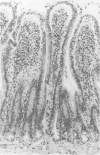
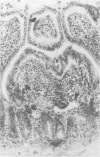
Abstract
Short term effects of indomethacin on intestinal permeability were studied on a model of rat isolated vascularly perfused terminal ileum. The objectives of this study were (a) to assess the effects of indomethacin on intestinal permeability and histology; (b) to assess the effects of prostaglandins, leukotrienes, and platelet activating factor (PAF) on the same parameters; (c) to evaluate the role of these inflammation mediators on indomethacin induced permeability modifications. Intravascular administration of 1.25 and 2.5 mM indomethacin induced a significant increase of 51Cr-EDTA transfer rate. Histological analysis showed only mucosal oedema. Pretreatment with 16,16 dimethyl-prostaglandin E2 did not reverse these changes. Intravascular administration of PAF, leukotrienes B4 and D4 provoked a significant rise in 51Cr-EDTA transfer rate and intraluminal protein leakage, with an intense vascocongestion of the mucosal capillaries. These changes were completely prevented by perfusion of the respective specific antagonists (BN52021 for PAF, LY255,583 for leukotriene B4 and MK571 for leukotriene D4). None of these three antagonists, however, or MK886, a selective 5'-lipo-oxygenase inhibitor, could reverse the indomethacin induced permeability changes. Indomethacin induced increased intestinal permeability at these high concentrations does not seem to be a result of changed prostanoid or PAF metabolism. Alternative mechanisms of the initial damage of non-steroid anti-inflammatory drugs should be sought.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aabakken L. Review article: non-steroidal, anti-inflammatory drugs--the extending scope of gastrointestinal side effects. Aliment Pharmacol Ther. 1992 Apr;6(2):143–162. doi: 10.1111/j.1365-2036.1992.tb00258.x. [DOI] [PubMed] [Google Scholar]
- Abramson S. B., Weissmann G. The mechanisms of action of nonsteroidal antiinflammatory drugs. Arthritis Rheum. 1989 Jan;32(1):1–9. doi: 10.1002/anr.1780320102. [DOI] [PubMed] [Google Scholar]
- Anthony A., Dhillon A. P., Nygard G., Hudson M., Piasecki C., Strong P., Trevethick M. A., Clayton N. M., Jordan C. C., Pounder R. E. Early histological features of small intestinal injury induced by indomethacin. Aliment Pharmacol Ther. 1993 Feb;7(1):29–39. doi: 10.1111/j.1365-2036.1993.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Beaven M. A., Bayer B. M. Factors influencing the uptake and disposition of indomethacin-[14C] in cell cultures. Biochem Pharmacol. 1980 Jul 15;29(14):2055–2061. doi: 10.1016/0006-2952(80)90491-8. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Peters T. J. Intestinal permeability, non-steroidal anti-inflammatory drug enteropathy and inflammatory bowel disease: an overview. Gut. 1989 Nov;30(Spec No):22–28. doi: 10.1136/gut.30.spec_no.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I., Smethurst P., Clark P., Menzies I., Levi J., Peters T. Effect of prostaglandin on indomethacin-induced increased intestinal permeability in man. Scand J Gastroenterol Suppl. 1989;164:97–103. doi: 10.3109/00365528909091195. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Williams P., Smethurst P., Peters T. J., Levi A. J. Effect of non-steroidal anti-inflammatory drugs and prostaglandins on the permeability of the human small intestine. Gut. 1986 Nov;27(11):1292–1297. doi: 10.1136/gut.27.11.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crissinger K. D., Kvietys P. R., Granger D. N. Pathophysiology of gastrointestinal mucosal permeability. J Intern Med Suppl. 1990;732:145–154. doi: 10.1111/j.1365-2796.1990.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Cuber J. C., Herrmann C., Kitabgi P., Bosshard A., Bernard C., De Nadai F., Chayvialle J. A. Neuromedin-N is not released with neurotensin from rat ileum. Endocrinology. 1990 Mar;126(3):1584–1592. doi: 10.1210/endo-126-3-1584. [DOI] [PubMed] [Google Scholar]
- Duffey M. E., Hainau B., Ho S., Bentzel C. J. Regulation of epithelial tight junction permeability by cyclic AMP. Nature. 1981 Dec 3;294(5840):451–453. doi: 10.1038/294451a0. [DOI] [PubMed] [Google Scholar]
- Fang W. F., Broughton A., Jacobson E. D. Indomethacin-induced intestinal inflammation. Am J Dig Dis. 1977 Sep;22(9):749–760. doi: 10.1007/BF01694504. [DOI] [PubMed] [Google Scholar]
