Abstract
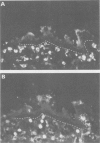
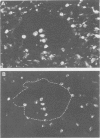
Mucosal specimens from active Crohn's disease (ileum, n = 6; colon, n = 6), active ulcerative colitis (n = 9), normal ileum (n = 6), and normal colon (n = 6) were subjected to paired immunofluorescence staining for characterisation of macrophage subsets in situ. In the normal state, only few CD68+ macrophages (< 10%) expressing the myelomonocytic L1 antigen (calprotectin) were seen. In inflamed mucosa, especially near small vessels, the CD68+L1+ fraction increased with the degree of inflammation, near ulcers to median 65% (range 35-91%). Cells reactive with the monoclonal antibody RFD7 were also increased in inflammation but less than 5% of them costained for L1 antigen. It is concluded that L1 producing macrophages are distinct from the RFD7+ subset and probably recently recruited from peripheral blood monocytes. Like granulocytes, L1+ macrophages may be important in non-specific defence, providing calprotectin with putative anti-microbial and anti-proliferative properties.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison M. C., Cornwall S., Poulter L. W., Dhillon A. P., Pounder R. E. Macrophage heterogeneity in normal colonic mucosa and in inflammatory bowel disease. Gut. 1988 Nov;29(11):1531–1538. doi: 10.1136/gut.29.11.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison M. C., Poulter L. W. Changes in phenotypically distinct mucosal macrophage populations may be a prerequisite for the development of inflammatory bowel disease. Clin Exp Immunol. 1991 Sep;85(3):504–509. doi: 10.1111/j.1365-2249.1991.tb05757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerke K., Halstensen T. S., Jahnsen F., Pulford K., Brandtzaeg P. Distribution of macrophages and granulocytes expressing L1 protein (calprotectin) in human Peyer's patches compared with normal ileal lamina propria and mesenteric lymph nodes. Gut. 1993 Oct;34(10):1357–1363. doi: 10.1136/gut.34.10.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P., Baklien K., Fausa O., Hoel P. S. Immunohistochemical characterization of local immunoglobulin formation in ulcerative colitis. Gastroenterology. 1974 Jun;66(6):1123–1136. [PubMed] [Google Scholar]
- Brandtzaeg P., Dale I., Fagerhol M. K. Distribution of a formalin-resistant myelomonocytic antigen (L1) in human tissues. I. Comparison with other leukocyte markers by paired immunofluorescence and immunoenzyme staining. Am J Clin Pathol. 1987 Jun;87(6):681–699. doi: 10.1093/ajcp/87.6.681. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Dale I., Gabrielsen T. O. The leucocyte protein L1 (calprotectin): usefulness as an immunohistochemical marker antigen and putative biological function. Histopathology. 1992 Aug;21(2):191–196. doi: 10.1111/j.1365-2559.1992.tb00374.x. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Mucosal and glandular distribution of immunoglobulin components. Immunohistochemistry with a cold ethanol-fixation technique. Immunology. 1974 Jun;26(6):1101–1114. [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P. Prolonged incubation time in immunohistochemistry: effects on fluorescence staining of immunoglobulins and epithelial components in ethanol- and formaldehyde-fixed paraffin-embedded tissues. J Histochem Cytochem. 1981 Nov;29(11):1302–1315. doi: 10.1177/29.11.7033362. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Rognum T. O. Evaluation of tissue preparation methods and paired immunofluorescence staining for immunocytochemistry of lymphomas. Histochem J. 1983 Jul;15(7):655–689. doi: 10.1007/BF01002987. [DOI] [PubMed] [Google Scholar]
