
The energy yield of uracil from the 1-h irradiation was 7.1 × 10−12 mol⋅J−1. Taking this value and the energy flux of cosmic rays as 0.046 J⋅cm−2⋅yr−1 (26), the production rate of uracil from a CO-N2-H2O atmosphere is estimated to be 3.3 × 10−13 mol⋅cm−2⋅yr−1. The production rate allows a calculation of the steady-state concentration in the primitive ocean with a volume assumed to be 300 liter⋅cm−2, its current value. At steady state, the production rate equals the rate of decomposition. Using the rate constant of decomposition of uracil at pH 7 and 25°C as 4.0 × 10−7 yr−1 (28), the steady-state concentration of uracil in the primitive ocean is estimated to be 2.8 × 10−9 mol⋅liter−1. This concentration may be too low to produce more complex biomolecules. However, uracil could have been concentrated by evaporation or eutectic freezing, as uracil is relatively stable [t1/2 = 1.7 × 106 yr at pH 7 and 25°C (28)].
The reaction mechanisms for the syntheses of compounds detected here are uncertain. Although uracil, 5-hydroxyuracil, orotic acid, and 4,5-dihydroxypyrimidine are synthesized from a HCN polymerization (29, 30), this is not likely to be a major mechanism in this case, because yields of adenine and guanine would be much higher than that of uracil if these compounds were derived exclusively from HCN polymerization. Uracil can also be obtained from the hydrolysis of cytosine synthesized by reaction of cyanoacetaldehyde and urea (31). However, this is also not likely to be the source of uracil in this case, because only 3% of a cytosine standard was hydrolyzed to uracil under the hydrolysis conditions, and cytosine was not detected in the proton-irradiated sample. Nicotinic acid can be synthesized by a number of reaction pathways (32, 33). For example, nicotinonitrile, which hydrolyzes to nicotinic acid, can be synthesized from the reaction of cyanoacetaldehyde, propiolaldehyde, and ammonia (32), which are in turn synthesized from a spark discharge (32, 34, 35).
CO Atmospheres vs. Strongly Reducing Atmosphere.
A CO-dominant atmosphere can give bioorganic compounds with yields comparable to those obtained from a strongly reducing atmosphere. The uracil yield from proton irradiation in a CO-N2-H2O mixture is slightly higher than that from proton irradiation in CH4-N2-H2O (36). Uracil synthesis from a spark discharge in CH4-N2-H2O or CO-N2-H2O has not been reported.
Adenine has been detected in a sample prepared from a spark discharge experiment using CH4-N2-NH3-H2O and frozen at −20°C for 5 yr (37). The carbon yield was 6 × 10−4 %, which is slightly lower than the 1.1 × 10−3 % of uracil synthesized from the proton irradiation of CO-N2-H2O. A room temperature electric discharge solution contained no detectable adenine.
A CO-N2-H2O atmosphere gives comparable amounts of amino acids as a CH4-N2-H2O atmosphere. The energy yield of glycine from proton irradiation of CO-N2-H2O is 2 × 10−9 mol⋅J−1, which is comparable to the 2 × 10−9 mol⋅J−1 from proton irradiation in CH4-N2-H2O (26) and the 8.7 × 10−10 mol⋅J−1 from a spark discharge acting on CH4-N2-NH3-H2O (15). The production rate of amino acids from proton irradiation of CO-N2-H2O is estimated to be 1.2 × 10−10 mol⋅cm−2⋅yr−1, which is comparable with the 1.5 × 10−10 mol⋅cm−2⋅yr−1 estimated from a spark discharge acting on CH4-N2-NH3-H2O mixtures.
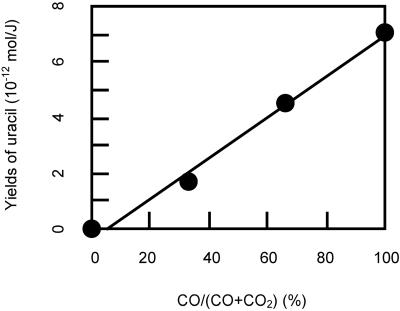
Since it is generally held that CO2 would have been present in the primitive atmosphere, the effect of CO2 on the formation of uracil was investigated. Fig. 1 shows the energy yield of uracil with various mixing ratios of CO/CO2 in CO-CO2-N2-H2O gas mixtures (36). The yield of uracil is approximately proportional to CO/(CO+CO2). This finding suggests that CO2 does not inhibit the reaction that forms uracil. This is also true of glycine synthesis (38).
