Abstract
Photosystem II (PSII) catalyzes the light-driven oxidation of water and the reduction of plastoquinone; the oxidation of water occurs at a cluster of four manganese. The PSII CP43 subunit functions in light harvesting, and mutations in the fifth luminal loop (E) of CP43 have established its importance in PSII structure and/or assembly [Kuhn, M. G. & Vermaas, V. F. J. (1993) Plant Mol. Biol. 23, 123–133]. The sequence A350PWLEPLR357 in luminal loop E is conserved in CP43 genes from 50 organisms. To map important posttranslational modifications in this sequence, tandem mass spectrometry (MS/MS) was used. These data show that the indole side chain of Trp-352 is posttranslationally modified to give mass shifts of +4, +16, and +18 daltons. The masses of the modifications suggest that the tryptophan is modified to kynurenine (+4), a keto-/amino-/hydroxy- (+16) derivative, and a dihydro-hydroxy- (+18) derivative of the indole side chain. Peptide synthesis and MS/MS confirmed the kynurenine assignment. The +16 and +18 tryptophan modifications may be intermediates formed during the oxidative cleavage of the indole ring to give kynurenine. The site-directed mutations, W352C, W352L, and W352A, exhibit an increased rate of photoinhibition relative to wild type. We hypothesize that Trp-352 oxidative modifications are a byproduct of PSII water-splitting or electron transfer reactions and that these modifications target PSII for turnover. As a step toward understanding the tertiary structure of this CP43 peptide, structural modeling was performed by using molecular dynamics.
Keywords: mass spectrometry, collision-induced dissociation, tryptophan, kynurenine, photoinhibition
Photosystem II (PSII) is a protein–pigment complex located in thylakoid membranes of plants, eukaryotic algae, and cyanobacteria. PSII catalyzes the light-driven oxidation of water to O2, and the reduction of plastoquinone. PSII contains both intrinsic and extrinsic polypeptides. The intrinsic polypeptides include chlorophyll-binding proteins, CP47, CP43, and the D1 and D2 polypeptides (reviewed in ref. 1). The D2/D1 heterodimer binds P680, pheophytin, and the quinone receptors, QA and QB (2). Three extrinsic subunits, the manganese stabilizing, 24-kDa, and 18-kDa proteins, are required for maximum oxygen evolution in plants (3, 4). Recently, a 3.8-Å structure of the cyanobacterial PSII reaction center has been reported (5, 6).
The intrinsic PSII subunits, CP43 and CP47, function as light-harvesting proteins and play a role in PSII assembly and activity (7–11). CP47 and CP43 have similar tertiary and secondary structures (5). Each polypeptide has six membrane-spanning regions and a large luminal, hydrophilic loop (E) between helix V and VI (5, 7). In Synechocystis sp. PCC 6803, loop E of the CP43 subunit extends from residue Asn-280 to Trp-412 (9). Mutations or deletions in this loop inactivate or impair PSII activity in Synechocystis (9, 11–13).
Posttranslational modifications can play important roles in the assembly, degradation, structure, and function of proteins. However, little is known about the roles of such modifications in membrane proteins. For example, in cytochrome c oxidase, a crosslinked tyrosine–histidine cofactor has been identified at the binuclear metal site (14); the function of this cofactor has not yet been definitively established. Recently, it has been suggested that posttranslationally modified amino acids, containing carbonyl groups, covalently bind hydrazines and amines at the catalytic site of PSII (10, 15). Because amines and hydrazines are inhibitors of photosynthetic water oxidation, it was suggested that these carbonyl-containing amino acids play roles in the structure, function, or assembly of PSII.
To obtain more information about posttranslational modifications in PSII, we have used tandem mass spectrometry (MS/MS; ref. 16). MS/MS has been used previously to obtain structural information about intrinsic membrane proteins (for examples, see refs. 17–20). MS is one of the few techniques that can detect altered amino acid side chains in membrane proteins, which are difficult to study by other high resolution structural techniques. Previously, MS has detected stable oxidation products of D1/D2 in a PSII reaction center preparation (21). Electrospray ionization MS data have also suggested posttranslational modifications in CP47 and CP43 (18).
In this study, we report that a tryptophan in a highly conserved region of CP43 is posttranslationally modified. As assessed by the observed mass-to-charge ratios, multiple types of tryptophan modifications are present. We hypothesize that these modifications play a role in signaling the turnover of the PSII reaction center.
Materials and Methods
Reagents.
14C-phenylhydrazine (specific activity, 2.65 mCi/mmol; 1 Ci = 37 GBq) was obtained from California Bionuclear Corporation (Los Angeles). 2-Br-phenylhydrazine was purchased from Aldrich, and sequencing-grade trypsin was purchased from Promega.
Sample Preparation.
