Abstract
The effect of various chemical modifications of nitrogen atoms on the efficiency of polyethylenimines (PEIs) as synthetic vectors for the delivery of plasmid DNA into monkey kidney cells in vitro has been systematically investigated. The resultant structure–activity relationship has both provided mechanistic insights and led to PEI derivatives with markedly enhanced performance. For example, N-acylation of PEI with the molecular mass of 25 kDa (PEI25, one of the most potent polycationic gene delivery vectors) with alanine nearly doubles its transfection efficiency in the presence of serum and also lowers its toxicity. Furthermore, dodecylation of primary amino groups of 2-kDa PEI yields a nontoxic polycation whose transfection efficiency in the presence of serum is 400 times higher than the parent's and which exceeds 5-fold even that of PEI25.
Interest in gene therapy has soared in recent years because of its great promise in treating diseases ranging from inherited disorders to acquired conditions and cancer (1–3). However, much progress remains to be made before safe and reliable gene delivery vehicles are developed (4). Side reactions provoked by the viral elements, e.g., immune response and insertional mutagenesis (5), have prompted efforts to improve nonviral delivery systems (vectors) (6–8). Synthetic vectors based on polycations are particularly attractive because of their potential safety, nucleic acid cargo capacity, and designability (9–11). And yet, currently available polycations are severely limited by their low efficiencies (4, 8).
Improvements in gene delivery by polyplexes (polycation/DNA complexes) may come from a detailed examination of their mechanism of action, establishing the underlying structure–activity relationships, and manipulation of the vectors accordingly (12–16). Polyethylenimine (PEI), a readily available synthetic polycation introduced for transfection a few years ago (17), is an ideal candidate for such mechanistic investigations owing to its already relatively high transfection efficiency and ease of functionalization.
Elucidation of the mechanism of PEI-mediated gene delivery has received much attention in recent years (18–24). Since every third atom in PEI is a protonatable nitrogen, the overall protonation level should rise when the pH drops to ≈5 inside the lysosomal compartment, leading to the influx of chloride ions and, consequently, to osmotic swelling and rupture of the lysosome. The early escape of the PEI/DNA complex from the lysosome, and thus the avoidance of lysosomal degradation arising from the “proton sponge effect,” was postulated to be the cause of the high transfection efficiency exhibited by PEIs (17); this concept, however, has been recently challenged (22).
In the present study, we carried out systematic chemical modifications of commercial branched PEIs with the goal of understanding and enhancing PEI-mediated transfection. PEIs with molecular masses of 25 and 2 kDa were selected because they exhibit high and low transfection efficiencies, respectively (17–20). The chemical modifications were designed to affect the proton sponge capacity, hydrophobic-hydrophilic balance, and lipophilicity. Through transfection experiments using these modified PEIs on monkey kidney (COS-7) cells, we reaffirmed the importance of the proton sponge effect. Also, the efficiency of PEI25 in the presence of serum was doubled with concurrent appreciable reduction in cytotoxicity by rational fine-tuning of the hydrophobic-hydrophilic balance. Finally, the transfection efficiency of PEI2 was enhanced some 400-fold by increasing its lipophilicity (with the efficiencies of these modified PEI2s in the presence of serum being five times higher than even that of PEI25).
Experimental Procedures
Materials.
The 25-kDa PEI was used for reactions as received, while its 2-kDa counterpart, obtained as a 50% aqueous solution, was lyophilized before being subjected to chemical reactions. The PEIs, as well as N-hydroxysuccinimide, dicyclohexylcarbodiimide, 1-iodoalkanes, di-tert-butyl dicarbonate, and 2-(bromoethyl)trimethylammonium bromide were purchased from Aldrich. Trifluoroacetic acid was purchased from Sigma, and Boc-Ala-OSu, Boc-His(Boc)-OH, and Boc-Leu-OSu were purchased from Advanced ChemTech. All solvents used, purchased from Aldrich, were of the highest purity available. Dialyses were carried out by using Spectra/Por CE dialysis membranes with a molecular mass cutoff of 500 Da (Spectrum Laboratories, Houston). D2O was purchased from Cambridge Isotope Laboratories (Cambridge, MA), and CDCl3 was from Sigma. Elemental analyses were performed by M-H-W Laboratories (Phoenix).
Perquaternized PEI25.
One gram (23.3 mmol) of PEI25 was dissolved in 15 ml of dry MeOH and treated with excess (17.2 ml) 1-iodomethane at room temperature (used elsewhere henceforth unless stated otherwise) (Fig. 1, route A). The resulting mixture was heated at 65°C for 2 h, 928 mg of NaOH was added after cooling, and the heating was applied for an additional 12-h period, followed by removal of the solvent and unreacted methyl iodide by evaporation under reduced pressure. The residue obtained was dissolved in water and dialyzed four times against water. Subsequent lyophilization yielded the product as a white, hygroscopic solid. A similar procedure was adopted for the quaternization of PEI25 with 1-iodoethane, except that EtOH was used as the solvent and the reaction mixture was heated for 24 h after the addition of NaOH. The extent of quaternization, determined from the C/N ratio obtained from elemental analysis, was found to be 87% and 77%, respectively, for the permethylation and perethylation. Quaternization was confirmed by NMR (25–27).