Fig 1.
Energy yields of uracil with varying CO/CO2 mixing ratios in CO-CO2-15N2-H2O. The gas mixture had been irradiated for 1 h.
A large amount of molecular hydrogen may have been present in the primitive atmosphere if the oxidation state of the upper mantle were reducing. A H2-CO-N2-H2O or H2-CO2-N2-H2O atmosphere is more favorable for amino acid synthesis than an atmosphere without H2 (15, 39, 40). For example, in case of reaction of a H2-CO-N2-H2O atmosphere with a spark discharge, the carbon yields of amino acids are 0.05% and 0.9% at H2/CO = 0 and H2/CO = 1, respectively (15). The amino acid yields from H2-CH4-N2-H2O are approximately independent of the H2/CH4 ratio. H2 seems to inhibit the synthesis of the nucleic acid bases (41, 42).
Ammonia could not have been more than a minor component in the primitive atmosphere, even if the oxidation state of the upper mantle were highly reducing. Ammonia is photochemically labile and its half-life in the primitive atmosphere would have been very short on a geological time scale (10). Ammonia is very soluble in water and some NH4+ may have been adsorbed on clay minerals. Bada and Miller (43) estimated that the maximum value of the partial pressure of NH3 in the primitive atmosphere would have been 7.3 × 10−6 atm at 25°C with a pH 8 ocean. They suggested that most of the ammonia would have been dissolved in the oceans and thus could have contributed to the synthesis of amino acids by the Strecker synthesis. The carbon yield of amino acids from a spark discharge acting on a CO-N2-H2O gas mixture over 0.05 M aqueous NH4Cl is 10 times higher than that without NH4Cl, although the effect of gaseous NH3 on the reaction (1 × 10−4 atm in that case) is unclear (15). The amino acid yields from spark discharge experiments on NH3-H2-CH4-N2-H2O gas mixtures are approximately equal to those from mixtures without NH3 (15). The yield of uracil from CO-N2-H2O was four times higher than that from CO-NH3-H2O in proton irradiation experiments (36).
It has been shown here that a CO-CO2-N2-H2O atmosphere can yield comparable amounts of bioorganic compounds as a strongly reducing atmosphere. Considering that a CO2-N2-H2O atmosphere does not yield bioorganic compounds efficiently, and that it is difficult to accept a strongly reducing atmosphere from models of early atmospheric chemistry and geology, a CO-CO2-N2-H2O atmosphere may be more favorable for prebiotic synthesis and the origin of life.
Other Models for the Origin of Life.
Recently, Kasting and Brown (14) suggested that a CO2-N2-H2O atmosphere containing tens to hundreds of ppm of CH4 might have existed. Such amounts of CH4 could have produced a small amount of HCN and also contributed to early greenhouse warming of the Earth. If the atmospheric ratio of CH4 had been 100 ppm, the production rate of HCN can be estimated to have been 108 cm−2⋅s−1 (estimated from ref. 44). However, biologically important molecules, such as nucleic acid bases and amino acids, might not have been synthesized sufficiently via HCN polymerizations with such a low production rate of HCN if at least portions of the Earth were not frozen (45). The steady-state concentration of HCN in the primitive ocean at pH 8 and 25°C with the production rate of 108 cm−2⋅s−1 is estimated to be 8 × 10−10 M. HCN can effectively polymerize to form biologically important molecules when its concentration is higher than 0.01 M (46). At lower concentration, hydrolysis of HCN is predominant to form ammonia and formic acid (46). If the production rate of HCN were approximately 1,000 times higher, it would still be too low to produce significant amount of biologically important molecules unless portions of the Earth were frozen (45). Therefore, it seems unlikely that a CO2-N2-H2O atmosphere containing a small amount of CH4 played an important role for the synthesis of bioorganic compounds via HCN polymerizations.
