Abstract
In 1912, Franz Boas published a study demonstrating the plastic nature of the human body in response to changes in the environment. The results of this study have been cited for the past 90 years as evidence of cranial plasticity. These findings, however, have never been critiqued thoroughly for their statistical and biological validity. This study presents a reassessment of Boas' data within a modern statistical and quantitative genetic framework. The data used here consist of head and face measurements on over 8,000 individuals of various European ethnic groups. By using pedigree information contained in Boas' data, narrow sense heritabilities are estimated by the method of maximum likelihood. In addition, a series of t tests and regression analyses are performed to determine the statistical validity of Boas' original findings on differentiation between American and European-born children and the prolonged effect of the environment on cranial form. Results indicate the relatively high genetic component of the head and face diameters despite the environmental differences during development. Results point to very small and insignificant differences between European- and American-born offspring, and no effect of exposure to the American environment on the cranial index in children. These results contradict Boas' original findings and demonstrate that they may no longer be used to support arguments of plasticity in cranial morphology.
The appearance in the early 20th century of Franz Boas' studies on descendents of immigrants (1–5) introduced the idea that environment could modify cranial morphology extensively. Boas' findings were based on the analysis of anthropometric data collected between 1909 and 1910 on European-born immigrants and their American-born children in New York. Although the results of Boas' study were critiqued initially by some (6, 7), they are now widely accepted and cited frequently in papers dealing with race (8–10), human evolution (11), and social anthropology. Unfortunately, Boas' research design was deficient, and his findings were never critiqued in a systematic way until recently (12).
Cranial plasticity refers to the idea that the cranium responds to environmental forces during growth and development, and thus the cranium can be shaped primarily by environmental forces. This is the key argument for many that critically view the use of cranial measurements in population studies (9) and forensic settings (10). Despite many studies that have demonstrated a substantial genetic component to cranial dimensions (13–18), the notion that cranial plasticity is the dominant force in determining cranial form persists, mainly because of the ideas presented in the work of Boas and his students (19).
Here, we describe our work on Boas' published data (20), which addresses the issues with modern statistical and quantitative genetic methods. We show that divergence between parents and offspring and between European- and American-born children is negligible in comparison to the differentiation between ethnic groups. In addition, the prolonged effect of the environment on cranial form proposed by Boas is shown to be insignificant.
Materials and Methods
The data analyzed here are from Boas (20) and consist of anthropometric measurements taken from 1909 to 1910 on ≈13,000 European-born immigrants and their American-born children in the New York area. Several European ethnic/geographic groups are present in the data including Bohemians, Central Italians, Hebrews,§ Poles, Hungarians, Scots, and Sicilians. The anthropometrics collected include head length, head breadth, facial breadth, stature, eye color, and hair color. Because this paper deals with the notion of cranial plasticity, we used only measurements on the head and face.
In an attempt to replicate Boas' original findings, univariate t tests and least-squares regression are used. The t tests represent tests that Boas could have used if the formulations had been available for comparison of same-age American and European children in the final Immigration Commission report (1). These tests are performed to assess differentiation in the three raw cranial variables and the cranial index between same-age European- and American-born children. Children from age 4 to 19 are compared, producing 896 possible tests in the seven ethnic groups. To bypass the complication of small sample sizes in many situations, age-specific sexes are pooled by z scoring the variables by sex, leading to 448 tests. To account for the large number of tests, an α significance level of 0.001 is used to reduce the type I error rate. Least-squares regression is used to test the effect of duration of environmental exposure on the cranial index. Boas (2) claimed that there were dramatic effects on cranial form depending on the time of exposure to the American environment. We calculate environmental exposure as the difference between immigration year and 1910 for the European-born children and as age for the American-born children. For the European-born children, age and environmental exposure are both entered into a multiple-regression model, and type II sums of squares are used to test for the partial regression effects. A two-factor ANOVA model with interaction is used to test for ethnic group (genetic effect) and birthplace (environmental effect) on all cranial variables and the cranial index. The benefit of the ANOVA model is that it allows testing of individual model effects by using a standard F ratio. In this case, the proportion of variance in each variable attributable to birthplace and ethnic group are tested for equality. If Boas' conclusions are correct, then birthplace should account for most of the variance, whereas the contrary should be true if the effect of the American environment is small.
