Abstract
Biomolecular self-assembly can be used as a powerful tool for nanoscale engineering. In this paper, we describe the development of building blocks for nanobiotechnology, which are based on the fusion of streptavidin to a crystalline bacterial cell surface layer (S-layer) protein with the inherent ability to self-assemble into a monomolecular protein lattice. The fusion proteins and streptavidin were produced independently in Escherichia coli, isolated, and mixed to refold and purify heterotetramers of 1:3 stoichiometry. Self-assembled chimeric S-layers could be formed in suspension, on liposomes, on silicon wafers, and on accessory cell wall polymer containing cell wall fragments. The two-dimensional protein crystals displayed streptavidin in defined repetitive spacing, and they were capable of binding d-biotin and biotinylated proteins. Therefore, the chimeric S-layer can be used as a self-assembling nanopatterned molecular affinity matrix to arrange biotinylated compounds on a surface. In addition, it has application potential as a functional coat of liposomes.
Nanotechnology faces the challenge to provide innovative approaches for the assembly of molecular structures and devices with dimensions of a few to tens of nanometers, because this size range is not routinely accessible with current technologies. It is too small for conventional lithographic structuring methods but too large for chemical synthesis. Nanofabrication techniques based on the atomic force microscope (AFM) or electron beam (e-beam) lithography work in a serial rather than in a parallel way, and, therefore, do not offer adequate throughput. However, the most complex functional nanoscale structures are built efficiently from biomolecules in biological systems. Therefore, nanobiotechnology, a new discipline that seeks to employ biomolecules as building blocks and biomolecular self-assembly as the construction principle, offers exciting possibilities for the defined assembly of functional nanostructures. Research on designing biomolecules for nanobiotechnology has included both proteins and nucleic acids. Two-dimensional DNA lattices (1), three-dimensional cages and networks of DNA (2), and structures made of DNA and streptavidin (3) were constructed based on the highly specific base-pairing properties of DNA. Efforts to use proteins as patterning elements and building blocks in nanotechnology included making fusion proteins that self-assemble into large symmetrical nanomaterials (4). In this study, we designed chimeric proteins by fusing streptavidin to a crystalline bacterial cell surface layer (S-layer) protein. S-layer proteins have evolved to form two-dimensional protein crystals as the outermost component of bacterial cell envelopes (5–7). Their intrinsic ability to self-assemble allows the in vitro formation of monomolecular protein lattices in suspension, on lipid films, on liposomes, and on solid supports including silicon wafers, metals, and polymers (8, 9). Self-assembly of the S-layer protein moiety was exploited to arrange streptavidin in defined order and orientation in two-dimensional protein crystals. Owing to the versatile applications of the streptavidin–biotin interaction as a biomolecular coupling system, the chimeric S-layer was designed to serve as a compatible patterning element for any biotinylated targets.
In this work, the S-layer protein SbsB (10) of Geobacillus stearothermophilus PV72/p2 (11) was used. SbsB is generated from a 920-aa preprotein by cleavage of a 31-aa signal peptide (10). It contains an S-layer homology (SLH) domain (12) at its N terminus, which is responsible for anchoring the protein to the cell surface by binding to an accessory cell wall polymer (13). SbsB forms a 4.5-nm thick p1 lattice with the lattice parameters a = 10.4 nm, b = 7.9 nm, and base angle γ = 81°. Because in a p1 lattice one morphological unit consists of a single protein and there are no internal symmetries, a protein moiety that is fused to any position of SbsB, provided that it does not interfere with lattice formation, will be presented with this same spacing. When SbsB crystallizes in vitro on solid supports or lipid films, its orientation is reversed, and the inner face with the SLH domain is exposed to the solvent. However, on surfaces presenting the specific accessory cell wall polymer, SbsB crystallizes in its natural orientation. Therefore, two types of building blocks with streptavidin at the outer and at the inner face of SbsB were desired. Until now, no structural model at atomic resolution of SbsB or any other S-layer protein has been available. Therefore, an understanding of the protein's structure–function relationship was gained in a preliminary study from the production and analysis of truncated forms, sequence analysis, and electron microscopy. The knowledge was used to select sequence positions for functional fusion. Six different fusion constructs were made, four with streptavidin fused to N-terminal positions of SbsB (referred to as “N-terminal fusion proteins”), and two with streptavidin attached to the C terminus of SbsB (“C-terminal fusion proteins”). The proteins were refolded to heterotetramers consisting of one chain of fusion protein and three chains of streptavidin. The self-assembly capability of chimeric S-layer proteins in suspension, on liposomes, on silicon wafers, and on cell wall fragments was demonstrated, and their ability to bind d-biotin and two biotinylated marker proteins, peroxidase and ferritin, was studied.