- Dale I., Brandtzaeg P., Fagerhol M. K., Scott H. Distribution of a new myelomonocytic antigen (L1) in human peripheral blood leukocytes. Immunofluorescence and immunoperoxidase staining features in comparison with lysozyme and lactoferrin. Am J Clin Pathol. 1985 Jul;84(1):24–34. doi: 10.1093/ajcp/84.1.24. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Allan W., Hogan P. G., Doe W. F. Immunohistochemical characterisation of macrophages in human liver and gastrointestinal tract: expression of CD4, HLA-DR, OKM1, and the mature macrophage marker 25F9 in normal and diseased tissue. J Leukoc Biol. 1987 Nov;42(5):474–484. doi: 10.1002/jlb.42.5.474. [DOI] [PubMed] [Google Scholar]
- LEDER L. D. UBER DIE SELEKTIVE FERMENTCYTOCHEMISCHE DARSTELLUNG VON NEUTROPHILEN MYELOISCHEN ZELLEN UND GEWEBSMASTZELLEN IM PARAFFINSCHNITT. Klin Wochenschr. 1964 Jun 1;42:553–553. doi: 10.1007/BF01486688. [DOI] [PubMed] [Google Scholar]
- Mahida Y. R., Jewell D. P. Macrophage activity in inflammatory bowel disease. Gut. 1990 Sep;31(9):1086–1087. doi: 10.1136/gut.31.9.1086-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Patel S., Gionchetti P., Vaux D., Jewell D. P. Macrophage subpopulations in lamina propria of normal and inflamed colon and terminal ileum. Gut. 1989 Jun;30(6):826–834. doi: 10.1136/gut.30.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Wu K. C., Jewell D. P. Respiratory burst activity of intestinal macrophages in normal and inflammatory bowel disease. Gut. 1989 Oct;30(10):1362–1370. doi: 10.1136/gut.30.10.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Wu K., Jewell D. P. Enhanced production of interleukin 1-beta by mononuclear cells isolated from mucosa with active ulcerative colitis of Crohn's disease. Gut. 1989 Jun;30(6):835–838. doi: 10.1136/gut.30.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murao S., Collart F. R., Huberman E. A protein containing the cystic fibrosis antigen is an inhibitor of protein kinases. J Biol Chem. 1989 May 15;264(14):8356–8360. [PubMed] [Google Scholar]
- Poulter L. W., Campbell D. A., Munro C., Janossy G. Discrimination of human macrophages and dendritic cells by means of monoclonal antibodies. Scand J Immunol. 1986 Sep;24(3):351–357. doi: 10.1111/j.1365-3083.1986.tb02104.x. [DOI] [PubMed] [Google Scholar]
- Pryzwansky K. B., Martin L. E., Spitznagel J. K. Immunocytochemical localization of myeloperoxidase, lactoferrin, lysozyme and neutral proteases in human monocytes and neutrophilic granulocytes. J Reticuloendothel Soc. 1978 Sep;24(3):295–310. [PubMed] [Google Scholar]
- Pulford K. A., Sipos A., Cordell J. L., Stross W. P., Mason D. Y. Distribution of the CD68 macrophage/myeloid associated antigen. Int Immunol. 1990;2(10):973–980. doi: 10.1093/intimm/2.10.973. [DOI] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Jewell D. P. Immunohistological characterisation of intraepithelial lymphocytes of the human gastrointestinal tract. Gut. 1981 Mar;22(3):169–176. doi: 10.1136/gut.22.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Mason D. Y., Jewell D. P. Expression of HLA-DR antigens by colonic epithelium in inflammatory bowel disease. Clin Exp Immunol. 1983 Sep;53(3):614–618. [PMC free article] [PubMed] [Google Scholar]
- Selby W. S., Poulter L. W., Hobbs S., Jewell D. P., Janossy G. Heterogeneity of HLA-DR-positive histiocytes in human intestinal lamina propria: a combined histochemical and immunohistological analysis. J Clin Pathol. 1983 Apr;36(4):379–384. doi: 10.1136/jcp.36.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbakk M., Naess-Andresen C. F., Lingaas E., Dale I., Brandtzaeg P., Fagerhol M. K. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet. 1990 Sep 29;336(8718):763–765. doi: 10.1016/0140-6736(90)93237-j. [DOI] [PubMed] [Google Scholar]
- van Furth R., Raeburn J. A., van Zwet T. L. Characteristics of human mononuclear phagocytes. Blood. 1979 Aug;54(2):485–500. [PubMed] [Google Scholar]