PSII was isolated from market spinach (22) with the modifications described (15). PSII samples were maintained under dim light (0.6–16 μE m−2·s−1). Chlorophyll and oxygen assays were performed (23, 24). Steady-state rates of oxygen evolution were ≥700 μmol O2 (mg of chl)−1·h−1. PSII membranes were Tris-washed, generating PSII-3 and removing the manganese cluster and extrinsic subunits (15).
In Situ Trypsin Digests.
In situ trypsin digestion of spinach PSII was performed by a previously described procedure (20). Briefly, 1.1 nmol of oxygen evolving PSII or PSII-3 was illuminated (3620 μE s−1·m−2) or incubated in the dark for 30 min. The buffer was 50 mM Hepes–NaOH, pH 7.5. Illumination was from a Dolan–Jenner annular illuminator equipped with a red filter and a heat filter. The membranes were then precipitated with 95% ethanol. After resuspending pellets in 4 M urea, trypsin digestion (12.5 ng/μl) was performed for 17–25 h at 37°C in a buffer containing 50 mM NH4HCO3 and 5 mM CaCl2. The dried samples were resuspended in 0.5% formic acid or 0.2% acetic acid before MS/MS analysis.
In Gel Trypsin Digests.
PSII-3 samples (35 nmol) were incubated with 56 μM 14C- or 12C-phenylhydrazine, 56 μM 2-Br-phenylhydrazine, or no phenylhydrazine under room light for 30 min in buffer containing 50 mM Hepes–NaOH, pH 7.5/52 mM Na2CO3/2.6 M urea/2% SDS (15). Samples were subjected to SDS/PAGE, and the portion of the gel, containing CP43 and CP47, was excised (20).
For in gel digestion, the gel blocks were rehydrated at room temperature with a solution containing 50 mM NH4HCO3, 5 mM CaCl2, 12.5 ng/μl trypsin and incubated at 37°C. The peptides were extracted from the gel pieces, as described (20), except that 0.3% TEAA (triethylammonium acetate), pH 7.0, and acetonitrile were used in a 1:1 ratio. The peptide solution was concentrated with argon or dried in vacuo.
In gel digested samples were filtered and injected onto a phenyl S 5-μm, 300-Å, 3.0 × 250-mm column (Waters), which had been equilibrated with 0.3% TEAA, pH.7.0 (solvent A), on an HPLC system (Beckman Coulter). Peptides were eluted with buffer B, containing 0.3% TEAA, pH 7.0, and 90% acetonitrile; elution of peptides was monitored at 214 nm. The fraction used for these experiments eluted between 21% and 23% buffer B.
Tandem Mass Spectrometric Measurements.
Microcapillary columns containing reverse-phase packing material were constructed as described (20). The column was equilibrated with aqueous 0.2% acetic acid and 2% acetonitrile (solvent A) before injecting the sample. Liquid chromatography was carried out at 400–600 nl/min by using a Magic 2002 capillary HPLC system plumbed to a Magic Variable Splitter (Michrom BioResources, Auburn, CA) or by using a Hewlett–Packard 1100 series HPLC system. The column was coupled online with the mass spectrometer using a PicoView nanospray ionization source (New Objective, Cambridge, MA) or as described (25). The peptides were eluted with a gradient program of 0–80% solvent B (0.2% aqueous acetic acid and 90% acetonitrile) in 90 min. In some experiments, multidimensional protein identification technology, in which the peptide mixture is separated by two liquid chromatography steps, was used (25).
The peptides were eluted directly into the ThermoFinnigan (San Jose, CA) LCQ ion trap mass spectrometer. The LCQ heated capillary temperature was 190°C, and the spray voltage was 1.2–2.4 kV. MS/MS data were collected in an automatic data-dependent scanning mode (isolation width, 3 mass to charge ratio (m/z); normalized collision energy, 35). The spectrometer collected cycles of four scans: one full scan (200–2,000 m/z) and three subsequent MS/MS scans. Collision-induced dissociation (CID) was performed on the three most abundant ions detected in the full scan. In some experiments, specific m/z values were selected, the ZoomScan mode was used, direct sample infusion was used, or an Applied Biosystems QSTAR Pulsar (quadrupole-time of flight) mass spectrometer was used.
Database Searches.
The SEQUEST algorithm (distributed by ThermoFinnigan) correlates experimentally obtained CID spectra to the theoretical CID spectra of peptide sequences from a specified database and assigns a cross-correlation (Xcorr) score (26). A subset database, containing Spinacia oleracea entries, was constructed by using the Database Manager Program in ThermoFinnigan's BioTools software package for initial searches. This subset database was extracted from the National Center for Biotechnology Information (NCBI) nonredundant database (www.ncbi.nlm.nih.gov). After initial identification using this database, searches were then conducted against the complete NCBI database.
The protein basic local alignment search tool (BLASTP) was used to search the complete, nonredundant NCBI database for CP43 sequences that contain APWLEPLR (27). The search gave 51 complete sequences for CP43. Alignment of the CP43 sequences was carried out by using Genetics Computer Group software (WISCONSIN PACKAGE, Accelrys, San Diego; refs. 28 and 29).