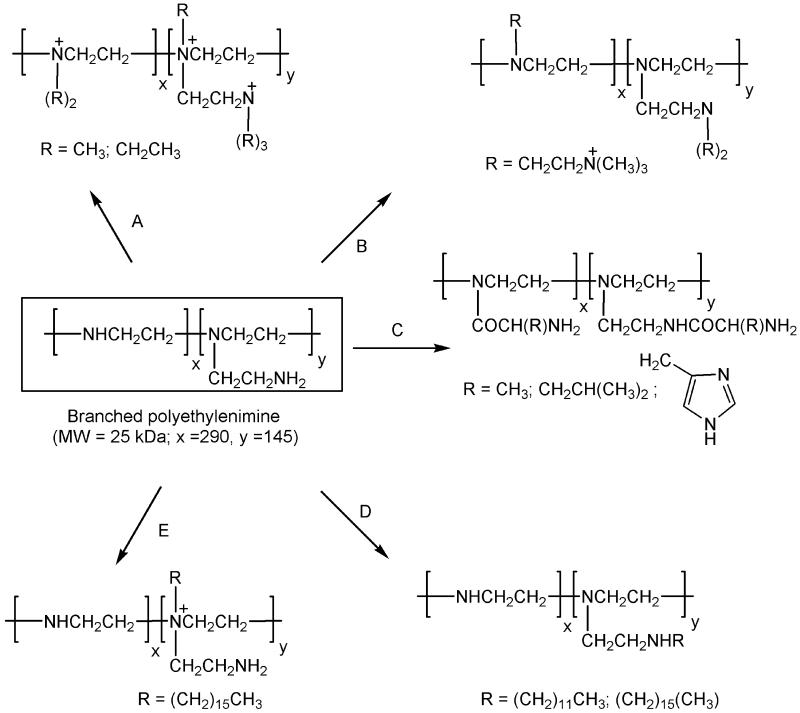
Fig 1.
Schematic representation of various synthetic routes toward chemically modified PEIs. Route A, quaternization of amines with methyl or ethyl iodides. Route B, alkylation of primary and secondary amines with 2-bromocholine. Route C, acylation of primary and secondary amines with amino acids. Route D, alkylation of primary amines with dodecyl or hexadecyl iodides. Route E, quaternization of tertiary amines with hexadecyl iodide. Routes A–D were applied to PEI25; route D was also adapted for the alkylation of PEI2. See Experimental Procedures for details.
PEI25 Alkylated with 2-(Bromoethyl)trimethylammonium Bromide.
One gram (23.2 mmol) of PEI25 was dissolved in 30 ml of MeOH, followed by addition of 8.58 g (35.8 mmol) of 2-(bromoethyl)trimethylammonium bromide and heating at 65°C for 24 h (Fig. 1, route B). The reaction mixture was cooled, 928 mg of NaOH was added, and the heating was applied for 6 more days. Thereafter, the solvent was evaporated, and the residue obtained was dissolved in water and dialyzed four times against water. Subsequent lyophilization yielded 2.11 g (43%) of a solid that was characterized by NMR (D2O). Note that NMR examination of the crude product before dialysis showed the formation of CH2=CH2N+(CH3)3Br− as the byproduct resulting from the elimination of HBr from the alkyl bromide.
PEI25 Derivatized with Amino Acids.
Grafting of Ala, Leu, and His residues onto PEI25 was carried out by using N-hydroxysuccinimide (HOSu) esters of the NH2-protected amino acids, as exemplified below with histidine (Fig. 1, route C). For preparing the HOSu ester of Boc-His(Boc)-OH, the latter (4.9 g, 13.8 mmol) was dissolved in 20 ml of dry dimethylformamide, and 2.38 g (20.7 mmol) of HOSu and 3.13 g (15.2 mmol) of dicyclohexylcarbodiimide were added. After stirring for 12 h, the mixture was diluted with ethyl acetate. Dicyclohexylurea that precipitated was filtered off, and the filtrate was washed successively with water, 5% NaHCO3 and saturated brine. EtOAc was removed under reduced pressure, the residue was dissolved in CH2Cl2, additional crystals of dicyclohexylurea were filtered off, and the solvent was evaporated under reduced pressure to obtain Boc-His(Boc)-OSu as a white solid.