Materials and Methods
Construction of Expression Vectors.
This section can be found in Supporting Materials and Methods, which is published as supporting information on the PNAS web site, www.pnas.org.
Chimeric Gene Expression and Fusion Protein Isolation, Refolding, Purification, SDS/PAGE Analysis, and Self-Assembly.
Gene expression was carried out as described in the pET System Manual (Novagen) by using E. coli strain BL21(DE3) carrying an expression vector. SDS/PAGE analysis of biomass was performed according to standard procedures (14). Biomass was fixed, embedded, and ultrathin-sectioned as described (15). Core streptavidin and N-terminal fusion proteins were isolated according to a published protein-isolation protocol (16) from half a liter of expression culture each, mixed and dissolved in 10 ml of 7 M guanidine hydrochloride (GHCl; pH 1.5), dialyzed twice against 100 ml of 7 M GHCl (pH 1.5) for 3 h at room temperature, and injected as described (17) into 700 ml of ice-cold 20 mM phosphate buffer (pH 6.2). The C-terminal fusion proteins were applied to the same procedure after lysis of whole cells in 7 M GHCl (pH 1.5). Tetramers of the desired stoichiometry were separated by gel permeation chromatography (Superdex 200 resin, Amersham Pharmacia) and purified by affinity chromatography (2-iminobiotin resin, Sigma) according to the manufacturers' instructions, except that all buffers for N-terminal fusion proteins contained 2 M GHCl. SDS/PAGE analysis of streptavidin homo- and heterotetramers was performed by incubating parallel samples in loading buffer containing 5% SDS in boiling water and at room temperature, respectively, for 5 min, and applying them to adjacent lanes of SDS/15% PAGE separation gels. Self-assembly products of heterotetrameric fusion proteins were formed in suspension by dialyzing a 10 μM protein solution in 2 M GHCl/50 mM Tris⋅HCl buffer (pH 7.2) against Aqua purificata (A. purif.; Milli-Q, Millipore) at 4°C for at least 24 h. Self-assembly on accessory cell wall polymer containing cell wall fragments was accomplished by mixing 1 mg of fusion protein with 1 mg of cell wall fragments, which were prepared as described (13), in 2 M GHCl/50 mM Tris⋅HCl buffer (pH 7.2) and dialyzing against A. purif. at 4°C for at least 24 h. Self-assembly on liposomes, which were prepared as described (18) from a lipid mixture composed of 20 μmol 1,2-dipalmitoyl-sn-glycero-3-phosphatidyl choline (DPPC), 10 μmol cholesterol, and 8 μmol hexadecylamine, was performed as follows: a 10 μM protein solution in 2 M GHCl/50 mM Tris⋅HCl buffer (pH 7.2) was dialyzed against A. purif. for 1 h at room temperature. Self-assembly products were removed by centrifugation (10 min at 16,000 × g), and the solution was mixed with an equal volume of liposome suspension (0.646 mM DPPC) in 150 mM NaCl. After 3 h of incubation under rotation at room temperature, the liposomes were pelleted (10 min at 16,000 × g) and resuspended in half the volume of A. purif. For crystallization on silicon wafers (boran doped, MEMC Electronic Materials, Novara, Italy), a 4 μM protein solution in 2 M GHCl/50 mM Tris⋅HCl buffer (pH 7.2) was dialyzed at 4°C against 10 mM CaCl2 and A. purif. for 3 h each. After centrifugation (10 min at 16,000 × g), the solution was mixed with an equal volume of 20 mM phosphate buffer (pH 7.2). Silicon wafers were cut to 0.5-cm2 pieces, cleaned with ethanol and A. purif., added to 500 μl of protein solution for a 16-h incubation period at room temperature, and then transferred to 10 mM phosphate buffer (pH 7.2).
Fluorescence Titration with d-Biotin to Determine Biotin-Binding Ability.
Protein concentrations were determined by absorption spectroscopy of native proteins at 280 nm (Hitachi U-3300 spectrophotometer) and calculated by using extinction coefficients of 211,230 M−1cm−1 for heterotetrameric fusion proteins and 167,280 M−1cm−1 for core streptavidin [www.expasy.ch/tools/protparam.html (19)]. Fluorescence titrations (20) were carried out with 2 ml of protein solution at a typical concentration of 100 nM protein in 50 mM Tris⋅HCl buffer (pH 7.2) with or without 2 M GHCl in a stirred 1-cm quartz cuvette set to 20°C in a Perkin–Elmer Luminescence Spectrometer (LS 50 B). The decrease in tryptophan fluorescence was monitored (excitation, 280 nm; emission, 340 nm; 15-nm slit) while a 20 μM d-biotin solution, which was prepared as a primary standard, was added in 5-μl increments at 1-min intervals. Recordings were made 30 s after each addition over a 10-s integration time. In the titration profiles, the breakpoint between progressive quenching and the subsequent plateau was used to calculate the amount of d-biotin needed for saturation of all biotin-binding sites.
