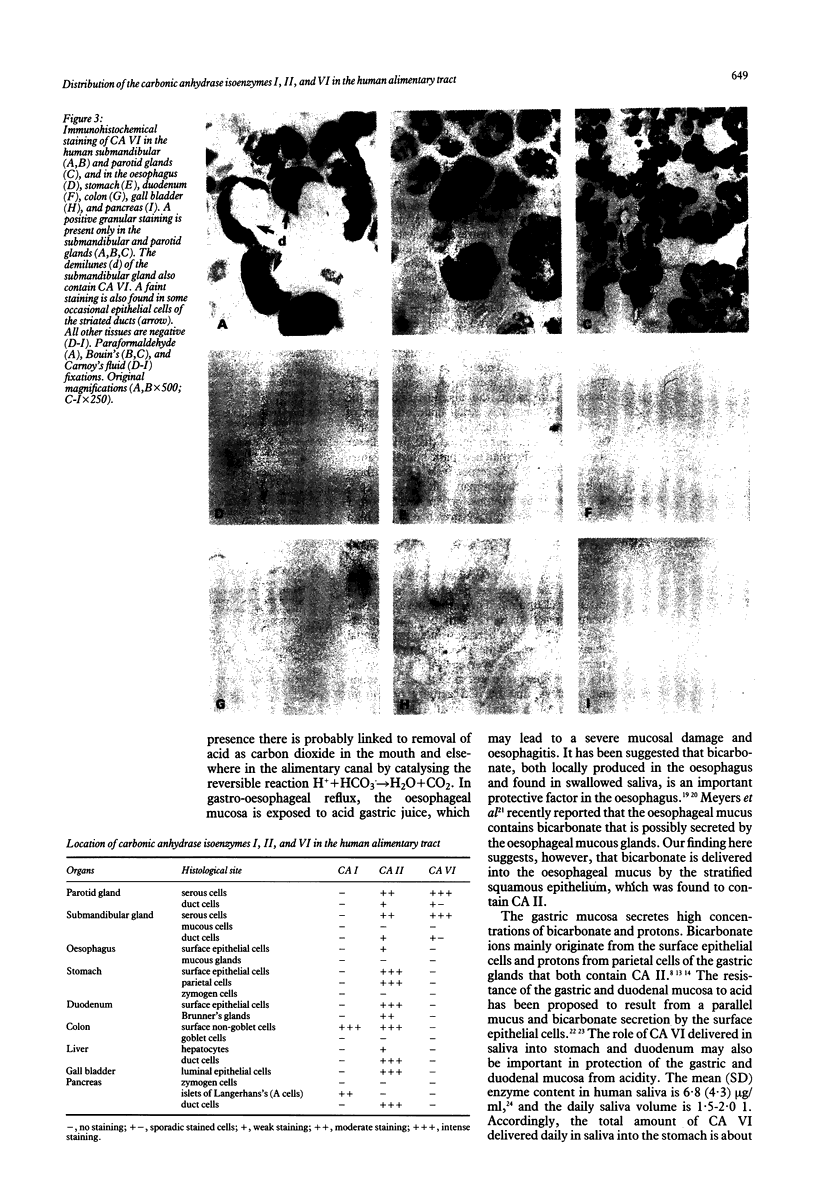
Abstract
The distribution of carbonic anhydrase isoenzymes I, II, and VI was studied in the human alimentary tract using specific antibodies to human isoenzymes in conjunction with the immunoperoxidase technique to elucidate the physiological role and possible functional interplay of carbonic anhydrases (CAs) in alimentary canal functions. From the isoenzymes studied, CA II was found to be the most widely distributed in the various epithelia throughout the alimentary canal. In addition to the acinar cells of the parotid and submandibular glands and the duodenal Brunner's glands, it was present in the mucosal epithelium of the oesophagus, stomach, duodenum, and colon. The epithelial cells of the hepatic bile ducts, gall bladder, and pancreatic ducts also contained CA II in abundance. In contrast, CA VI was present only in the serous acinar and ductal cells of the parotid and submandibular glands, and CA I in the mucosal epithelium of the colon and the A cells of the pancreatic Langerhans's islets. These results suggest that CA II as a widely distributed isoenzyme in the epithelia of the alimentary canal and CA VI as secreted into saliva, may form a mutually complementary system protecting oesophageal, gastric, and intestinal mucosa from acidity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLEYL U., MASCH B. HISTOCHEMISCHER NACHWEIS DER CARBOANHYDRATASE IN PANKREAS UND PLACENTA. (METHODE AM FLOTTIERENDEN SCHNITT) Klin Wochenschr. 1964 Apr 15;42:402–405. doi: 10.1007/BF01488639. [DOI] [PubMed] [Google Scholar]
- Bleyl U. Zur Spezifität des histochemischen Carboanhydratase-nachweises im Inselorgan der Bauchspeicheldrüse. Histochemie. 1964 Nov 12;4(4):286–311. doi: 10.1007/BF00282499. [DOI] [PubMed] [Google Scholar]
- Boquist L., Hagström S. Carbonic anhydrase activity in mouse endocrine pancreas. Acta Pathol Microbiol Scand A. 1979 May;87A(3):157–164. doi: 10.1111/j.1699-0463.1979.tb00037.x. [DOI] [PubMed] [Google Scholar]
- Carter M. J. Carbonic anhydrase: isoenzymes, properties, distribution, and functional significance. Biol Rev Camb Philos Soc. 1972 Nov;47(4):465–513. doi: 10.1111/j.1469-185x.1972.tb01079.x. [DOI] [PubMed] [Google Scholar]
- Carter M. J., Parsons D. S. The isoenzymes of carbonic anhydrase: tissue, subcellular distribution and functional significance, with particular reference to the intestinal tract. J Physiol. 1971 May;215(1):71–94. doi: 10.1113/jphysiol.1971.sp009458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter N., Wistrand P. J., Lönnerholm G. Carbonic anhydrase localization to perivenous hepatocytes. Acta Physiol Scand. 1989 Feb;135(2):163–167. doi: 10.1111/j.1748-1716.1989.tb08563.x. [DOI] [PubMed] [Google Scholar]