For the coupling of Boc-His(Boc)-OSu and PEI25, 5.27 g (11.6 mmol) of the former was added to a solution of 500 mg (11.6 mmol) of PEI25 in 20 ml of a 1:1 (vol/vol) dry mixture of tetrahydrofuran and CH2Cl2 containing 1.8 ml of triethylamine. After stirring for 36 h, the solvent was removed under reduced pressure, and the residue obtained was suspended in EtOAc and filtered. The filtrate was washed successively with water and saturated brine. Evaporation of the solvent yielded solid Boc-His(Boc)-PEI25 confirmed by NMR (CDCl3). For the removal of the t-Boc group, 4.84 g of the solid was dissolved in 33 ml of a 1:1 (vol/vol) mixture of trifluoroacetic acid and CH2Cl2, followed by a 1.5-h incubation and solvent removal by evaporation under reduced pressure. The residue thus obtained was dissolved in water and filtered. The filtrate was dialyzed three times against water and lyophilized to obtain His-PEI25 as a white solid. The extent of amino acylation per PEI unit was found by NMR (D2O) to be 54%, 49%, and 64%, for Ala, His, and Leu, respectively.
Alkylation of Primary Amino Groups in PEI25 and PEI2.
The PEIs were reacted with 10 mol % of 1-iodododecane in ethanol following a literature procedure (25) (Fig. 1, route D). The crude products were dissolved in water, treated with 11 mol % of NaOH, and dialyzed thrice against water. Pure products (11% dodecyl substitution) were obtained as gummy solids on lyophilization and confirmed by NMR (CDCl3). Hexadecyl-PEIs were synthesized in a similar manner, except that the alkylation was carried out in refluxing CHCl3 in the presence of triethylamine. The crude products were dissolved in 10% EtOH/H2O, dialyzed first against it, and then thrice against water. Pure products, obtained as white, hygroscopic solids on lyophilization of the dialyzates, were confirmed by NMR (CDCl3).
Quaternization of Tertiary Amino Groups in PEI25.
Protection of the primary and secondary amino groups of PEI25 was achieved by using di-tert-butyl dicarbonate (Fig. 1, route E). To this end, 3.72 g of PEI25 (86.5 mmol) was dissolved in 30 ml of dry MeOH, the solution was cooled in an ice bath, and 18.8 g (86.5 mmol) of di-tert-butyl dicarbonate was added with vigorous stirring. The ice bath was removed, and the reaction mixture was stirred overnight. Methanol was then evaporated, and the residue was washed thrice with hexane and dried under reduced pressure to obtain the Boc derivative of PEI25 as a white solid. This compound exhibited an Rf of 0.4 in TLC (10% CH3OH/CHCl3, silica, iodine staining), as compared with 0 for PEI25. Also, the product spot was ninhydrin-inactive, whereas the PEI25 spot was active. The product was further confirmed by NMR (CDCl3). Quaternization of the tertiary amino groups of Boc-protected PEI25 was achieved by reacting with hexadecyl iodide. To this end, 3 g of Boc-PEI25 (21 mmol) was dissolved in 15 ml of EtOH and treated with 7.39 g (21 mmol) of 1-iodohexadecane. The resulting mixture was heated at 65°C for 6 days. Note that the progress of the reaction was monitored by using NMR by withdrawing small aliquots. No further change was observed between samples recorded on the third and sixth days. The solvent was evaporated, and the residue was dissolved in MeOH. This solution was washed thrice with hexane, MeOH was evaporated, and the solid product was confirmed by NMR (D2O). Removal of the t-Boc protecting group was carried out by using a 1:1 mixture of trifluoroacetic acid and CH2Cl2, as described above for PEIs derivatized with amino acids. The solid product (a trifluoroacetate salt) obtained on solvent removal was dried under vacuum for 48 h. The extent of quaternization was found by NMR (D2O) to be 11%.
Plasmid.
gWiz Beta-gal (8,278 bp) encoding the β-galactosidase gene was purchased from Aldevron, Fargo, ND. This plasmid contains the β-galactosidase gene under the control of a modified promoter from the cytomegalovirus immediate early gene. This ready-to-use reporter plasmid was obtained as a 1.0 mg/ml stock solution in aqueous Tris⋅HCl-EDTA buffer.
Gel Retardation Assay.
Binding of polycations to plasmid DNA results in neutralization of negative charges in the phosphate backbone of DNA, and, in turn, in the formation of large electroneutralized complexes unable to migrate toward the anode in agarose gel (20). Complexes for this assay were formed at nitrogen to phosphate ratios of 0, 0.5, 1, 1.5, 2, 2.5, 5, and 7.5. In each case, an appropriate amount of the PEI in 7.5 μl of distilled water containing 150 mM NaCl was mixed with 0.75 μg of the plasmid DNA (gWiz Beta-gal) in 7.5 μl of PBS buffer containing 5% glucose. These solutions were incubated at 37°C for 20 min, mixed with 3 μl of the loading dye (bromophenol blue/xylene cyanol) solution, and loaded into agarose gel wells (0.8% agarose in Tris-borate EDTA buffer). Electrophoresis was carried out at 100 V for 75 min, and DNA bands were visualized by staining the gels with ethidium bromide (Sigma) solution for 90 min at room temperature, followed by UV illumination.