If the primitive Earth had a CO2-dominant atmosphere, bioorganic compounds would not have been efficiently synthesized in the atmosphere. Hydrothermal vents may have been potential environments for the synthesis of bioorganic compounds (47). However, similar problems that affect atmospheric syntheses would have affected hydrothermal vent syntheses. If the upper mantle had been near its current oxidation state, most of the carbon available for synthesis of bioorganic compounds in the hydrothermal vents would have been in the form of CO2 and bioorganic compounds would not have been effectively synthesized. Fluids discharged from present hydrothermal vents are composed of H2O and CO2 combined with lesser amount of H2, H2S, N2, CH4, CO, and NH3 (48).
Some organic compounds, such as acetic acid and pyruvic acid, can be synthesized from CO in the presence of sulfide minerals under simulated hydrothermal conditions (49, 50). However, hydrothermal vent syntheses are not very efficient with respect to the synthesis of important biological compounds compared with atmospheric syntheses. Glycine and alanine can be synthesized from CH4 and N2 under simulated hydrothermal conditions (51), but the carbon yields are 1,000 times lower than those from simulated atmospheric syntheses. Shock (52) suggested that formation of amino acids from CO2 was favorable under disequilibrium hydrothermal conditions. However, there is as yet no experimental evidence showing that amino acids can be synthesized from CO2 under hydrothermal conditions (53).
Another potential source of prebiotic bioorganic compounds may have been delivery of extraterrestrial material by comets and carbonaceous chondrites, although it is uncertain how significant this source may have been (54). Using data from the Murchison meteorite (55, 56) and a production rate of intact exogenous organics from airbursts estimated by Chyba and Sagan (27), the delivery rates of uracil and glycine by meteorites 3.8 billion years ago are estimated to be 20 mol⋅yr−1 and 3 × 103 mol⋅yr−1, respectively. These values are much smaller than the estimated production rates of 2 × 106 mol⋅yr−1 for uracil and 5 × 108 mol⋅yr−1 for glycine synthesized from the proton irradiation in CO-N2-H2O. Assuming that the production rate of uracil from cosmic rays is proportional to CO/(CO+CO2) as shown in Fig. 1, when the CO/CO2 ratio is 10−5, its production rate is equal to that from delivery by meteorites.
Conclusions
A CO-dominant atmosphere may have existed when life originated. This atmosphere could have produced a variety of bioorganic compounds with yields comparable to those obtained from a strongly reducing atmosphere. A small amount of CO2 could have allowed the primitive Earth to freeze. This could mean that CO would have been more stable in the atmosphere than previously thought because of the reduced vapor pressure of water. Methane and ammonia would have been also more stable and could have contributed to the synthesis of bioorganic compounds. CO2 is likely to have been present, but it might not have been significantly involved in the synthesis of bioorganic compounds.
Acknowledgments
We are grateful to Dr. J. L. Bada, Dr. J. F. Kasting, and Dr. G. D. McDonald for many helpful discussions. We are also grateful to Mr. K. Kawasaki for van de Graaff operation and Mr. S. Takeda and Mr. T. Kaneko for help in analysis. This work was supported by a National Aeronautics and Space Administration Specialized Center of Research and Training in Exobiology (NSCORT) grant (to S.L.M.) and a predoctoral fellowship (to H.J.C.). S.M. is supported by JSPS Research Fellowships for Young Scientists.
References
- 1.Schopf J. W. (1993) Science 260, 640-646. [DOI] [PubMed] [Google Scholar]
- 2.Mojzsis S. J., Arrhenius, G., McKeegan, K. D., Harrison, T. M., Nutman, A. P. & Friend, C. R. L. (1996) Nature 384, 55-59. [DOI] [PubMed] [Google Scholar]
- 3.Maher K. A. (1988) Nature 331, 612-614. [DOI] [PubMed] [Google Scholar]
- 4.Schopf J. W., Kudryavtsev, A. B., Agresti, D. G., Wdowiak, T. J. & Czaja, A. D. (2002) Nature 416, 73-76. [DOI] [PubMed] [Google Scholar]
- 5.Brasier M. D., Green, O. R., Jephcoat, A. P., Kleppe, A. K., Van Kranendonk, M. J., Lindsay, J. F., Steele, A. & Grassineau, N. V. (2002) Nature 416, 76-81. [DOI] [PubMed] [Google Scholar]
- 6.Fedo C. M. & Whitehouse, M. J. (2002) Science 296, 1448-1452. [DOI] [PubMed] [Google Scholar]
- 7.van Zuilen M. A., Lepland, A. & Arrhenius, G. (2002) Nature 418, 627-630. [DOI] [PubMed] [Google Scholar]
- 8.Holland H. D., (1984) The Chemical Evolution of the Atmosphere and Oceans (Princeton Univ. Press, Princeton, NJ).