Binding Studies with Biotinylated Peroxidase and Biotinylated Ferritin.
Liposomes coated with an S-layer were suspended in PBS at a concentration corresponding to 32.3 μM DPPC. Peroxidase-biotinamidocaproyl conjugate (Sigma) was added to a concentration of 2.5 μg/ml. After a 30-min incubation at room temperature, the liposomes were pelleted (10 min at 16,000 × g) and resuspended in the same volume of PBS. Ten microliters of pellet and supernatant fraction, respectively, were added to 800 μl of tetramethylbenzidine dihydrochloride solution (Sigma), which was prepared according to the manufacturer's instructions. After a 20-min incubation at room temperature, the reaction was stopped by adding 200 μl of 2 M H2SO4, and the absorption was read at 450 nm in a Hitachi U-2000 spectrophotometer. Ferritin was conjugated with sulfo-NHS-LC-LC-biotin (Pierce) according to the manufacturer's instructions and purified by ultrafiltration over a YM 100 filter (Millipore). Biotinylated ferritin was incubated with S-layer-carrying liposomes (32.3 μM DPPC in PBS) and cell wall fragments (0.2 mg/ml protein in PBS), respectively, at a concentration of 1.6 mg/ml. Liposomes and cell wall fragments were pelleted and washed four times with PBS (10 min at 8,000 × g) and negatively stained with uranyl acetate for transmission electron microscopy (TEM). Liposomes and cell wall fragments carrying an S-layer of recombinant SbsB served as negative controls.
Atomic Force Microscopy, Transmission Electron Microscopy, and Digital Image Enhancement.
This section can be found in Supporting Materials and Methods.
Results
Design and Preparation of Streptavidin-SbsB Heterotetrameric Fusion Proteins.
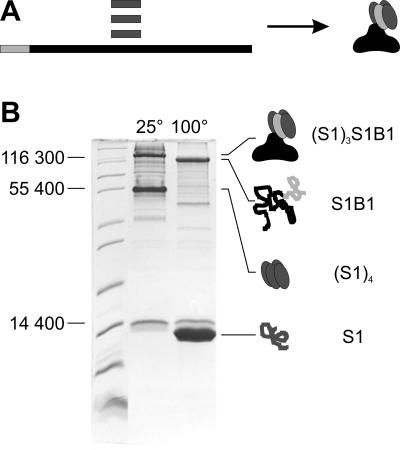
A preliminary study revealed that truncated forms of SbsB lacking the N-terminal SLH domain self-assembled to form an S-layer lattice with unchanged lattice parameters, whereas deletion of 15 amino acids or more from the C terminus prevented lattice formation. Based on these results, the following six fusion proteins were designed (a schematic illustration can be found in Fig. 6, which is published as supporting information on the PNAS web site). The amino acid numbers refer to the mature protein sequences of SbsB (10) and streptavidin (21) with reverse and negative numbering for the signal peptide sequences. S1B1 (Stv16–133/SbsB1–889) had minimum-sized core streptavidin attached to the N terminus of mature full-length SbsB. S1B-30 (Stv16–133/SbsB-30–889) included the signal peptide of SbsB as a linker between the two fusion partners. In S1B63 (Stv16–133/SbsB63–889), the first one of the three SLH motifs was deleted, and in S1B177 (Stv16–133/SbsB177–889), the whole SLH domain was deleted. The two C-terminal fusion proteins, BS1 (SbsB1–889/Stv16–133) and BS2 (SbsB1–889/Stv8–133), differed in the streptavidin chain length. The additional eight amino acids in BS2 were intended to serve as a flexible linker (22). The expression levels of all six fusion protein constructs in E. coli BL21(DE3) were in the range of 50 mg of protein per liter of bacterial culture. The four N-terminal fusion proteins formed self-assembly products in the cytoplasm of the host cells, which could be visualized by TEM of ultrathin-sectioned preparations (Fig. 7, which is published as supporting information on the PNAS web site). The crystalline sheets readily endured an enzymatic lysis procedure and could be isolated by centrifugation and washing. However, the C-terminal fusion proteins appeared in the soluble protein fraction, and no assembly-like structures were observed. Without a fusion partner, core streptavidin accumulated as inclusion bodies. Cytoplasmic self-assembly products and inclusion bodies were isolated, mixed, and subjected to a refolding procedure. The two C-terminal fusion proteins were applied to the same refolding procedure as crude cell lysates. Refolding was performed by denaturing and renaturing a mixture of fusion protein with excess core streptavidin, allowing the formation of two dominant tetramer species; one consisted of the fusion protein and core streptavidin at a 1:3 ratio (heterotetramer, Fig. 1A), and the other consisted of core streptavidin alone (homotetramer). Tetramer formation was confirmed by SDS/PAGE analysis (Fig. 1B), which was based on the fact that streptavidin tetramers are stable in 5% SDS at room temperature but denature upon boiling (23). Heterotetramers of the desired stoichiometry were purified by gel permeation chromatography and affinity chromatography on 2-iminobiotin resin. The N-terminal heterotetrameric fusion proteins were termed (S1)3S1B1 (144,973 Da), (S1)3S1B-30 (148,012 Da), (S1)3S1B63 (138,309 Da), and (S1)3S1B177 (125,636 Da). The C-terminal heterotetramers were termed BS1(S1)3 (144,784 Da) and BS2(S1)3 (145,512 Da).