Cell Culture and Transfection.
COS-7 cells (simian virus 40-transformed kidney cells of an African green monkey) were cultured in DMEM containing glutamine (GIBCO) supplemented with 10% heat-inactivated FBS (GIBCO) and antibiotics (100 units/ml penicillin and 100 μg/ml streptomycin, Sigma). Cells were grown at 37°C in humidified air containing 5% CO2 and passaged every 3–4 days. The cells (3 × 105 per well) were plated on Costar 6-well tissue culture clusters 24 h before transfection. Immediately before the initiation of transfection experiments, the medium was removed from each well, and the cells were washed twice with DMEM without serum and antibiotics and treated with the polyplexes as described below.
In each well, 2.5 μg of the plasmid DNA (gWiz Beta-gal) was used. Polyplexes were formed in PBS buffer containing 5% glucose unless otherwise specified. Quantities of plasmid and PEIs given below correspond to experiments done in triplicate.
The polyplexes were prepared by adding, under agitation, appropriate amounts of the polycation in 75 μl of PBS buffer containing 5% glucose to 7.5 μg of plasmid DNA in 75 μl of the same buffer. The resulting solutions containing the polyplexes were incubated at 37°C for 20 min, and then diluted to 4.5 ml with DMEM with or without 10% FBS. To each well 1.5 ml of this transfection medium was added, followed by incubation at 37°C in a humidified air (5% CO2) atmosphere for 6 h. The transfection medium was then removed, and the cells were further incubated under the same conditions in a complete medium (3 ml per well) for 42 h. Thereafter, the medium was removed from each well, and the cells were washed twice with Dulbecco's PBS without CaCl2 and MgCl2 (Sigma). The cells in each well were lysed with 560 μl of Reporter Lysis Buffer (Promega) following the manufacturer's protocol, and the lysates were assayed for β-galactosidase activity spectrophotometrically by following the absorbance of o-nitrophenolate at 420 nm (28, 29). The results were expressed as relative β-galactosidase activity per mg of protein. Total protein was estimated from the BCA (bicinchoninic acid, Sigma) assay (30).
The optimal ratios of DNA and polycations, expressed as nitrogen/phosphate (N/P) ratios, were established from an initial screening. Thus, PEI25 was inefficient for transfection up to an N/P of 2.5. Its transfection efficiency increased in the 5–10 N/P range and then leveled off. This result was true with the derivatives of PEI25 as well. Therefore, the N/P ratio was kept constant at 10 in further experiments. In the case of PEI2, the transfection efficiency increased in the 5–40 N/P range, and with its derivatives, the N/P ratio of 20 was found to be optimal.
Cytotoxicity Measurements.
Cell culture and transfection were performed under the same conditions as outlined above. Control cells were treated with 1.5 ml/well DMEM containing 50 μl of PBS buffer containing 5% glucose, while other cells with treated with either the plasmid or the polyplexes. Cytotoxicities were evaluated by measuring the metabolic activity of the cells, 48 h posttransfection by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (31). Briefly, the culture medium was removed, and the cells were treated with 1.5 ml of DMEM containing 0.5 mg/ml MTT (Sigma). After incubation at 37°C in a humidified air atmosphere (5% CO2) for 4 h, the medium was removed and 560 μl of DMSO was added to each well to dissolve the formazan crystals (32) produced from the reduction of MTT by viable cells. After incubation overnight, the absorbance of the dimethyl sulfoxide solution from each well was measured at 570 nm after appropriate dilutions. The results were expressed as percentages relative to control cells (mean ± SD, n = 3).
Results and Discussion
Although polycations are a leading class of synthetic gene delivery vehicles, their efficiency is dwarfed by that of viral ones (4, 8). Improvements may be achieved by bringing about chemical modifications of a given polycation to enhance one or more steps involved in the polyplex-mediated gene delivery, namely, binding to the cell surface and endocytosis, escape from the endosomal-lysosomal network, translocation to the cell nucleus, and vector unpacking (12, 16), and thereby generating a basic structure-efficiency relationship. In the present work, we selected PEIs for such an investigation because of their superior performance compared with other nonviral vectors and the presence of primary, secondary, and tertiary amino groups (17–19) amenable to diverse and selective chemical modifications.