Site-Directed Mutagenesis and Photoinhibition Studies.
Site-directed mutants were constructed and confirmed in the cyanobacterium, Synechocystis sp. PCC 6803, and photoinhibition studies were performed by methods previously described (11, 13).
Peptide Synthesis.
Two peptides, APWLEPLR and APXLEPLR, where X refers to kynurenine (Advanced ChemTech, Louisville, KY), were chemically synthesized at the University of Minnesota Microchemical Facility (Minneapolis). The general stepwise Merrifield's solid-phase method, adapted by Perkin–Elmer, was used for synthesis on a Perkin–Elmer/Applied Biosystems peptide synthesizer (30).
Peptide Modeling.
Molecular minimization and dynamics studies were performed on the sequence “TMRFWDLRAPWLEPLRGPNGLDLS” by using the Discover and Charmm packages of INSIGHT II (Version 98.0). The biopolymer module in INSIGHT II was used to build the peptides. The N terminus was an uncharged amino group, and the C terminus was modeled as NH(CH3). From an extended structure, initial minimizations were performed for the sequence by using the Amber force field in the Discover package. Dynamics were then performed by using the Charmm or the Amber force field to generate the random structures used for the final energy minimization.
Results
MS/MS.
In the experiments presented here, a CP43 tryptic peptide, APWLEPLR, was found to be posttranslationally modified. In Fig. 1A, a diagram of the predicted CID cleavage pattern of the unmodified parent ion, APWLEPLR, is shown. The subscripts on the b and y ions (31) indicate the number of amino acid residues in that peptide fragment. For ions of the same charge, the sequence of amino acids can be assigned from the y ion series, and the b series provides complementary sequence information. Posttranslational modification of a peptide leads to changes in m/z for the peptide fragments carrying the modification. Low energy CID can also cause loss of carbon monoxide, water, and ammonia from certain amino acid side chains (for review, see ref. 32). In this paper, assignments of the PSII-derived CID spectra are based on comparison to synthesized peptides.
Fig 1.
Diagram showing the predicted, monoisotopic m/z values for singly charged b- and y-ions derived from the CP43 tryptic peptide, APWLEPLR (A), the structure of kynurenine (W+4) (B), and structures of the +16 and +18 tryptophan modifications (C).
Synthesized Peptides.
The CID spectrum in Fig. 2A
was acquired from the synthesized peptide, APWLEPLR. The +2 parent ion
had a mass to charge ratio of 491.5. In the tandem mass spectrum, ions
were observed at m/z 175, 385, 514, 627, 813,
and 910, which were assigned to the predicted y ions,
y1, y3,
y4, y5,
y6, and y7 (Fig.
1A). Confirmatory b ions were observed at
m/z 169 (b2), 355
(b3), 468 (b4), 597
(b5), and 807 (b7). In
addition, +2 product ions, y and
y
and
y (Fig. 2A) and other ions
arising from the loss of NH3 (not labeled) were
assigned. Overall, the majority of the expected b and y ion series
(Fig. 1A) was observed, except that the
y2 ion predicted at m/z
288 and the b6 ion predicted at
m/z 694 (Fig. 1A) did not
have significant intensity. The predicted b1 ion
at m/z 72 was below the detection range.
(Fig. 2A) and other ions
arising from the loss of NH3 (not labeled) were
assigned. Overall, the majority of the expected b and y ion series
(Fig. 1A) was observed, except that the
y2 ion predicted at m/z
288 and the b6 ion predicted at
m/z 694 (Fig. 1A) did not
have significant intensity. The predicted b1 ion
at m/z 72 was below the detection range.
Fig 2.
CID mass spectra derived from the synthesized peptide, APWLEPLR (A), from the synthesized peptide, APXLEPLR, where X is kynurenine (B), and from PSII tryptic peptides (C–F). (C) The PSII spectrum is assigned to the tryptic CP43 peptide, APWLEPLR. (D–F) The PSII spectra are assigned to tryptic CP43 peptides derived by posttranslational modification, APW*LEPLR, where the tryptophan mass shift is +4 (D), +16 (E), and +18 (F). (A–F) The (M+2H)+2 parent ions had m/z values of 491.5, 493.3, 491.7, 493.5, 499.2, and 500.9, respectively. Each PSII assignment (C–F) was the top choice when SEQUEST searches were performed against the nonredundant database. The Xcorr scores were 2.5 (C), 2.4 (D), 2.4 (E), and 2.1 (F). For comparison, the CID spectrum in A, which was derived from the synthesized peptide, had an Xcorr score of 2.2. For peaks with good ion statistics, the mass accuracy in these <2,000 m/z ion-trap experiments is 250–500 ppm.