Fig 1.
Refolding of heterotetrameric fusion protein (S1)3S1B1 (A) and confirmation by SDS/PAGE analysis (B). (A) Core streptavidin (S1, dark gray) and fusion protein S1B1 (streptavidin moiety S1, light gray; SbsB moiety B1, black) were produced in E. coli and isolated independently, mixed, and refolded to heterotetramers (S1)3S1B1. (B) For SDS/PAGE analysis, samples were recovered from a refolding batch, and SDS was added to a concentration of 5%. Without boiling of the sample (lane 25°C), streptavidin–SbsB heterotetramers (S1)3S1B1 (144,973 Da) and core streptavidin homotetramers (S1)4 (49,840 Da) migrated at apparent molecular masses of 125,000 and 55,000 Da, respectively, on an SDS/15% PAGE gel. After boiling (lane 100°C), the tetramers of both the fusion protein and core streptavidin were denatured to monomers (S1B1, 107,593 Da; S1, 12,460 Da).
Self-Assembly of the Chimeric S-Layer Proteins.
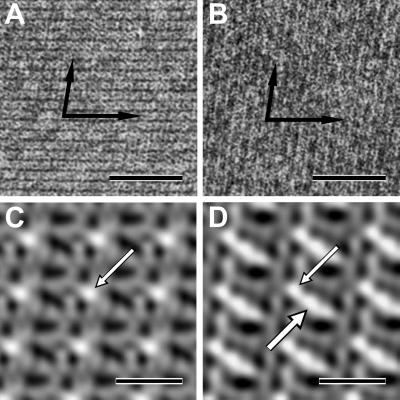
Similar to SbsB (Fig. 2A), all four N-terminal heterotetrameric fusion proteins were able to self-assemble into crystalline chimeric S-layer sheets in suspension (Fig. 2B and Fig. 8, which is published as supporting information on the PNAS web site). After Fourier transformation of electron micrographs taken from negatively stained (uranyl acetate) preparations of self-assembly products, analysis showed that the lattice parameters of SbsB were unchanged in all four chimeric S-layers. Digital image reconstructions of the (S1)3S1B1 lattice in comparison with the SbsB lattice showed the additional protein mass of streptavidin linked to the SLH-domain, to which it was fused (Fig. 2 C and D). (S1)3S1B1 could also crystallize on silicon wafers (Fig. 3) and on liposomes (Fig. 4A), leading to coherent monolayers. Monocrystalline areas on silicon were typically in the range of 400 nm. The two C-terminal fusion proteins could not self-assemble in suspension. However, both of them formed a monomolecular lattice on accessory cell wall polymer-containing cell wall fragments of G. stearothermophilus PV72/p2 (Fig. 5A). The lattice parameters were identical to those of SbsB, as was confirmed by Fourier processing of electron micrographs taken from negatively stained preparations.
Fig 2.
Self-assembly products and digital image reconstructions of SbsB (see A and C for comparison) and fusion protein (S1)3S1B1 (B and D). (A and B) The crystalline sheets were formed in suspension and negatively stained with uranyl acetate for TEM. The arrows indicate the base vectors of the oblique p1 lattice. (Bars = 50 nm.) (C and D) The digital image reconstructions were made by Fourier processing of electron micrographs (not identical to the ones shown in A and B). The region of highest protein mass in the SbsB lattice is the SLH-domain (C, arrow). In the lattice of the fusion protein, streptavidin showed up as additional protein mass (D, thick arrow) attached to the SLH domain. (Bars = 10 nm.)
Fig 3.