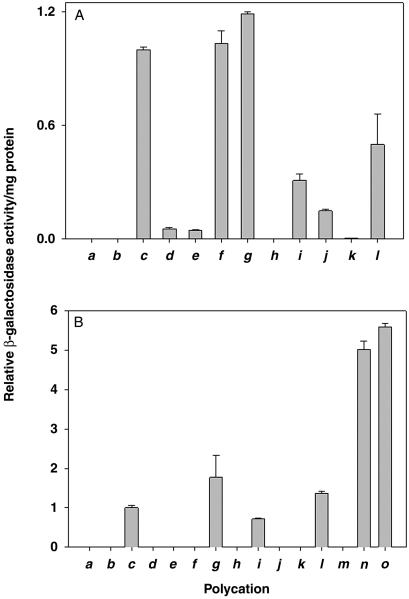
Based on confocal microscopy experiments with fluorescently labeled lysosomes and PEI, it was recently concluded that PEI/DNA complexes do not enter lysosomes during transfection (22). If true, this conclusion invalidates the current views of how PEI functions during DNA transfection (17, 22). It occurred to us that N-quaternization of PEI would abolish its ability to undergo further protonation and thus would independently test the proton sponge effect, which, in turn, stems from the idea that PEI-containing endosomes and lysosomes fuse, resulting in osmotic swelling and bursting of the lysosomes (17, 22). To pursue this rationale, PEI25 was permethylated and perethylated (Fig. 1, route A). As seen in Fig. 2A c–e, the quaternization slashed the transfection efficiency more than 20-fold (note that, expectedly, no appreciable transfection was observed without PEI, Fig. 2Ab). Since the peralkylated PEIs are more positively charged at neutral pH than PEI itself, they should bind DNA tighter. Indeed, when assayed by gel retardation (20), the 2.5 N/P ratio was required for the unmodified PEI25 to ensure essentially complete DNA binding, whereas even the 1.5 ratio was sufficient in the case of the peralkylated PEI25s. That the latter nevertheless exhibit far poorer transfection efficiencies points to the importance of having protonatable nitrogens for efficient transfection.
Fig 2.
Expression in COS-7 cell culture of β-galactosidase gene mediated in the absence of serum by PEI25 and its derivatives (A) and in the presence of serum by PEI25, PEI2, and their derivatives (B). (a) Nontransfected cells. (b) Cells transfected with the plasmid in the absence of polycation. (c–l) Cells transfected with the plasmid in the presence of PEI25 and its N-derivatives: underivatized PEI (c), permethylated PEI (d), perethylated PEI (e), PEI alkylated with choline (f), PEI acylated with alanine (g), PEI acylated with leucine (h), PEI acylated with histidine (i), PEI with dodecylated primary amines (j), PEI with hexadecylated primary amines (k), and PEI with dodecylated tertiary amines (l). (m) Unmodified PEI2. (n) PEI2 with dodecylated primary amines. (o) PEI2 with hexadecylated primary amines. The N/P ratio was 10 for all of the polyplexes of PEI25 and its derivatives and 20 in the case of PEI2 and its derivatives. All measurements were done in triplicate; the bar height represents the mean value with the SD shown. See Experimental Procedures for conditions and details.
To test this conclusion further, transfection experiments were performed with PEI alkylated with choline (Fig. 1, route B). Since the ensuing polymer is only partially derivatized with a moiety bearing a permanent positive charge, its proton sponge propensity should be retained due to the presence of secondary and tertiary amino groups that are protonatable at lower pH. As seen in Fig. 2Af, the transfection ability of this polycation was similar to that of PEI25, thus again confirming the existence of the proton sponge effect.
Serum is rich in anionic proteins that can interact with polycations (33, 34). Such interactions, stronger for highly charged polycations, should result in adsorption of the negatively charged proteins on the polyplexes, and consequently, in their diminished endocytosis (34) and gene expression. Indeed, one can see in Fig. 2B d and e, that in the presence of serum transfection efficiencies of the peralkylated PEI25s were further suppressed compared with those without serum (Fig. 2A). Likewise, PEI25's choline derivative, as competent as PEI25 in the absence of serum (Fig. 2Af), became much less so in its presence (Fig. 2Bf).
We next explored how the hydrophilic-hydrophobic balance of the polycation influences the transfection efficiency. Amino acid grafting (Fig. 1, route C) was used toward this end because (i) the hydrophilic-hydrophobic balance could be varied by a judicious choice of the side chains and (ii) the total number of protonatable hydrogens could be maintained the same as in PEI. In the absence of serum, the transfection efficiency of the alanyl derivative was marginally higher, and of the leucyl derivative far lower (≈0.2%), than that of PEI25 (Fig. 2A g and h). In the presence of serum, the transfection efficiency of the alanyl derivative of PEI25 was twice as high as that of its parent (Fig. 2Bg), whereas the leucine derivative exhibited no appreciable activity (Fig. 2Bh). These results reveal that moderate enhancement in hydrophobicity (as in the case of Ala) increases the transfection efficiency of PEI25, whereas a much greater increase (the Leu derivative) is grossly deleterious.