The CID spectrum in Fig. 2B was obtained from the synthesized peptide, APXLEPLR (Fig. 2B), where X indicates a kynurenine at position 3. Kynurenine is +4 daltons, compared with tryptophan (Fig. 1B). As expected, the +2 parent ion, (M+2H)+2, had a mass to charge ratio of 493.3. Comparison of the ions in Fig. 2 (A and B) shows that y1, y3, y4, and y5 have the same m/z values in each spectrum. However, the y6 and y7 ions, which contain X, were +4 m/z compared with the y6 and y7 ions in Fig. 2A. In addition, the b ions at m/z 359 (b3), 472 (b4), 601 (b5), and 811 (b7) were +4 m/z compared with the b3, b4, b5, and b7 ions in Fig. 2A. The observed m/z increments are consistent with the replacement of tryptophan with kynurenine in the synthesized peptide.
CP43-Derived Peptides.
Fig. 2
C–F shows representative CID spectra acquired from
PSII peptides. These peptides were generated by an in gel tryptic
digest of PSII-3. The CID spectrum presented in Fig. 2C is
similar to the CID spectrum obtained for the synthesized peptide,
APWLEPLR (Fig. 2A). The +2 parent ion (Fig.
2C) had a similar mass to charge ratio, 491.7
m/z. Ions, assignable to
y1,
y3–y6,
b2–b5, and
b7, had identical m/z
values in the PSII peptide (Fig. 2C) and in the chemically
synthesized peptide (Fig. 2A). Additional ions with
the same m/z values include the +2 product
ions, y and y
and y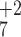 (Fig.
2C), and ions arising from the loss of
NH3 (not labeled). Because of the similarity of
the spectra, we conclude that the PSII peptide, giving rise to Fig.
2C, and the synthesized peptide, APWLEPLR (Fig.
2A), have the same sequence.
(Fig.
2C), and ions arising from the loss of
NH3 (not labeled). Because of the similarity of
the spectra, we conclude that the PSII peptide, giving rise to Fig.
2C, and the synthesized peptide, APWLEPLR (Fig.
2A), have the same sequence.
The CID spectrum presented in Fig. 2D was also acquired from
a PSII peptide. The spectrum is similar to the CID spectrum obtained
from the synthesized peptide, APXLEPLR (Fig. 2B),
where X is kynurenine. The +2 parent ion had a similar mass to charge
ratio, 493.5 m/z (Fig. 2D).
Comparison of Fig. 2
B and D shows that the
y1–y7,
b2–b5, and
b7 ions have the same m/z
values in both spectra. An intense y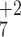 ion was also
observed in both CID data sets (Fig. 2
B and D).
These CID data are consistent with a +4 posttranslational modification
on Trp-352 in CP43. We conclude that the PSII peptide giving rise to
Fig. 2D has the sequence, APW*LEPLR, where W* corresponds to
kynurenine (Fig. 1B). Kynurenine can be generated
posttranslationally from tryptophan through oxidative cleavage of the
indole ring (33).
ion was also
observed in both CID data sets (Fig. 2
B and D).
These CID data are consistent with a +4 posttranslational modification
on Trp-352 in CP43. We conclude that the PSII peptide giving rise to
Fig. 2D has the sequence, APW*LEPLR, where W* corresponds to
kynurenine (Fig. 1B). Kynurenine can be generated
posttranslationally from tryptophan through oxidative cleavage of the
indole ring (33).
The CID spectrum presented in Fig. 2E was derived
from a +2 parent ion at m/z 499.2. The mass to
charge ratio of the parent ion and the tandem mass spectrum are
consistent with an assignment to APW*LEPLR, where W* has a mass of +16
compared with W. For example, in Fig. 2E, the
unmodified sequence, LEPLR, can be identified because ions, assignable
to y1, y2,
y3, y4, and
y5 (Fig. 1A), were observed.
However, the 813 (y6) m/z
ion in Fig. 2C was not observed in Fig.
2E, but an ion with a similar intensity was observed
at 829 m/z. If the 829 ion in Fig.
2E is assigned to y6, then the
m/z change is consistent with +16 modification
of Trp-352. This interpretation is supported by assignment of
b3, b4,
b5, and b6 ions at 371,
484, 613, and 712 m/z (Fig.
2E). These b ions are +16 compared with the b ion
series observed for the unmodified APWLEPLR peptide (Fig.
2C). Two confirmatory +2 ions, y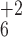 and
y
and
y , were assigned (Fig. 2E).
, were assigned (Fig. 2E).
In these ion trap experiments, low abundant fragment ions, such as a putative y7 ion in Fig. 2E, may have m/z values that differ by 1 m/z unit when compared with the theoretical prediction. This is caused by poor ion statistics (34). Therefore, to support the W+16 assignment, high mass resolution CID spectra were obtained from this PSII peptide by using a quadrupole/time-of-flight mass spectrometer (data not shown). The m/z values for the y3–y7 ions were measured with a mass accuracy of 50 ppm, and these data support the assigned APW*LEPLR sequence. For example, the y7 ion was observed with a m/z of 926.489, which confirms the +16 modification of the tryptophan. A+16 modification of tryptophan is consistent with the expected mass for oxindolylalanine, the keto derivative of tryptophan, which has been described (Fig. 1C) (35).