- Dodgson S. J., Forster R. E., 2nd, Storey B. T., Mela L. Mitochondrial carbonic anhydrase. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5562–5566. doi: 10.1073/pnas.77.9.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernley R. T., Wright R. D., Coghlan J. P. A novel carbonic anhydrase from the ovine parotid gland. FEBS Lett. 1979 Sep 15;105(2):299–302. doi: 10.1016/0014-5793(79)80634-1. [DOI] [PubMed] [Google Scholar]
- Flemström G. Gastroduodenal mucosal secretion of bicarbonate and mucus. Physiologic control and stimulation by prostaglandins. Am J Med. 1986 Aug 18;81(2A):18–22. doi: 10.1016/s0002-9343(86)80005-5. [DOI] [PubMed] [Google Scholar]
- Gleeson D., Hood K. A., Murphy G. M., Dowling R. H. Calcium and carbonate ion concentrations in gallbladder and hepatic bile. Gastroenterology. 1992 May;102(5):1707–1716. doi: 10.1016/0016-5085(92)91734-l. [DOI] [PubMed] [Google Scholar]
- Helm J. F., Dodds W. J., Hogan W. J., Soergel K. H., Egide M. S., Wood C. M. Acid neutralizing capacity of human saliva. Gastroenterology. 1982 Jul;83(1 Pt 1):69–74. [PubMed] [Google Scholar]
- Helm J. F., Dodds W. J., Pelc L. R., Palmer D. W., Hogan W. J., Teeter B. C. Effect of esophageal emptying and saliva on clearance of acid from the esophagus. N Engl J Med. 1984 Feb 2;310(5):284–288. doi: 10.1056/NEJM198402023100503. [DOI] [PubMed] [Google Scholar]
- KURATA Y. Histochemical demonstration of carbonic anhydrase activity. Stain Technol. 1953 Sep;28(5):231–233. doi: 10.3109/10520295309105238. [DOI] [PubMed] [Google Scholar]
- Korhonen L. K., Korhonen E., Hyyppä M. Histochemical demonstration of carbonic anhydrase activity in the alimentary canal. Histochemie. 1966;6(2):168–172. doi: 10.1007/BF00308189. [DOI] [PubMed] [Google Scholar]
- Kumpulainen T. Immunohistochemical localization of human carbonic anhydrase isozymes. Ann N Y Acad Sci. 1984;429:359–368. doi: 10.1111/j.1749-6632.1984.tb12360.x. [DOI] [PubMed] [Google Scholar]
- Kumpulainen T., Jalovaara P. Immunohistochemical localization of carbonic anhydrase isoenzymes in the human pancreas. Gastroenterology. 1981 Apr;80(4):796–799. [PubMed] [Google Scholar]
- Lönnerholm G., Selking O., Wistrand P. J. Amount and distribution of carbonic anhydrases CA I and CA II in the gastrointestinal tract. Gastroenterology. 1985 May;88(5 Pt 1):1151–1161. doi: 10.1016/s0016-5085(85)80074-3. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967 Oct;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Meyers R. L., Orlando R. C. In vivo bicarbonate secretion by human esophagus. Gastroenterology. 1992 Oct;103(4):1174–1178. doi: 10.1016/0016-5085(92)91501-t. [DOI] [PubMed] [Google Scholar]