Previous work had shown that the transfection efficiency of another polycation, polylysine, could be greatly enhanced, essentially to the level of PEI, by grafting of histidyl (14) or imidazole (35) residues. In contrast, herein the efficiency of histidyl-PEI25 was found to be lower than the parent's, both in the absence and presence of serum (Fig. 2Ai and Bi). Apparently, modification with His residues provides no advantage to PEI25 because it is already a good proton sponge.
Differential scanning calorimetry and electron microscopy studies had demonstrated that PEI bearing pendant hexyl and dodecyl chains interacted strongly with phospholipids, whereas the unmodified polymer did not (36). The presence of long lipophilic substituents on PEI can therefore be expected to strengthen the interaction of PEI/DNA complexes with the cell membrane, hence to enhance endocytosis of the complexes, and, in turn, to increase the transfection efficiency.
Partial alkylation of the primary amino groups with dodecyl and hexadecyl halides (Fig. 1, route D) did not raise the transfection efficiency of PEI25 in the absence or presence of serum and, in fact, resulted in its marked decline (Fig. 2A j and k). However, when the tertiary amino groups of PEI25 were quaternized with hexadecyl iodide (Fig. 1, route E), the resulting polycation displayed higher transfection efficiency compared with the former's previous hexadecyl derivative (Fig. 2Al) and, in the presence of serum, even greater efficiency than PEI25 (Fig. 2Bl). These results indicate that the position of alkylation (primary vs. tertiary nitrogen) has a profound effect on the transfection efficiency.
It has been proposed that hydrophobic groups, including dodecyl, attached to the PEI's primary amino groups form hydrophobic clusters that affect the protonation of amino groups (37, 38). In the present case, in PEI with its tertiary amines quaternized, the hydrophobic hexadecyl substituents are expected to be located toward the interior of the polycation (because tertiary amines are the branching points of the polymer and hence are oriented toward its interior). In contrast, in PEI with its primary amino groups alkylated, the hydrophobic substituents should be situated on the periphery of the polymer and, to avoid thermodynamically costly exposure to water, should undergo clustering. These distinctions in the disposition of the alkyl groups attached to primary vs. tertiary amino groups of PEI25 may be responsible for the observed differences in transfection efficiencies of the two generated types of polyplexes (Fig. 2).
The low-molecular-weight PEI2 is an inferior transfection agent compared with PEI25 (18–20). We found that as the N/P ratio is raised from 10 to 20 to 30 to 40, the transfection efficiencies grow 2.0-, 2.6-, and 1.4-fold, respectively. This pattern differs from the behavior of PEI25 whose efficiency did not increase beyond the N/P ratio of 10. Note, however, that even at the highest polycation to DNA ratio used, PEI2 was still some 2 orders of magnitude less efficient than PEI25 at the N/P of 10. In contrast to PEI25 though (see above), primary amine alkylation of PEI2 resulted in a dramatic rise in the transfection efficiency. For example, at the N/P ratio of 20, the dodecyl and hexadecyl derivatives of PEI2 were 110 and 170 times more efficient than the parent at the same N/P ratio. Additionally, the efficiencies of these PEI2 derivatives reached the transfection efficiencies of ≈30% and 50%, respectively, of that of PEI25. Finally, in the presence of serum, dodecyl- and hexadecyl-PEI2s were 400 and 550 times more efficient, respectively, than PEI2 itself (Fig. 2B m–o). Moreover, their transfection efficiencies under these conditions exceeded that of PEI25 more than 5-fold (Fig. 2B c, n, and o), thereby making these polymers “better than the best.”
Phospholipid formulations incorporating lipophilic PEI2 (polycation liposomes) have been recently reported to possess enhanced transfection efficiency compared with PEI2 (39, 40). This finding was attributed to liposome anchoring of PEI derivatives resulting in enhanced interaction with the cell membrane, which, in turn, leads to facile endocytosis of the DNA-vector complexes (39, 40). These systems, however, are no more efficient than PEI25 in the presence of serum (40). Also, the requirement of large molar excess of phospholipids (39) is a major drawback of such formulations. In contrast, the systems described in the present work are highly efficient even in the presence of serum and require no additives.
The cytotoxicities of the polyplexes formed from the unmodified and modified PEIs are presented in Table 1. It can be seen that PEI25 derivatized with Ala as well as dodecyl-PEI2 displayed no cytotoxicity. Hexadecyl-PEI2 was moderately cytotoxic, but no more so than PEI25. In addition, it appears that the decrease in the transfection efficiency of certain PEI25 derivatives (Fig. 2) was not caused by an increase in cytotoxicity as they were no more cytotoxic than the parent PEI.