The CID spectrum presented in Fig. 2F was derived
from a +2 parent ion at m/z 500.9. The mass to
charge ratio of the parent ion and the tandem mass spectrum are
consistent with an assignment to APW*LEPLR, where W* has a mass of +18
compared with W. For example, in Fig. 2F, the
unmodified sequence, LEPLR, can be identified because ions, assignable
to y1, y3,
y4, and y5 (Fig.
1A), were observed. However, the 813
(y6) ion in Fig. 2C was not observed
in Fig. 2F, but an ion with a similar intensity was
observed at 831 m/z. If the 831 ion is assigned
to y6, then the m/z
change is consistent with +18 modification of Trp-352. This
interpretation is supported by assignment of b3,
b4, and b5 ions at 373,
486, and 615 m/z (Fig. 2F),
which are +18 compared with the b ion series observed for the
unmodified peptide in Fig. 2C. A confirmatory
y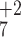 ion was also assigned (Fig. 2F).
The +18 derivative of tryptophan may correspond to a dihydro,
3-hydroxyl indolylalanine (Fig. 1C). Other possible
structures with the same mass cannot be excluded.
ion was also assigned (Fig. 2F).
The +18 derivative of tryptophan may correspond to a dihydro,
3-hydroxyl indolylalanine (Fig. 1C). Other possible
structures with the same mass cannot be excluded.
PSII Preparations.
The presence or absence of hydrazines (15) had no apparent influence on the CID results. For example, there was no evidence for covalent binding of hydrazine to this tryptic peptide. A similar set of Trp-352 modifications was detected in oxygen-evolving PSII and in PSII-3 and was detected in illuminated and in dark-maintained PSII and PSII-3. Analysis of representative ion chromatograms suggests that all three modified peptides were at least as abundant as the unmodified peptide.
The same modifications were detected in samples that had been purified by SDS/PAGE electrophoresis (in gel) and in samples that had not been subjected to electrophoresis (in situ). For in situ PSII, SEQUEST Xcorr scores ranged from 1.4 to 2.0. Higher Xcorr scores were observed when samples were resuspended in formic acid, and specific ions were selected in the scanning mode. This data acquisition scheme ensures that modified peptides, which elute over a short time span in the chromatogram, are detected. Taken together, these results imply that CP43 posttranslational modifications are an intrinsic property of PSII and are not induced by our handling procedures.
CP43 Sequence Alignment.
An amino acid sequence alignment was performed for 51 complete CP43 sequences in the nonredundant database. Fig. 3A presents representative sequences for 15 species. The overall level of sequence identity for loop E was >60%. The A350PWLEPLR357 sequence (Synechocystis sp. PCC 6803) of CP43 was found to be very highly conserved. Trp-352 was conserved in all 51 species, except in Synechococcus PCC 7942, in which this position was assigned as a cysteine. Pro-355 was completely conserved in all CP43 sequences in the nonredundant database. Pro-351 was also highly conserved in CP43 sequences (Fig. 3), with only one substitution in Heterocapsa, whereas Pro-359 had three substitutions in Prochlorococcus, Cyanophora, and Heterocapsa.
Fig 3.
Alignment of derived amino acid sequences for luminal loop E of the CP43 subunit (A) and a structural model of the spinach CP43 sequence, TMRFWDLRAPWLEPLRGPNGLDLS (B). (A) Representative CP43 sequences from 15 species are presented. The species, listed in order of appearance, are as follows (accession numbers are given in parentheses): Synechocystis sp. PCC 6803 (BAA17799), Anabaena sp. (L21857), Synechococcus sp. PCC 7942 (U30252), Secale cereale (S03436), Zea mays (S58537), Pisum sativum (P06004), Nicotiana tabacum (P06413), Marchantia polymorpha (F2LV44), Spinacia oleracea (7636084), Chlamydomonas reinhardtii (AB044528), Cyanophora (AAA81279), Guillardia theta (NP_050677), Euglena gracilis (S34498), Prochlorococcus marinus (AAK69277), and Heterocapsa triquetra (AAD44702). The Synechocystis 6803-aa numbering scheme was used; the first valine at the alternate start site is amino acid no. 1 (7). (B) Trp-352, Pro-351, Pro-355, and Pro-359 are indicated as wireframe.
Site-Directed Mutagenesis.
Previous work has shown that loop E is important in the assembly or function of PSII (9, 11, 12, 36). Two mutations, ΔF345-E354 and ΔW352-N360, deleted part of the A350PWLEPLR357 sequence (9). The deletion mutant, ΔF345-E354, had wild-type levels of CP43 but was unable to evolve oxygen or assemble functional PSII (9). The deletion mutant, ΔW352-N360, was unable to evolve oxygen or assemble functional PSII (9). Also, the site-directed mutant, E354Q, lost the capacity for photoautotrophic growth, contained no detectable CP43 protein, and was unable to evolve oxygen or assemble functional PSII (11).