Narrow sense heritabilities for the cranial traits are estimated by the method of maximum likelihood following Konigsberg and Ousley (21) and Blangero and Konigsberg (22). The maximum-likelihood method is suitable here, because the data consist of often multigenerational pedigrees, and also has the ability to accommodate missing data within pedigrees. This method has an advantage over methods of parent–offspring regression in that it uses all kinship information within the pedigrees simultaneously instead of a series of pairwise regressions. The method also produces multivariate heritabilities and estimates of genetic and environmental correlations between traits. Before analysis, the data are converted into residuals around a mean of zero by age and sex for the total sample to control the effects of growth and sexual dimorphism while maintaining ethnic group-specific means and variances. Heritabilities are estimated for the total sample as well as for each of the subsamples. Pooled within-group heritabilities are produced by extracting the diagonal of P−1G, where P and G represent the pooled within-groups phenotypic and genetic covariance matrices (21).
Results
Despite the pooling of sexes, only 156 of the 448 possible t tests can be performed because of small sample sizes. At the α level of 0.001, 11 of the 156 tests show significant differences between European- and American-born children. All the significant differences are in the Hebrew sample, and 73% of the tests relate to the cranial index. They indicate a general reduction in cranial index in American-born children between 7 and 14 years of age. Often associated with this in the Hebrew sample is an increase in cranial vault length in the American-born children.
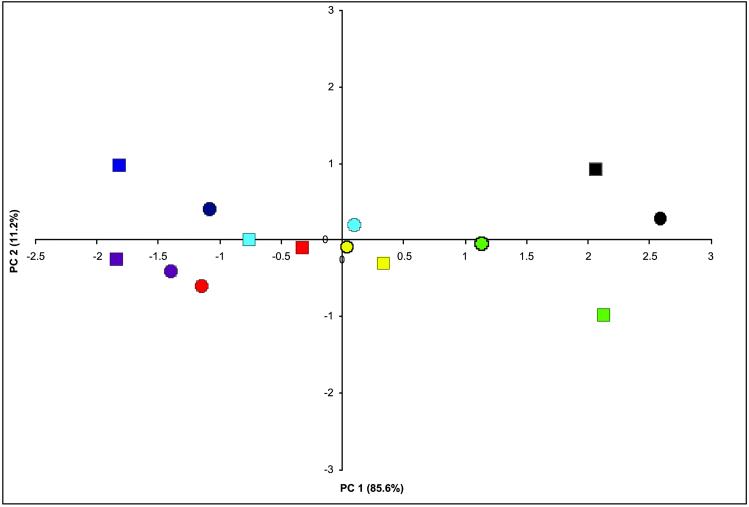
The regression of cranial index on age and environmental exposure (Table 1) shows a trivial effect of environmental exposure on the cranial index. In Scottish males, there is a slight increase in cranial index in response to duration of American residence (β1 = 0.0879, model R2 = 0.119), and Polish females show a decrease in cranial index in response to duration of American residence (β1 = −0.1923, model R2 = 0.234). The predominant trend in the data suggests a much more significant effect of age on the cranial index rather than of duration of American residence, which suggests an overall stability of the cranial index in response to changing environment and fails to support the propositions of Boas (2). Results of the ANOVA (Table 2) likewise show evidence that the propositions of Boas (2) are in error. Because significant interaction effects are present in all variables but face breadth, the individual effects of the model, by definition, are uninterruptible because of the interdependence of the main effects in the model (23). The presence of the significant interaction in this model indicates that the differences between means and the relative magnitude of the variation between the American- and European-born samples depend on the ethnic group. We discuss this further below. For the case of face breadth, the interaction effect is removed from the model, and the model is reformulated. Both effects are found to account for significant portions of the total sum of squares. A test for equality of effects produces an F value of 1.13 (P = 0.33, df = 1, 6), indicating a slightly higher but insignificant variance component for birthplace. To interpret the interaction effect in the model, Mahalanobis-generalized distances are computed for each ancestral group subdivided by birthplace. The distance matrix then is subjected to an eigenvalue decomposition following Gower (24). This method allows for the visualization of the interdependence of factors from the ANOVA model. The results are presented in Fig. 1. The first principal coordinate separates ancestral groups on an approximate East-to-West gradient, indicating some geographic patterning to the variation, whereas the second principal coordinate suggests a slight decrease in variation in the American-born subgroups due to their lower degree of divergence from the center of the coordinate space. The reduction in variation, however, is not present in all ancestry groups.
Table 1.