Fusion protein (S1)3S1B1 crystallized on silicon wafers. The atomic force microscopy deflection image was recorded in contact mode under 100 mM NaCl; z-range = 0.6 nm. (Bar = 100 nm.)
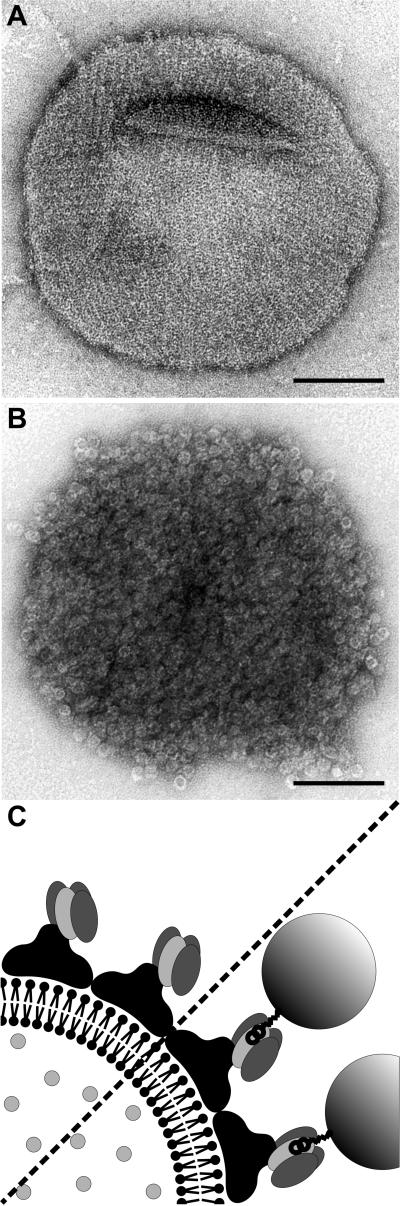
Fig 4.
Liposomes carrying a chimeric S-layer formed by the fusion protein (S1)3S1B1 (A) were capable of binding biotinylated ferritin (B). Preparations were negatively stained with uranyl acetate for TEM. (Bars = 100 nm.) (C) The cartoon shows the orientation of (S1)3S1B1 on liposomes with the streptavidin-carrying inner face of the S-layer exposed.
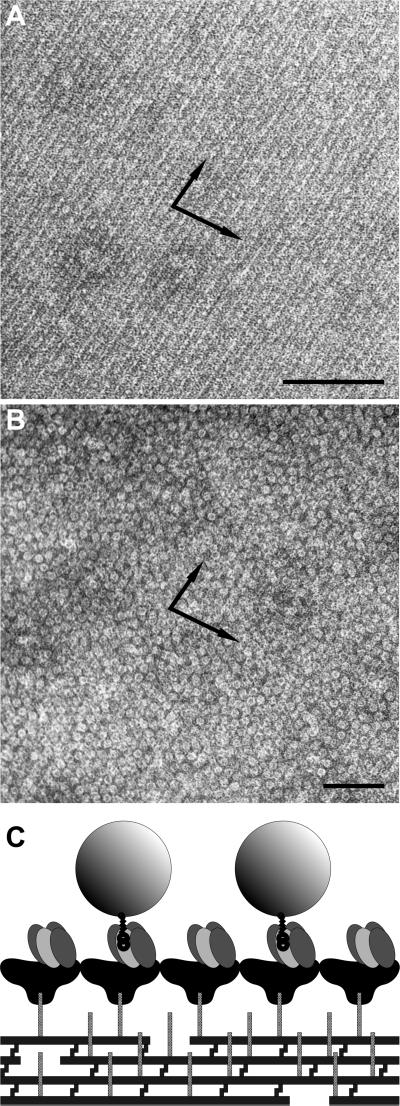
Fig 5.
Cell wall fragments carrying a chimeric S-layer formed by the fusion protein BS1(S1)3 (A) were capable of binding biotinylated ferritin (B). (A) Self-assembly was enabled by the specific interaction between an accessory cell wall polymer that is part of the cell wall of G. stearothermophilus PV72/p2 and the SLH domain of the fusion protein. (B) Bound biotinylated ferritin reflected the underlying S-layer lattice. The preparations were negatively stained with uranyl acetate for TEM. The arrows indicate the base vectors of the oblique p1 lattice. (Bars = 100 nm.) (C) The cartoon shows the orientation of BS1(S1)3 after SLH-enabled self-assembly with the streptavidin-carrying outer face of the S-layer exposed.
Determination of Biotin-Binding Ability.