Table 1.
Cytotoxicity to COS-7 cells of polyplexes formed by the plasmid DNA and PEIs and their N-derivatives
| PEI | Cell viability, % of control |
|---|---|
| None | 101 ± 3 |
| PEI25 | 81 ± 10 |
| Permethyl-PEI25 | 105 ± 2 |
| Perethyl-PEI25 | 105 ± 2 |
| Cholyl-PEI25 | 101 ± 5 |
| Alanyl-PEI25 | 98 ± 4 |
| Leucyl-PEI25 | 104 ± 2 |
| Histidyl-PEI25 | 105 ± 1 |
| Dodecyl-PEI25 | 105 ± 2 |
| Hexadecyl-PEI25 | 93 ± 2 |
| Hexadecyl-PEI25 | 87 ± 2 |
| PEI2 | 103 ± 3 |
| Dodecyl-PEI2 | 101 ± 1 |
| Hexadecyl-PEI2 | 79 ± 1 |
Cytotoxicity was measured by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay. The N/P ratio was 10 in the case of PEI25 and its derivatives and 20 in the case of PEI2 and its derivatives. The viability of the nontransfected cells in the absence of PEI is taken as 100%. See Experimental Procedures for other details.
Primary amines are alkylated.
Tertiary amines are alkylated.
In closing, several salient features pertinent to polycation-based gene delivery were addressed herein by using diverse chemical modifications of the best nonviral polycationic gene delivery class of agents, PEIs. As a result, two PEI derivatives were synthesized, alanyl-PEI25 and dodecyl-PEI2, whose transfection efficiencies were several times greater than that of PEI25 (one of the most potent nontargeted polycationic gene delivery vectors). Furthermore, these two derivatives, in contrast to PEI25, were not toxic. They could be further modified for targeted gene delivery by incorporating appropriate recognition elements, such as galactose or folic acid (41, 42). Insights obtained from these studies should help develop truly superior polyplexes with genuine utility in human gene therapy.
Acknowledgments
We are grateful to Professor Ram Sasisekharan for allowing us to use his tissue culture facility, and to Ms. Kristine Holley for helpful assistance. This work was financially supported by the Biotechnology Process Engineering Center at the Massachusetts Institute of Technology and National Institutes of Health Grant GM26698.
Abbreviations
PEI, polyethylenimine
References
- 1.Esmail D. Z. & Anderson, W. F. (1999) Science 285, 2084-2088. [DOI] [PubMed] [Google Scholar]
- 2.Abott A. (2001) Nature 411, 410-412. [DOI] [PubMed] [Google Scholar]
- 3.McCormick F. (2001) Nat. Rev. 1, 130-140. [DOI] [PubMed] [Google Scholar]
- 4.Verma I. M. & Somia, N. (1997) Nature 389, 239-242. [DOI] [PubMed] [Google Scholar]
- 5.Marshall E. (2000) Science 286, 2244-2245. [DOI] [PubMed] [Google Scholar]
- 6.Miller A. D. (1998) Angew. Chem. Int. Ed. Engl. 37, 1768-1785. [Google Scholar]
- 7.Henry C. M. (2001) Chem. Eng. News 20, 35-41. [Google Scholar]
- 8.Brown M. D., Schatzelin, A. G. & Uchegbu, I. F. (2001) Int. J. Pharm. 229, 1-21. [DOI] [PubMed] [Google Scholar]
- 9.Kabanov A. V. & Kabanov, V. A. (1995) Bioconjug. Chem. 6, 7-20. [DOI] [PubMed] [Google Scholar]
- 10.Kircheis R., Wightman, L. & Wagner, E. (2001) Adv. Drug Delivery Rev. 53, 341-358. [DOI] [PubMed] [Google Scholar]
- 11.Davis M. E. (2002) Curr. Opin. Biotechnol. 13, 128-131. [DOI] [PubMed] [Google Scholar]
- 12.Pouton C. W. & Seymour, L. W. (1998) Adv. Drug Delivery Rev. 34, 3-19. [DOI] [PubMed] [Google Scholar]
- 13.Fisher K. D., Ulbrich, K., Suber, V., Ward, C. M., Mautner, V., Blakey, D. & Seymour, L. W. (2000) Gene Ther. 7, 1337-1343. [DOI] [PubMed] [Google Scholar]
- 14.Pinchon C., Concalves, C. & Midoux, P. (2001) Adv. Drug Delivery Rev. 53, 75-94. [DOI] [PubMed] [Google Scholar]