Results of regression analysis of cranial index on age and environmental exposure
| Ancestry
|
Effect | |||
|---|---|---|---|---|
| European-born males | European-born females | |||
| Age | Exposure | Age | Exposure | |
| Bohemian | — | — | ||
| Hebrew | — | — | ||
| Polish | — | — | ||
| Scottish | — | — | ||
| Sicilian | — | — | ||
| Central Italian | — | — | ||
| Hungarian/Slovakian | — | — | — | — |
Exposure, 1910, immigration year;
, significant at 0.05;
, significant at 0.01;
, significant at 0.001; —, not significant.
Table 2.
Results of ANOVA model for ancestry and birthplace variance components
| Variable | Source | Type III mean square regression | F | P > F |
|---|---|---|---|---|
| Head length | Birth place | 422.312 | 11.73 | 0.0006 |
| Ancestry | 3163.804 | 87.91 | <0.0001 | |
| BP*A | 731.449 | 20.32 | <0.0001 | |
| Head breadth | Birth place | 184.799 | 7.15 | 0.0075 |
| Ancestry | 5682.794 | 220.01 | <0.0001 | |
| BP*A | 403.511 | 16.62 | <0.0001 | |
| Face breadth | Birth place | 939.755 | 33.06 | <0.0001 |
| Ancestry | 1834.542 | 64.53 | <0.0001 | |
| BP*A | 43.492 | 1.53 | 0.1639 | |
| Face breadth (no interaction) | Birth place | 2308.465 | 81.17 | <0.0001 |
| Ancestry | 2092.498 | 71.36 | <0.0001 | |
| Cranial index | Birth place | 2.561 | 0.24 | 0.6242 |
| Ancestry | 3535.548 | 331.40 | <0.0001 | |
| BP*A | 403.097 | 37.78 | <0.0001 |
Fig 1.
Principal coordinates plot of ancestral groups by birthplace showing a decrease in variation in American samples and interaction between ethnic group and degree of differentiation. American samples are represented by circles, and European samples are represented by squares. Dark blue, Bohemian; yellow, Central Italian; light blue, Hebrew; red, Polish; purple, Hungarian; black, Scottish; green, Sicilian.
Multivariate heritabilities were estimated by maximum likelihood (Table 3). Table 3 also shows the pooled within-groups phenotypic and genetic variance–covariance matrices and the sample sizes for the heritability estimates. Both head-length and -breadth measurements show heritabilities greater than 0.5, indicating that most of phenotypic variation in these traits can be attributed to genetic factors. Facial breadth shows a slightly lower heritability, indicating a slightly higher environmental variance component; this corresponds well with the results of the ANOVA, which show a slightly higher environmental component for facial breadth.
Table 3.
Phenotypic and genetic variance–covariance matrices, P−1G matrix, and sample sizes for heritability estimates
| Matrix | Head length | Head breadth | Face breadth |
|---|---|---|---|
| Phenotypic | 36.57 | — | — |
| 9.40 | 26.27 | — | |
| 9.81 | 15.22 | 28.73 | |
| Genetic | 19.88 | — | — |
| 5.14 | 15.34 | — | |
| 5.01 | 8.20 | 14.42 | |
| P−1G | 0.55 | — | — |
| 0.01 | 0.61 | — | |
| −0.02 | −0.03 | 0.49 |
Heritabilities appear on the diagonal of the P−1G matrix. Number of pedigrees, 1,482; n, 9,595.
Discussion
In this study, we conducted a modern statistical evaluation of Franz Boas' data (2) and attempted to replicate his findings of cranial plasticity under changing environmental conditions. Instead of the large plasticity component claimed by Boas and countless others who have cited his work, our analysis reveals high heritability in the family data and variation among the ethnic groups, which persists, in the American environment. Research on this topic has shown major influences of changing environmental conditions on human stature and body-fat patterning (25, 26), but the only studies capable of dealing with effects of these changing conditions on the cranium were published 50–90 years ago (1–3, 6). Uncritical acceptance of his findings has resulted in 90 years of misunderstanding about the magnitude of plasticity. Reanalysis of Boas' data not only fails to support his contention that cranial plasticity is a primary source of cranial variation but rather supports what morphologists and morphometricians have known for a long time: most of the variation is genetic variation.