- Gillard J., Ford-Hutchinson A. W., Chan C., Charleson S., Denis D., Foster A., Fortin R., Leger S., McFarlane C. S., Morton H. L-663,536 (MK-886) (3-[1-(4-chlorobenzyl)-3-t-butyl-thio-5-isopropylindol-2-yl]-2,2 - dimethylpropanoic acid), a novel, orally active leukotriene biosynthesis inhibitor. Can J Physiol Pharmacol. 1989 May;67(5):456–464. doi: 10.1139/y89-073. [DOI] [PubMed] [Google Scholar]
- Gumbiner B. Structure, biochemistry, and assembly of epithelial tight junctions. Am J Physiol. 1987 Dec;253(6 Pt 1):C749–C758. doi: 10.1152/ajpcell.1987.253.6.C749. [DOI] [PubMed] [Google Scholar]
- Jones T. R., Zamboni R., Belley M., Champion E., Charette L., Ford-Hutchinson A. W., Frenette R., Gauthier J. Y., Leger S., Masson P. Pharmacology of L-660,711 (MK-571): a novel potent and selective leukotriene D4 receptor antagonist. Can J Physiol Pharmacol. 1989 Jan;67(1):17–28. doi: 10.1139/y89-004. [DOI] [PubMed] [Google Scholar]
- Karasawa A., Guo J. P., Ma X. L., Tsao P. S., Lefer A. M. Protective actions of a leukotriene B4 antagonist in splanchnic ischemia and reperfusion in rats. Am J Physiol. 1991 Aug;261(2 Pt 1):G191–G198. doi: 10.1152/ajpgi.1991.261.2.G191. [DOI] [PubMed] [Google Scholar]
- Krugliak P., Hollander D., Le K., Ma T., Dadufalza V. D., Katz K. D. Regulation of polyethylene glycol 400 intestinal permeability by endogenous and exogenous prostanoids. Influence of non-steroidal anti-inflammatory drugs. Gut. 1990 Apr;31(4):417–421. doi: 10.1136/gut.31.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes P., Ibbotson G., Russell J., Wallace J. L., Granger D. N. Role of platelet-activating factor in ischemia/reperfusion-induced leukocyte adherence. Am J Physiol. 1990 Aug;259(2 Pt 1):G300–G305. doi: 10.1152/ajpgi.1990.259.2.G300. [DOI] [PubMed] [Google Scholar]
- Kubes P. Ischemia-reperfusion in feline small intestine: a role for nitric oxide. Am J Physiol. 1993 Jan;264(1 Pt 1):G143–G149. doi: 10.1152/ajpgi.1993.264.1.G143. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehr H. A., Guhlmann A., Nolte D., Keppler D., Messmer K. Leukotrienes as mediators in ischemia-reperfusion injury in a microcirculation model in the hamster. J Clin Invest. 1991 Jun;87(6):2036–2041. doi: 10.1172/JCI115233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. J., Zhang X. J., Gu X. A., Clark D. A. Acute intestinal injury induced by acetic acid and casein: prevention by intraluminal misoprostol. Gastroenterology. 1991 Jul;101(1):22–30. doi: 10.1016/0016-5085(91)90455-t. [DOI] [PubMed] [Google Scholar]
- Miura S., Suematsu M., Tanaka S., Nagata H., Houzawa S., Suzuki M., Kurose I., Serizawa H., Tsuchiya M. Microcirculatory disturbance in indomethacin-induced intestinal ulcer. Am J Physiol. 1991 Aug;261(2 Pt 1):G213–G219. doi: 10.1152/ajpgi.1991.261.2.G213. [DOI] [PubMed] [Google Scholar]
- Rainsford K. D. Mechanisms of gastrointestinal toxicity of non-steroidal anti-inflammatory drugs. Scand J Gastroenterol Suppl. 1989;163:9–16. [PubMed] [Google Scholar]
- Satoh H., Guth P. H., Grossman M. I. Role of food in gastrointestinal ulceration produced by indomethacin in the rat. Gastroenterology. 1982 Jul;83(1 Pt 2):210–215. [PubMed] [Google Scholar]
- Vaananen P. M., Meddings J. B., Wallace J. L. Role of oxygen-derived free radicals in indomethacin-induced gastric injury. Am J Physiol. 1991 Sep;261(3 Pt 1):G470–G475. doi: 10.1152/ajpgi.1991.261.3.G470. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Keenan C. M., Granger D. N. Gastric ulceration induced by nonsteroidal anti-inflammatory drugs is a neutrophil-dependent process. Am J Physiol. 1990 Sep;259(3 Pt 1):G462–G467. doi: 10.1152/ajpgi.1990.259.3.G462. [DOI] [PubMed] [Google Scholar]
- Whittle B. J., Morishita T., Ohya Y., Leung F. W., Guth P. H. Microvascular actions of platelet-activating factor on rat gastric mucosa and submucosa. Am J Physiol. 1986 Dec;251(6 Pt 1):G772–G778. doi: 10.1152/ajpgi.1986.251.6.G772. [DOI] [PubMed] [Google Scholar]
- Yamada T., Specian R. D., Granger D. N., Gaginella T. S., Grisham M. B. Misoprostol attenuates acetic acid-induced increases in mucosal permeability and inflammation: role of blood flow. Am J Physiol. 1991 Aug;261(2 Pt 1):G332–G339. doi: 10.1152/ajpgi.1991.261.2.G332. [DOI] [PubMed] [Google Scholar]