We have generated three site-directed mutations at Trp-352 (W352L, W352A, and W352C) in the Synechocystis CP43 subunit. Our preliminary data show that in all three mutants, the half-time for PSII photoinhibition decreases by the same factor of 3–4, relative to wild-type cells. These experiments suggest that the unmodified form of Trp-352 plays a role in the protection of PSII from light-induced damage.
Modeling of the Peptide Sequence.
The presence of three highly conserved prolines in the protein sequence, A350PWLEPLRGPN360, suggests a structural significance for this region of loop E, which contains the posttranslationally modified tryptophan. Fig. 3B shows the results of structural modeling of the CP43 peptide, TMRFWDLRAPWLEPLRGPNGLDLS. A representative model from a family of lowest energy conformations is presented. This peptide is not predicted to form α-helix or β-sheet, but adopts a compact structure with distinct turns at each proline. The turns imposed by the prolines may position the tryptophan for modification. This model predicts that Trp-352 and the totally conserved Pro-355 are exposed on the same face of the CP43 peptide. This result may be consistent with surface exposure of Trp-352.
Discussion
These MS/MS studies provide evidence for novel modifications in the CP43 subunit of PSII. These data are consistent with three Trp-352 modifications, which result in mass increases of +4, +16, and +18 daltons. Based on a comparison to a synthesized peptide, the +4 tryptophan modification is assigned to kynurenine (Fig. 1B). Kynurenine has been identified previously in a low density lipoprotein (37). In that protein, cleavage of the indole ring is a metal-catalyzed oxidation reaction. Kynurenine can also be generated by treatment of tryptophan-containing peptides and proteins with hydrogen peroxide (35). The +16 modification is tentatively assigned to oxindolylalanine (Fig. 1C). This modification has also been reported (35). To our knowledge, the +18 form of tryptophan has not been described previously, but it is tentatively assigned to a hydroxylated indole derivative of tryptophan (Fig. 1C).
Oxidative modifications to tryptophan are likely to be caused by reactive oxygen species (38). These reactive species can be produced as a byproduct of PSII water-splitting or in PSII centers in which oxygen evolution is disrupted (39). We observe multiple oxidative modifications of one tryptophan side chain. One possible explanation of our results is that the oxindolylalanine (+16) and the hydroxyindole (+18) derivatives are intermediates, which are produced during the oxidative cleavage of the indole ring to give kynurenine as a final product (+4).
Previous mutational studies support the conclusion that the sequence APWLEPLR plays a structural or functional role in PSII. Our own site-directed mutations at Trp-352 are also consistent with this conclusion. Therefore, we propose that posttranslational modification of this region is a key element in PSII structure, function, turnover, or assembly. In our experiments, both modified and unmodified AWPLEPR peptides were detected. Therefore, it seems likely that the modifications are transient or accumulated and that these modifications play a role in signaling and not a stable role in structure.
Previously, a triggering mechanism has been proposed to be important in signaling the turnover of PSII (see ref. 40 and references therein). A conformational change is one possible triggering mechanism (41). It has been proposed that covalent modification of D1 may be involved in signaling for D1 turnover (41, 42). This is intriguing because oxidative modifications have been implicated as the trigger of protein turnover in bacteria (43).
Therefore, we speculate that the accumulation of these oxidative modifications in CP43 plays a role in the turnover of CP43, D1, and/or other PSII subunits. Structural modeling of APWLEPLR may be consistent with exposure of Trp-352 on the face of loop E. The formation of kynurenine may serve as a signal for CP43 and/or D1 processing through an interaction with a luminal protease (for examples of identified luminal proteases, see refs. 44 and 45). Roles in the assembly of the PSII reaction center are also possible.
Previous studies of the turnover and repair of PSII have focused mainly on turnover in vitro under illumination, referred to as photoinhibition (for reviews, see refs. 40 and 46). During photoinhibition, PSII is susceptible to illumination-induced damage, and rapid turnover of PSII subunits can be detected. Two different mechanisms have been proposed: an acceptor side mechanism that occurs in oxygen-evolving PSII and a donor side mechanism that occurs in manganese-depleted PSII (40, 46).
The D1 subunit exhibits rapid turnover in vivo (see ref. 47 and references therein), and the turnover rate increases under photoinhibitory conditions (reviewed in ref. 48). Other PSII subunits, such as D2 (49) and CP43 (50), are also proteolyzed during photoinhibition. The proteolytic products produced from CP43 have not yet been characterized. Proteolysis of CP43 during photoinhibition has been reported to require high light intensities, removal of the manganese-stabilizing protein, and loss of manganese (51). Our site-directed mutagenesis experiments show a connection between Trp-352 in CP43 and photoinhibitory effects because all three substitutions at Trp-352 increased the susceptibility of PSII to photodamage. These experiments suggest that the unmodified indole side chain of Trp-352 plays a role in the protection of PSII at high light intensities.