We have shown that in this sample, the change in developmental environment produced a relatively minor effect on cranial dimensions relative to familial and ancestral effects. Temporal changes in human cranial morphology often occur in the absence of migration or major environmental changes (27). Large morphological change is observed in nonhuman populations where cultural manipulation of the environment is not possible (28). In America, both Blacks and Whites have experienced significant change in cranial morphology over the past 150 years but have not converged to a common morphology as might be expected if environmental plasticity plays a major role (29). Secular change rather than a specific immigration effect is an equally likely explanation for the changes that Boas observed. What is lacking in Boas' immigrant study, and what has not been demonstrated yet since Boas, is that environmental plasticity plays a more important role than genetic variation. What has been demonstrated is that cranial dimensions are capable of revealing “genetic” patterns in human populations over time and space (30–40).
Finally, we address the issue of why Boas published such seemingly erroneous conclusions. Although it might seem an insurmountable task to dissect Boas' motives or convictions for pursuing such a study, we can examine Boas' mindset as revealed in his publications from the time period. Some 10 years before the immigrant study, Boas was one of the most (if not the most) statistical and quantitatively oriented anthropologists, as seen in publications from the period predating the immigrant study (41–44). In the final report presented to congress, Boas' statistical fluency tends to disappear, perhaps in the face of such a large data set and the lack of proper statistical tests. For the period in which this study was published, the results were presented in a manner making the data look as convincing as possible. We also must consider the attitude of Boas toward the scientific racism of the day. Evidence of Boas' disdain for the often typological and racist ideas in anthropology have been reviewed previously (45) and are evident also in his later publications (46–48). Boas' motives for the immigrant study could have been entwined in his view that the racist and typological nature of early anthropology should end, and his argument for dramatic changes in head form would provide evidence sufficient to cull the typological thinking. We make no claim that Boas made deceptive or ill-contrived conclusions. In Fig. 1 it is evident that there are differences between American- and European-born samples. What we do claim is that when his data are subjected to a modern analysis, they do not support his statements about environmental influence on cranial form.
Acknowledgments
We thank an anonymous reviewer for comments on earlier drafts of this article.
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 14622.
The Hebrew data consist of individuals of Jewish ancestry from Western Russian, Poland, Germany, Austria, Switzerland, and Romania. The term “Hebrew” is used here only for continuity with Boas' original publication.
References
- 1.Boas F., (1910) Report Presented to the 61st Congress on Changes in Bodily Form of Descendants of Immigrants (Government Printing Office, Washington, DC).
- 2.Boas F. (1912) Am. Anthropol. 14, 530-562. [Google Scholar]
- 3.Boas F. (1940) Am. Anthropol. 42, 183-189. [Google Scholar]
- 4.Boas F. (1965) in Source Book in Anthropology, eds. Kroeber, A. L. & Waterman, T. T. (Harcourt Brace, New York), pp. 141–154.
- 5.Boas F., (1966) Race, Language and Culture (The Free Press, New York), pp. 60–75.
- 6.Fisher R. A. & Gray, H. (1938) Ann. Eugen. 8, 74-93. [Google Scholar]
- 7.Morant G. M. & Samson, O. (1936) Biometrika 28, 1-31. [Google Scholar]
- 8.Cole F. C. (1931) in Methods in Social Sciences: A Case Book, ed. Rice, S. A. (Univ. of Chicago, Chicago), pp. 582–585.
- 9.Goodman A. H. (1995) in Biological Anthropology: The State of the Science, eds. Boaz, N. T. & Wolfe, L. D. (Int. Inst. Hum. Evol. Res., Bend, OR), pp. 215–240.
- 10.Goodman A. H. (1997) Sciences (New York) 37, 20-31. [Google Scholar]
- 11.Carlson D. S. & Van Gerven, D. P. (1977) Am. J. Phys. Anthropol. 46, 495-506. [DOI] [PubMed] [Google Scholar]
- 12.Sparks C. S., (2001) Department of Anthropology (Univ. of Tennessee, Knoxville).
- 13.Devor E. J., McGue, M., Crawford, M. H. & Lin, P. M. (1986) Am. J. Phys. Anthropol. 69, 71-82. [DOI] [PubMed] [Google Scholar]
- 14.Devor E. J., McGue, M., Crawford, M. H. & Lin, P. M. (1986) Am. J. Phys. Anthropol. 69, 83-92. [DOI] [PubMed] [Google Scholar]
- 15.Devor E. J. (1987) J. Craniofac. Genet. Dev. Biol. 7, 95-106. [PubMed] [Google Scholar]
- 16.Susanne C. (1977) Hum. Biol. 49, 573-580. [PubMed] [Google Scholar]
- 17.Susanne C. (1975) Ann. Hum. Biol. 2, 279-287. [DOI] [PubMed] [Google Scholar]
- 18.Paganini-Hill A., Martin, A. O. & Spence, M. A. (1981) Am. J. Phys. Anthropol. 55, 55-67. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein M. S., (1943) Demographic and Bodily Changes in Descendants of Mexican Immigrants (Univ. of Texas Inst. of Latin American Studies, Austin).