Because the fusion proteins were constructed from the wild-type streptavidin sequence, the biotin-binding sites were assumed to show the typical high affinity to biotin. However, it remained to be investigated whether biotin-binding sites were sterically blocked by the large and unglobular fusion partner SbsB. The biotin-binding ability was determined by using a fluorescence titration method (20) that is based on the quenching of endogenous tryptophan residues upon biotin binding. The amount of d-biotin required for saturation of a known amount of streptavidin or fusion protein was calculated from the consumption of d-biotin up to the distinct breakpoint in the fluorescence titration profile, where progressive quenching stopped and a plateau began. (S1)3S1B1 showed a biotin-binding ability of 2.95 ligands per protein tetramer. In comparison, homotetrameric streptavidin, which was isolated from the very same refolding batch, showed a binding ability of 3.95 biotin molecules per tetramer. To determine whether lattice formation blocks biotin-binding sites, a suspension of (S1)3S1B1 self-assembly products was titrated directly and after dissolving the self-assembly products in 2 M GHCl, respectively. Between the two titration profiles, the breakpoint marking saturation was not shifted. The C-terminal fusion protein BS1(S1)3 was titrated in solution and as a crystalline protein monolayer bound to cell wall fragments, respectively. Profiles of both titrations showed breakpoints corresponding to a biotin-binding ability of 3.20 ligands per tetramer.
Binding Studies with Biotinylated Peroxidase and Biotinylated Ferritin.
It was critical to determine whether the streptavidin moieties of crystalline chimeric S-layer assemblies were sterically accessible for binding of biotinylated targets. To test this possibility, biotinylated peroxidase was used as an enzyme marker, and biotinylated ferritin was used as a marker that can be visualized by TEM. Liposomes with a coherent crystalline monolayer of (S1)3S1B1 were incubated with biotinamidocaproyl-peroxidase. After centrifugation, >95% (liposomes coated with recombinant SbsB < 3%) of the total enzyme activity was associated with the liposomes in the pellet fraction, and <5% was found in the supernatant. For TEM analysis, (S1)3S1B1-coated liposomes and BS1(S1)3-carrying cell wall fragments, respectively, were incubated with biotinylated ferritin, centrifuged, and washed. Negatively stained preparations showed the liposomes (Fig. 4B) and the cell wall fragments (Fig. 5B) densely covered with ferritin, whereas virtually no binding to liposomes coated with recombinant SbsB was observed.
Discussion
The aim of this work was to design building blocks and patterning elements for nanobiotechnology by fusing streptavidin to an S-layer protein, and thus to form chimeric S-layers with streptavidin arranged in a two-dimensional crystalline order. The fusion proteins could be designed so that self-assembly of the S-layer protein SbsB was not prevented, and streptavidin was accessible and functional in the chimeric S-layer lattice. Moreover, two types of building blocks were obtained by fusing streptavidin to the N terminus (inner face) and C terminus (outer face) of SbsB. To be a useful patterning element and nano-building block, an S-layer-streptavidin fusion protein must be able to self-assemble on technologically relevant surfaces and display streptavidin at the accessible face of the chimeric S-layer. Both of these were demonstrated for the N-terminal fusion protein (S1)3S1B1. The C-terminal fusion proteins, BS1(S1)3 and BS2(S1)3, only self-assembled on cell wall fragments, but because the accessory cell wall polymer that enabled crystallization is a glycan that can be isolated (13), chemically coupled, and immobilized to surfaces (C. Mader, C. Holzy, U.B.S., and M.S., unpublished work), they are candidates for highly specific anchoring and crystallization on prefunctionalized surfaces. The model system with cell wall fragments served to demonstrate the accessible display of streptavidin.
Chilkoti et al. (24) reported refolding of streptavidin heterotetramers by the mixing of differently mutated subunits, and Reznik et al. (25) refolded heterotetramers of defined 1:1 stoichiometry by the mixing of streptavidin subunits with complementary mutations. In the present paper, an alternative way of preparing heterotetramers of defined stoichiometry is described. The refolding procedure produced a mixed population of tetramers, although the population was biased toward two predominant species by the applied molar ratio of subunits. The differences in molecular size that were conferred by the large fusion partner SbsB were then exploited to isolate the heterotetramer species of desired stoichiometry.
Because the lattice constants of all six chimeric S-layers remained unchanged, streptavidin must have been located either above or below the plane of assembly. It did not seem to interfere with the self-assembly process of the N-terminal fusion proteins. However, streptavidin compromised the self-assembly properties of the C-terminal fusion proteins. A study preliminary to this work showed that the C-terminal amino acids of SbsB were essential for lattice formation, which presumably makes this region of the protein sensitive to modifications. In BS2(S1)3, use of a flexible protein region of streptavidin (22) as a linker did not make a notable difference. Without this flexible region, BS1(S1)3 was assumed to be more stable and was studied in more detail. Because all four N-terminal fusion proteins were capable of self-assembling in suspension, (S1)3S1B1 was chosen for biotin binding and crystallization studies because it was assumed to be more stable than the constructs including truncated versions of SbsB or its signal peptide.