In summary, mass spectral analysis shows that CP43 can be modified to give a kynurenine derivative of tryptophan. Intermediates in the indole cleavage reaction have also been detected. We hypothesize that these oxidative modifications are mediated by reactive oxygen species and that the generation of kynurenine in the CP43 sequence, APWLEPLR, signals the turnover of PSII subunits.
Acknowledgments
We acknowledge the University of Minnesota Mass Spectrometry Consortium for the Life Sciences, the University of Minnesota Microchemical facility, Bengt Svensson, Dinesha Walek, W. Hayes MacDonald, and Lara Hays. We also thank Professors Anath Das and Janet Schottel for equipment use. This work was supported by National Institutes of Health Grants GM43273 (to B.A.B.) and DK59731 (to M.J.M.) and National Science Foundation Grant MCB 9982981 (to C.P.-E.).
Abbreviations
CID, collision-induced dissociation
PSII, photosystem II
TEAA, triethylammonium acetate
Xcorr, cross-correlation
References
- 1.Barry B. A., Boerner, R. J. & de Paula, J. C. (1994) in The Molecular Biology of the Cyanobacteria, ed. Bryant, D. (Kluwer, Dordrecht, The Netherlands), Vol. 1, pp. 215–257. [Google Scholar]
- 2.Nanba O. & Satoh, K. (1987) Proc. Natl. Acad. Sci. USA 84 109-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyao M. & Murata, N. (1989) Biochim. Biophys. Acta 977 315-321. [DOI] [PubMed] [Google Scholar]
- 4.Bricker T. & Frankel, L. (1998) Photosynth. Res. 56 157-173. [Google Scholar]
- 5.Zouni A., Witt, H. T., Kern, J., Fromme, P., Krauss, N., Saenger, W. & Orth, P. (2001) Nature 409 739-743. [DOI] [PubMed] [Google Scholar]
- 6.Shen J.-R. & Kamiya, N. (2000) Biochemistry 39 14739-14744. [DOI] [PubMed] [Google Scholar]
- 7.Bricker T. M. (1990) Photosynth. Res. 24 1-13. [DOI] [PubMed] [Google Scholar]
- 8.Rogner M., Chisholm, D. A. & Diner, B. A. (1991) Biochemistry 30 5387-5395. [DOI] [PubMed] [Google Scholar]
- 9.Kuhn M. G. & Vermaas, V. F. J. (1993) Plant Mol. Biol. 23 123-133. [DOI] [PubMed] [Google Scholar]
- 10.Ouellette A. J. A., Anderson, L. B. & Barry, B. A. (1998) Proc. Natl. Acad. Sci. USA 95 2204-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg C., Christian, J., Bricker, T. M. & Putnam-Evans, C. (1999) Biochemistry 38 15994-16000. [DOI] [PubMed] [Google Scholar]
- 12.Dzelzkalns V. A. & Bogorad, L. (1988) EMBO J. 7 333-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knoepfle N., Bricker, T. M. & Putnam-Evans, C. (1999) Biochemistry 38 1582-1588. [DOI] [PubMed] [Google Scholar]
- 14.Yoshikawa S., Shinzawa-Itoh, K., Nakashima, R., Yaono, R., Yamashita, E., Inoue, N., Yao, M., Fei, M. J., Libeu, C. P., Mizushima, T., et al. (1998) Science 280 1723-1729. [DOI] [PubMed] [Google Scholar]
- 15.Anderson L. B., Ouellette, A. J. A. & Barry, B. A. (2000) J. Biol. Chem. 275 4920-4927. [DOI] [PubMed] [Google Scholar]
- 16.Dongré A. R., Eng, J. & Yates, J. R., III (1997) Trends Biotechnol. 15 383-439. [DOI] [PubMed] [Google Scholar]
- 17.Hufnagel P., Schweiger, U., Eckerskorn, C. & Oesterhelt, D. (1996) Anal. Biochem. 243 46-54. [DOI] [PubMed] [Google Scholar]
- 18.Whitelegge J. P., Faull, K. F., Gundersen, C. B. & Gomez, S. M. (1998) in Photosynthesis: Mechanisms and Effects, ed. Garab, G. (Kluwer, Dordrecht, The Netherlands), pp. 4381–4384.