- 9.Miller S. L. & Orgel, L. E., (1974) The Origins of Life on the Earth (Prentice–Hall, Englewood Cliffs, NJ).
- 10.Levine J. S., Augustsson, T. R. & Natarajan, M. (1982) Origins Life 12, 245-259. [DOI] [PubMed] [Google Scholar]
- 11.Kasting J. F. (1993) Science 259, 920-926. [DOI] [PubMed] [Google Scholar]
- 12.Delano J. W. (2001) Origins Life Evol. Biosphere 31, 311-341. [DOI] [PubMed] [Google Scholar]
- 13.Walker J. C. G. (1985) Origins Life 16, 117-127. [DOI] [PubMed] [Google Scholar]
- 14.Kasting J. F. & Brown, L. L. (1998) in The Molecular Origins of Life, ed. Brack, A. (Cambridge Univ. Press, New York), pp. 35–56.
- 15.Schlesinger G. & Miller, S. L. (1983) J. Mol. Evol. 19, 376-382. [DOI] [PubMed] [Google Scholar]
- 16.Wilde S. A., Valley, J. W., Peck, W. H. & Graham, C. M. (2001) Nature 409, 175-178. [DOI] [PubMed] [Google Scholar]
- 17.Mojzsis S. J., Harrison, T. M. & Pidgeon, R. T. (2001) Nature 409, 178-181. [DOI] [PubMed] [Google Scholar]
- 18.Sleep N. H. & Zahnle, K. (2001) J. Geophys. Res. 106, 1373-1399. [Google Scholar]
- 19.Rye R., Kuo, P. H. & Holland, H. D. (1995) Nature 378, 603-605. [DOI] [PubMed] [Google Scholar]
- 20.DiSanti M. A., Mumma, M. J., Russo, N. D., Magee-Sauer, K., Novak, R. & Rettig, T. W. (1999) Nature 399, 662-665. [DOI] [PubMed] [Google Scholar]
- 21.Kasting J. F. (1990) Origins Life Evol. Biosphere 20, 199-231. [DOI] [PubMed] [Google Scholar]
- 22.Vantrump J. E. & Miller, S. L. (1973) Earth Planet Sci. Lett. 20, 145-150. [Google Scholar]
- 23.Abelson P. H. (1966) Proc. Natl. Acad. Sci. USA 55, 1365-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyakawa S., Sawaoka, A. B., Ushio, K. & Kobayashi, K. (1999) J. Appl. Phys. 85, 6853-6857. [Google Scholar]
- 25.Kobayashi K., Tsuchiya, M., Oshima, T. & Yanagawa, H. (1990) Origins Life Evol. Biosphere 20, 99-109. [Google Scholar]
- 26.Kobayashi K., Kaneko, T., Saito, T. & Oshima, T. (1998) Origins Life Evol. Biosphere 28, 155-165. [DOI] [PubMed] [Google Scholar]
- 27.Chyba C. & Sagan, C. (1992) Nature 355, 125-132. [DOI] [PubMed] [Google Scholar]
- 28.Levy M. & Miller, S. L. (1998) Proc. Natl. Acad. Sci. USA 95, 7933-7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferris J. P., Joshi, P. C., Edelson, E. H. & Lawless, J. G. (1978) J. Mol. Evol. 11, 293-311. [DOI] [PubMed] [Google Scholar]
- 30.Miyakawa S., Cleaves, H. J. & Miller, S. L. (2002) Origins Life Evol. Biosphere 32, 209-218. [DOI] [PubMed] [Google Scholar]
- 31.Robertson M. P. & Miller, S. L. (1995) Nature 375, 772-774. [DOI] [PubMed] [Google Scholar]
- 32.Dowler M. J., Fuller, W. D., Orgel, L. E. & Sanchez, R. A. (1970) Science 169, 1320-1321. [DOI] [PubMed] [Google Scholar]
- 33.Cleaves H. J. & Miller, S. L. (2001) J. Mol. Evol. 52, 73-77. [DOI] [PubMed] [Google Scholar]
- 34.Ferris J. P., Sanchez, R. A. & Orgel, L. E. (1968) J. Mol. Biol. 33, 693-704. [DOI] [PubMed] [Google Scholar]