In the N-terminal fusion protein (S1)3S1B1, 2.95 biotin-binding sites per tetramer were found to be able to bind biotin. Theoretically, residual biotin originating from the bacterial expression culture could occupy and block biotin-binding sites. Nonetheless, 3.95 binding sites per tetramer were available in a preparation of core streptavidin, which was isolated from the very same refolding batch as (S1)3S1B1. Therefore, both protein preparations must have been almost free from contaminating biotin, and one of the four biotin-binding sites of (S1)3S1B1 must have been inaccessible because of steric hindrance by the large and unglobular fusion partner SbsB. Because a crude cell lysate was applied to the refolding procedure for the preparation of the C-terminal fusion proteins, a higher amount of contaminating biotin and reduced biotin-binding ability was expected. Chimeric S-layer assembly did not cause a steric hindrance for biotin binding, because titration profiles of self-assembled and disassembled proteins showed no shift of the titration breakpoint. Biotinylated ferritin was used as a model compound for binding studies because it is a proteinaceous particle with uniform properties, and it can be visualized by TEM. However, ferritin is 12 nm in diameter, which is bigger than the morphological unit of the SbsB lattice. Therefore, it was not suitable to test for the formation of superlattice arrays. Nevertheless, bound biotinylated ferritin reflected the oblique p1 lattice symmetry. Superlattice array formation with suitable biotinylated targets will be the subject of further investigations.
The digital image reconstruction of the (S1)3S1B1 lattice confirmed that streptavidin was arranged in a defined repetitive position in the crystal. Interestingly, it appeared in an ellipsoidal rather than in the expected round shape. However, the comparison with the SbsB lattice showed that streptavidin was positioned on top of an arm of SbsB that separates two elongated pores, and a protein mass located above a stain-filled pore would not show up in a negatively stained preparation. To our knowledge, this is the first report of a digital image reconstruction of a chimeric S-layer lattice with an integrated functional protein.
Two-dimensional crystals of streptavidin can also be formed without an S-layer protein as a fusion partner. Such crystals display two accessible biotin-binding sites per tetramer that can be used to bind biotinylated proteins (26). However, contrary to streptavidin, S-layer proteins form two-dimensional protein crystals by a robust self-assembly process that has been optimized by evolution. Therefore, with an S-layer protein as a fusion partner, there are many compatible surfaces for crystallization, which makes technological applications of streptavidin in two-dimensional crystalline arrangement feasible.
Because of the ability of SbsB to self-assemble on many surfaces and interfaces, and because of the versatile applications of streptavidin and biotin as a biomolecular coupling system, streptavidin–SbsB fusion proteins have a broad application potential. The functional biomolecular matrix with repetitive features in the nanometer range allows a unique approach to immobilizing compounds with defined spacing on a surface. Because fusion proteins could be crystallized on silicon wafers, the chimeric S-layer can be used as a patterning element for molecular electronics applications such as arrays of quantum dots. Moreover, it can function as an interface in biosensor elements to arrange functional biomolecules in a defined way. The chargeable nanopatterned surface or interface also allows new approaches for diagnostics, affinity matrices, enzyme membranes, biocompatible surfaces, biological templating, and composite vaccines (5, 27, 28). Furthermore, the S-layer-coated liposomes mimic the cell envelope of archaea, and the lipid membrane is stabilized by the S-layer (18). In combination with the affinity to biotinylated binding partners, 5-layer-stabilized liposomes offer new prospects for liposome targeting, drug-delivery systems, and the design of biomimetic virus envelopes and vehicles for gene therapy. The results with S-layer-streptavidin fusion proteins demonstrate that biomolecular building blocks and nanobiotechnology can be used for the engineering of defined functional structures in the size range that cannot be routinely accessed by other micro- and nanofabrication techniques.
Supplementary Material
Acknowledgments
We thank Charles R. Cantor and Takeshi Sano for the gift of pUC8-SZ. We thank Seta Küpcü for help with liposomes, Dominik Rünzler for help with fluorescence spectroscopy, and Erika Györvary and Oliver Stein for their care of the atomic force microscopy work. We also thank Friedrich Srienc for critical review of the manuscript. This work was supported in part by Austrian Science Foundation Project 14689, European Union Project IST-1999-11974 (BIOAND), and the Competence Center for Biomolecular Therapeutics.