- 20.Boas F., (1928) Materials for the Study of Inheritance in Man (Columbia Univ. Press, New York).
- 21.Konigsberg L. W. & Ousley, S. D. (1995) Hum. Biol. 67, 481-498. [PubMed] [Google Scholar]
- 22.Blangero J. & Konigsberg, L. W. (1991) Genet. Epidemiol. 8, 299-316. [DOI] [PubMed] [Google Scholar]
- 23.Neter J., Kutner, M. H., Nachtsheim, C. J. & Wasserman, W., (1996) Applied Linear Statistical Models (WCB McGraw–Hill, Boston).
- 24.Gower J. C. (1966) Biometrika 53, 325-338. [Google Scholar]
- 25.Bogin B., (1999) Patterns of Human Growth (Cambridge Univ. Press, Cambridge, U.K.).
- 26.Bogin B. & Loucky, J. (1997) Am. J. Phys. Anthropol. 102, 17-32. [DOI] [PubMed] [Google Scholar]
- 27.Zellner K., Jaeger, U. & Kromeyer-Hauschild, K. (1998) Anthropol. Anz. 56, 301-312. [PubMed] [Google Scholar]
- 28.Pergams O. R. W. & Ashley, M. V. (1999) Evolution (Lawrence, Kans.) 53, 1573-1581. [DOI] [PubMed] [Google Scholar]
- 29.Jantz R. L. & Meadows Jantz, L. (2000) Am. J. Hum. Biol. 12, 327-338. [DOI] [PubMed] [Google Scholar]
- 30.Harding R. M. (1990) Hum. Biol. 62, 733-745. [PubMed] [Google Scholar]
- 31.Jantz R. L., Hunt, D. R., Falsetti, A. B. & Key, P. J. (1992) Hum. Biol. 64, 435-461. [PubMed] [Google Scholar]
- 32.Jantz R. L. & Meadows, L. (1995) Hum. Biol. 67, 375-386. [PubMed] [Google Scholar]
- 33.Jantz R. L. & Owsley, D. W. (2001) Am. J. Phys. Anthropol. 114, 146-155. [DOI] [PubMed] [Google Scholar]
- 34.Relethford J. H., Crawford, M. H. & Blangero, J. (1997) Hum. Biol. 69, 443-465. [PubMed] [Google Scholar]
- 35.Relethford J. H. & Harpending, H. C. (1994) Am. J. Phys. Anthropol. 95, 249-270. [DOI] [PubMed] [Google Scholar]
- 36.Relethford J. H. & Crawford, M. H. (1995) Am. J. Phys. Anthropol. 96, 25-38. [DOI] [PubMed] [Google Scholar]
- 37.Relethford J. H. & Blangero, J. (1990) Hum. Biol. 62, 5-25. [PubMed] [Google Scholar]
- 38.Relethford J. H. (1994) Am. J. Phys. Anthropol. 95, 53-62. [DOI] [PubMed] [Google Scholar]
- 39.Relethford J. H. (1991) Hum. Biol. 63, 155-165. [PubMed] [Google Scholar]
- 40.Sciulli P. W. & Schneider, K. N. (1985) Am. J. Phys. Anthropol. 66, 429-443. [DOI] [PubMed] [Google Scholar]
- 41.Boas F. (1899) Am. Anthropol. 1, 448-461. [Google Scholar]
- 42.Boas F. (1899) Am. Anthropol. 1, 98-106. [Google Scholar]
- 43.Boas F. (1905) Science 21, 862-863. [DOI] [PubMed] [Google Scholar]
- 44.Boas F. (1908) Am. Anthropol. 5, 530-538. [Google Scholar]
- 45.Ousley S. D. (2000) Eur. Rev. Native Am. Stud. 14, 9-15. [Google Scholar]
- 46.Boas F. (1916) Proc. Natl. Acad. Sci. USA 2, 713-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boas F. (1936) Science 84, 522-525. [DOI] [PubMed] [Google Scholar]
- 48.Boas F., (1940) Race, Language, and Culture (Collier MacMillan, Toronto).