- Parkkila A. K., Parkkila S., Juvonen T., Rajaniemi H. Carbonic anhydrase isoenzymes II and I are present in the zona glomerulosa cells of the human adrenal gland. Histochemistry. 1993 Jan;99(1):37–41. doi: 10.1007/BF00268018. [DOI] [PubMed] [Google Scholar]
- Parkkila S., Kaunisto K., Rajaniemi L., Kumpulainen T., Jokinen K., Rajaniemi H. Immunohistochemical localization of carbonic anhydrase isoenzymes VI, II, and I in human parotid and submandibular glands. J Histochem Cytochem. 1990 Jul;38(7):941–947. doi: 10.1177/38.7.2113069. [DOI] [PubMed] [Google Scholar]
- Parkkila S., Parkkila A. K., Vierjoki T., Ståhlberg T., Rajaniemi H. Competitive time-resolved immunofluorometric assay for quantifying carbonic anhydrase VI in saliva. Clin Chem. 1993 Oct;39(10):2154–2157. [PubMed] [Google Scholar]
- Rege R. V., Moore E. W. Evidence for H+ secretion by the in vivo canine gallbladder. Gastroenterology. 1987 Feb;92(2):281–289. doi: 10.1016/0016-5085(87)90118-1. [DOI] [PubMed] [Google Scholar]
- Sato A., Spicer S. S., Tashian R. E. Ultrastructural localization of carbonic anhydrase in gastric parietal cells with the immunoglobulin-enzyme bridge method. Histochem J. 1980 Nov;12(6):651–659. doi: 10.1007/BF01012020. [DOI] [PubMed] [Google Scholar]
- Spicer S. S., Sens M. A., Tashian R. E. Immunocytochemical demonstration of carbonic anhydrase in human epithelial cells. J Histochem Cytochem. 1982 Sep;30(9):864–873. doi: 10.1177/30.9.6813372. [DOI] [PubMed] [Google Scholar]
- Sullivan B., Berndt W. O. Transport by isolated rabbit gallbladders in bicarbonate-buffered solutions. Am J Physiol. 1973 Oct;225(4):845–848. doi: 10.1152/ajplegacy.1973.225.4.845. [DOI] [PubMed] [Google Scholar]
- Weinman S. A., Reuss L. Na+-H+ exchange at the apical membrane of Necturus gallbladder. Extracellular and intracellular pH studies. J Gen Physiol. 1982 Aug;80(2):299–321. doi: 10.1085/jgp.80.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock R. T., Wheeler H. O. Hydrogen ion transport by isolated rabbit gallbladder. Am J Physiol. 1969 Jul;217(1):310–316. doi: 10.1152/ajplegacy.1969.217.1.310. [DOI] [PubMed] [Google Scholar]
- Zhu X. L., Sly W. S. Carbonic anhydrase IV from human lung. Purification, characterization, and comparison with membrane carbonic anhydrase from human kidney. J Biol Chem. 1990 May 25;265(15):8795–8801. [PubMed] [Google Scholar]