- 35.Schlesinger G. & Miller, S. L. (1983) J. Mol. Evol. 19, 383-390. [DOI] [PubMed] [Google Scholar]
- 36.Yamanashi H., Takeda, S., Murasawa, K., Miyakawa, S., Kaneko, T. & Kobayashi, K. (2001) Anal. Sci. 17,Suppl., 1639-1642. [Google Scholar]
- 37.Levy M., Miller, S. L., Brinton, K. & Bada, J. L. (2000) Icarus 145, 609-613. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi K., Masuda, H., Ushio, K., Ohashi, A., Yamanashi, H., Kaneko, T., Takahashi, J., Hosokawa, T., Hashimoto, H. & Saito, T. (2001) Adv. Space Res. 27, 207-215. [DOI] [PubMed] [Google Scholar]
- 39.Miyakawa S., Tamura, H., Sawaoka, A. B. & Kobayashi, K. (1998) Appl. Phys. Lett. 72, 990-992. [Google Scholar]
- 40.Miyakawa S., Kobayashi, K. & Sawaoka, A. B. (1999) Adv. Space Res. 24, 465-468. [DOI] [PubMed] [Google Scholar]
- 41.Ponnamperuma C., Lemmon, R. M., Mariner, R. & Calvin, M. (1963) Proc. Natl. Acad. Sci. USA 49, 737-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyakawa S., Murasawa, K., Kobayashi, K. & Sawaoka, A. B. (2000) Origins Life Evol. Biosphere 30, 557-566. [DOI] [PubMed] [Google Scholar]
- 43.Bada J. L. & Miller, S. L. (1968) Science 159, 423-425. [DOI] [PubMed] [Google Scholar]
- 44.Zahnle K. J. (1986) J. Geophys. Res. 91, 2819-2834. [Google Scholar]
- 45.Miyakawa S., Cleaves, H. J. & Miller, S. L. (2002) Origins Life Evol. Biosphere 32, 195-208. [DOI] [PubMed] [Google Scholar]
- 46.Sanchez R. A., Ferris, J. P. & Orgel, L. E. (1967) J. Mol. Biol. 30, 223-253. [PubMed] [Google Scholar]
- 47.Corliss J. B., Baross, J. A. & Hoffman, S. E. (1981) Oceanol. Acta 4,Suppl., 59-69. [Google Scholar]
- 48.Chiodini G. & Marini, L. (1998) Geochim. Cosmochim. Acta 62, 2673-2687. [Google Scholar]
- 49.Huber C. & Wachtershauser, G. (1997) Science 276, 245-247. [DOI] [PubMed] [Google Scholar]
- 50.Cody G. D., Boctor, N. Z., Filley, T. R., Hazen, R. M., Scott, J. H., Sharma, A. & Yoder, H. S., Jr. (2000) Science 289, 1337-1340. [DOI] [PubMed] [Google Scholar]
- 51.Yanagawa H. & Kobayashi, K. (1992) Origins Life Evol. Biosphere 22, 147-159. [Google Scholar]
- 52.Shock E. L. (1990) Origins Life Evol. Biosphere 20, 331-367. [Google Scholar]
- 53.Bada J. L., Miller, S. L. & Zhao, M. (1995) Origins Life Evol. Biosphere 25, 111-118. [DOI] [PubMed] [Google Scholar]
- 54.Whittet D. C. B. (1997) Origins Life Evol. Biosphere 27, 249-262. [PubMed] [Google Scholar]
- 55.Stoks P. G. & Schwartz, A. W. (1979) Nature 282, 709-710. [Google Scholar]
- 56.Kvenvolden K., Lawless, J., Pering, K., Peterson, E., Flores, J., Ponnamperuma, C., Kaplan, I. R. & Moore, C. (1970) Nature 228, 923-926. [DOI] [PubMed] [Google Scholar]
- 57.Miyakawa S., Murasawa, K., Kobayashi, K. & Sawaoka, A. B. (1999) J. Am. Chem. Soc. 121, 8144-8145. [Google Scholar]
- 58.Hirose Y., Ohmuro, K., Saigoh, M., Nakayama, T. & Yamagata, Y. (1990–1991) Origins Life Evol. Biosphere 20, 471-481. [Google Scholar]