- 15.Subramanian A., Ranganathan, P. & Diamond, S. (1999) Nat. Biotechnol. 19, 873-877. [DOI] [PubMed] [Google Scholar]
- 16.Schaffer D. V., Fidelman, N. A., Dan, N. & Lauffenburger, D. A. (2000) Biotechnol. Bioeng. 67, 598-606. [DOI] [PubMed] [Google Scholar]
- 17.Boussif O., Lezoualc'h, F., Zanta, A., Mergny, M. D., Scherman, D., Demeneix, B. & Behr, J.-P. (1995) Proc. Natl. Acad. Sci. USA 92, 7297-7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer D., Bieber, T., Li, Y., Elsasser, H.-P. & Kissel, T. (1999) Pharm. Res. 16, 1273-1279. [DOI] [PubMed] [Google Scholar]
- 19.Godbey W. T., Wu, K. K. & Mikos, A. G. (1999) J. Biomed. Mater. Res. 45, 268-275. [DOI] [PubMed] [Google Scholar]
- 20.Gebhart C. L. & Kabanov, A. V. (2001) J. Control. Release 73, 401-416. [DOI] [PubMed] [Google Scholar]
- 21.Godbey W. T., Wu, K. K. & Mikos, A. G. (1999) Proc. Natl. Acad. Sci. USA 96, 5177-5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Godbey W. T., Barry, M. A., Saggau, P., Wu, K. K. & Mikos, A. G. (2000) J. Biomed. Mater. Res. 51, 321-328. [DOI] [PubMed] [Google Scholar]
- 23.Lecocq M., Wattiaux-De Coninck, S., Laurent, N., Wattiaux, R. & Jadot, M. (2000) Biochem. Biophys. Res. Commun. 278, 414-418. [DOI] [PubMed] [Google Scholar]
- 24.Akinc A. & Langer, R. (2002) Biotechnol. Bioeng. 78, 503-508. [DOI] [PubMed] [Google Scholar]
- 25.Johnson T. W. & Klotz, I. M. (1974) Macromolecules 7, 149-153. [Google Scholar]
- 26.Noding G. & Heitz, W. (1998) Macromol. Chem. Phys. 199, 1637-1644. [Google Scholar]
- 27.Okahata Y. & Kunitake, T. (1978) J. Polym. Sci. Polym. Chem. Ed. 16, 1865-1881. [Google Scholar]
- 28.Alam J. & Cook, J. L. (1990) Anal. Biochem. 188, 245-254. [DOI] [PubMed] [Google Scholar]
- 29.Schenborn E. & Goiffon, V. (1993) Promega Notes Magazine 41, 11. [Google Scholar]
- 30.Smith P. K., Krohn, R. I., Hermanson, G. T., Mallia, A. K., Gartner, F. H., Provenzano, M. D., Fujimoto, E. K., Goeke, N. M., Olson, B. J. & Klenk, D. C. (1985) Anal. Biochem. 150, 76-85. [DOI] [PubMed] [Google Scholar]
- 31.Hansen M. B., Nielsen, S. E. & Berg, K. (1989) J. Immunol. Methods 119, 203-210. [DOI] [PubMed] [Google Scholar]
- 32.Ahn C.-H., Chae, S. Y., Bae, Y. H. & Kim, S. W. (2002) J. Control. Release 80, 273-282. [DOI] [PubMed] [Google Scholar]
- 33.Orgis M., Brunner, S., Schuller, S., Kircheis, R. & Wagner, E. (1999) Gene Ther. 6, 595-605. [DOI] [PubMed] [Google Scholar]
- 34.Roufai M. B. & Midoux, P. (2001) Bioconjug. Chem. 12, 92-99. [DOI] [PubMed] [Google Scholar]
- 35.Putman D., Gentry, C. A., Pack, D. W. & Langer, R. (2001) Proc. Natl. Acad. Sci. USA 98, 1200-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takigawa D. Y. & Tirrell, D. A. (1985) Macromolecules 18, 338-342. [Google Scholar]
- 37.Suh J., Lee, S. H., Kim, S. M. & Hah, S. S. (1997) Bioorg. Chem. 25, 221-231. [Google Scholar]
- 38.Suh J. & Hong, S. H. (1998) J. Am. Chem. Soc. 120, 12545-12552. [Google Scholar]
- 39.Yamazaki Y., Nango, M., Matsuura, M., Hasegawa, Y., Hasegawa, M. & Oku, N. (2000) Gene Ther. 7, 1148-1155. [DOI] [PubMed] [Google Scholar]
- 40.Kim S., Choi, J. S., Jang, H. S., Suh, H. & Park, J. (2001) Bull. Korean Chem. Soc. 22, 1069-1075. [Google Scholar]
- 41.Zanta M.-A., Bousiff, O., Adib, A. & Behr, J.-P. (1997) Bioconjug. Chem. 8, 839-844. [DOI] [PubMed] [Google Scholar]
- 42.Guo W. & Lee, R. L. (1999) Polym. Preprints 40, 290-291. [Google Scholar]