Abbreviations
SLH, S-layer homology
GHCl, guanidine hydrochloride
TEM, transmission electron microscopy
A. purif., Aqua purificata
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Winfree E., Liu, F., Wenzler, L. A. & Seeman, N. C. (1998) Nature 394, 539-544. [DOI] [PubMed] [Google Scholar]
- 2.Seeman N. C. (1999) Trends Biotechnol. 17, 437-443. [DOI] [PubMed] [Google Scholar]
- 3.Niemeyer C. M. (2000) Curr. Opin. Chem. Biol. 4, 609-618. [DOI] [PubMed] [Google Scholar]
- 4.Padilla J. E., Colovos, C. & Yeates, T. O. (2001) Proc. Natl. Acad. Sci. USA 98, 2217-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sleytr U. B., Sára, M. & Pum, D. (2000) in Supramolecular Polymers, ed. Ciferri, A. (Dekker, New York), pp. 177–213.
- 6.Sleytr U. B., Sára, M., Pum, D. & Schuster, B. (2001) Prog. Surf. Sci. 68, 231-278. [Google Scholar]
- 7.Sára M. & Sleytr, U. B. (2000) J. Bacteriol. 182, 859-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pum D. & Sleytr, U. (1999) Trends Biotechnol. 17, 8-12. [Google Scholar]
- 9.Sleytr U. B., Messner, P., Pum, D. & Sára, M. (1999) Angew. Chem. Int. Ed. Engl. 38, 1034-1054. [DOI] [PubMed] [Google Scholar]
- 10.Kuen B., Koch, A., Asenbauer, E., Sára, M. & Lubitz, W. (1997) J. Bacteriol. 179, 1664-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sára M., Kuen, B., Mayer, H. F., Mandl, F., Schuster, K. C. & Sleytr, U. B. (1996) J. Bacteriol. 178, 2108-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelhardt H. & Peters, J. (1998) J. Struct. Biol. 124, 276-302. [DOI] [PubMed] [Google Scholar]
- 13.Ries W., Hotzy, C., Schocher, I., Sleytr, U. B. & Sára, M. (1997) J. Bacteriol. 179, 3892-3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sambrook J. & Russell, D. W., (2001) Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 15.Messner P., Hollaus, F. & Sleytr, U. B. (1984) Int. J. Syst. Bacteriol. 34, 202-210. [Google Scholar]
- 16.Jarosch M., Egelseer, E. M., Huber, C., Moll, D., Mattanovich, D., Sleytr, U. B. & Sára, M. (2001) Microbiology 147, 1353-1363. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt T. G. & Skerra, A. (1994) J. Chromatogr. A 676, 337-345. [DOI] [PubMed] [Google Scholar]
- 18.Mader C., Küpcü, S., Sára, M. & Sleytr, U. B. (1999) Biochim. Biophys. Acta 1418, 106-116. [DOI] [PubMed] [Google Scholar]
- 19.Gill S. C. & von Hippel, P. H. (1989) Anal. Biochem. 182, 319-326. [DOI] [PubMed] [Google Scholar]
- 20.Lin H. J. & Kirsch, J. F. (1979) Methods Enzymol. 62, 287-289. [DOI] [PubMed] [Google Scholar]
- 21.Argaraña C. E., Kuntz, I. D., Birken, S., Axel, R. & Cantor, C. R. (1986) Nucleic Acids Res. 14, 1871-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pähler A., Hendrickson, W. A., Kolks, M. A., Argaraña, C. E. & Cantor, C. R. (1987) J. Biol. Chem. 262, 13933-13937. [PubMed] [Google Scholar]
- 23.Bayer E. A., Ben-Hur, H. & Wilchek, M. (1990) Methods Enzymol. 184, 80-89. [DOI] [PubMed] [Google Scholar]
- 24.Chilkoti A., Schwartz, B. L., Smith, R. D., Long, C. J. & Stayton, P. S. (1995) Bio/Technology 13, 1198-1204. [DOI] [PubMed] [Google Scholar]
- 25.Reznik G. O., Vajda, S., Smith, C. L., Cantor, C. R. & Sano, T. (1996) Nat. Biotechnol. 14, 1007-1011. [DOI] [PubMed] [Google Scholar]
- 26.Darst S. A., Ahlers, M., Meller, P. H., Kubalek, E. W., Blankenburg, R., Ribi, H. O., Ringsdorf, H. & Kornberg, R. D. (1991) Biophys. J. 59, 387-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pum D., Neubauer, A., Györvary, E., Sára, M. & Sleytr, U. B. (2000) Nanotechnology 11, 100-107. [Google Scholar]
- 28.Sleytr U. B., Sára, M., Pum, D. & Schuster, B. (2002) in Nano-Surface Chemistry, ed. Rosoff, M. (Dekker, New York), pp. 333–389.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.