- 19.Corradini D., Huber, C. G., Timpero, A. M. & Zolla, L. (2000) J. Chromatogr. A 886 111-121. [DOI] [PubMed] [Google Scholar]
- 20.Ouellette A. J. A. & Barry, B. A. (2002) Photosynth. Res. 72 159-173. [DOI] [PubMed] [Google Scholar]
- 21.Sharma J., Panico, M., Barber, J. & Morris, H. R. (1997) J. Biol. Chem. 272 3935-3943. [DOI] [PubMed] [Google Scholar]
- 22.Berthold D. A., Babcock, G. T. & Yocum, C. F. (1981) FEBS Lett. 134 231-234. [Google Scholar]
- 23.Lichtenthaler H. K. (1987) Methods Enzymol. 148 350-382. [Google Scholar]
- 24.Barry B. A. (1995) Methods Enzymol. 258 303-319. [DOI] [PubMed] [Google Scholar]
- 25.Washburn M. P., Wolters, D. & Yates, J. R., III (2001) Nat. Biotechnol. 19 242-247. [DOI] [PubMed] [Google Scholar]
- 26.Eng J. K., McCormack, A. L. & Yates, J. R., III (1994) J. Am. Soc. Mass Spectrom. 5 976-989. [DOI] [PubMed] [Google Scholar]
- 27.Altshul S. F., Madden, T. L., Schäffer, A. A., Zhang, J., Zhang, Z., Miller, W. & Lipman, D. J. (1997) Nucleic Acids Res. 25 3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Needleman S. B. & Wunsch, C. D. (1970) J. Mol. Biol. 48 443-453. [DOI] [PubMed] [Google Scholar]
- 29.Feng D.-F. & Doolittle, R. F. (1987) J. Mol. Evol. 25 351-360. [DOI] [PubMed] [Google Scholar]
- 30.Atherton E., Logan, C. J. & Sheppard, R. C. (1981) J. Chem. Soc. Perkin Trans. 1 538-546. [Google Scholar]
- 31.Hunt D. F., Yates, J. R., III, Shabanowitz, J., Winston, S. & Hauer, C. R. (1986) Proc. Natl. Acad. Sci. USA 83 6233-6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papayannopoulos I. A. (1995) Mass Spectrom. Rev. 14 49-71. [Google Scholar]
- 33.Fowden L. (1964) Annu. Rev. Biochem. 33 173. [DOI] [PubMed] [Google Scholar]
- 34.Chernushevich I. V., Loboda, A. V. & Thomson, B. A. (2001) J. Mass Spectrom. 36 849-865. [DOI] [PubMed] [Google Scholar]
- 35.Simat T. J. & Steinhart, H. (1998) J. Agric. Food Chem. 46 490-498. [DOI] [PubMed] [Google Scholar]
- 36.Vermaas W. F. J., Ikeuchi, M. & Inoue, Y. (1988) Photosynth. Res. 17 97-113. [DOI] [PubMed] [Google Scholar]
- 37.Yang C.-Y., Gu, Z.-W., Yag, M., Lin, S.-N., Siuzdak, G. & Smith, C. V. (1999) Biochemistry 38 15903-15908. [DOI] [PubMed] [Google Scholar]
- 38.Berlett B. S. & Stadtman, E. R. (1997) J. Biol. Chem. 272 20313-20316. [DOI] [PubMed] [Google Scholar]
- 39.Krause G. H. (1994) in Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants, eds. Foyer, C. H. & Mullineaux, P. M. (CRC Press, Boca Raton, FL), pp. 43–75.
- 40.Aro E.-M., Virgin, I. & Andersson, B. (1993) Biochim. Biophys. Acta 1143 113-134. [DOI] [PubMed] [Google Scholar]
- 41.Prasil O., Adir, N. & Ohad, I. (1992) in The Photosystems: Structure, Function and Molecular Biology, ed. Barber, J. (Elsevier Science, Amsterdam), Vol. 11, pp. 295–348. [Google Scholar]
- 42.Callahan F. E., Ghirardi, M. L., Sopory, S. K., Mehta, A. M., Edelman, M. & Mattoo, A. K. (1990) J. Biol. Chem. 265 15357-15360. [PubMed] [Google Scholar]
- 43.Stadtman E. R. (1990) Biochemistry 29 6323-6331. [DOI] [PubMed] [Google Scholar]
- 44.Bowyer J. R., Packer, J. C. L., McCormack, B. A., Whitelegge, J. P., Robinson, C. & Taylor, M. A. (1992) J. Biol. Chem. 267 5424-5433. [PubMed] [Google Scholar]
- 45.Itzhaki H., Naveh, L., Lindahl, M., Cook, M. & Adam, S. (1998) J. Biol. Chem. 273 7094-7098. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto Y. (2001) Plant Cell Physiol. 42 121-128. [DOI] [PubMed] [Google Scholar]
- 47.Mattoo A. K., Pick, U., Hoffman-Falk, H. & Edelman, M. (1981) Proc. Natl. Acad. Sci. USA 78 1572-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kyle D. J. (1985) Photochem. Photobiol. 41 107-116. [Google Scholar]
- 49.Barbato R., Frisco, G., de Laureto, P. P., Frizzo, A., Rigoni, F. & Giacometti, G. (1992) FEBS Lett. 311 33-36. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto Y. & Akasaka, T. (1995) Biochemistry 34 9038-9045. [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto Y., Ishkawa, Y., Nakatami, E., Yamada, M., Zhang, H. & Wydrzynski, T. (1998) Biochemistry 37 1565-1574. [DOI] [PubMed] [Google Scholar]





