Abstract
When cells are exposed to death-inducing molecules such as tumor necrosis factor-α or Fas, caspase 8 is activated and cleaves an apoptotic facilitator, Bid, that is a member of the Bcl-2 family. After additional modification, the C-terminal moiety of Bid is translocated to the mitochondria and induces the release of cytochrome c into the cytoplasm. In an attempt to directly observe the cleavage of Bid and the following events in living cells, we constructed a vector that encoded Bid fused with yellow fluorescent protein (YFP) and cyan fluorescent protein (CFP) (YFP-Bid-CFP). On expression of YFP-Bid-CFP in mammalian cells, we were able to observe the efficient transfer of energy from excited CFP to YFP within the YFP-Bid-CFP molecule and, importantly, the fusion protein YFP-Bid-CFP was fully functional in cells. When YFP-Bid-CFP was cleaved by caspase 8, on activation by anti-Fas Abs but not by Aβ or tunicamycin, no such transfer of energy was detected. To our knowledge, this is the first report of (i) visualization of the activation of Bid by proteolytic cleavage, with direct observation of the cleavage of YFP-Bid-CFP in the cytoplasm and subsequent translocation of the cleaved Bid to mitochondria and (ii) the absence of Aβ- or tunicamycin-mediated significant activation of caspase 8 in individual living cells.
Apoptosis plays important roles in development and homeostasis, and caspases have been shown to play important roles in apoptosis. The complex phenotypes of “caspase-knockout” mice suggest that multiple mechanisms for activation of caspases operate in parallel and that the signal-transduction pathways to apoptosis are stimulus-specific and specific to individual types of cell (1).
Damage to mitochondria is a major signal-amplification step in the apoptotic pathway, and mitochondria are also the principal site of action of proteins in the Bcl-2 family (2). Many members of this family contain four conserved domains (2), designated BH1, BH2, BH3, and BH4 (BH, Bcl-2 homology domain), and although many of these proteins are proapoptotic [Bax, Bak, etc. (3, 4)], others are antiapoptotic [Bcl-2, Bcl-xL, etc. (5, 6)]. Irrespective of their pro- or antiapoptotic effectors, many of these proteins have a propensity to form homo- and heterodimers.
Bid is a proapoptotic member of the Bcl-2 family that contains only the BH3 domain. It was first noted for its ability to bind to Bcl-2 and Bax (3). Mutational analysis indicated that an intact BH3 domain is necessary for binding to Bcl-2 and Bax, and this binding activity is correlated with the ability of Bid to induce cell death. These observations suggested a model in which Bid serves as a death-inducing ligand that moves from the cytosol to the mitochondrial membrane to inactivate Bcl-2 or to activate Bax (3, 7). In proliferating cells, inactive Bid (a protein of 22 kDa; p22) is localized in the cytoplasm. On exposure of cells to tumor necrosis factor-α or Fas, both of which induce apoptosis, caspase 8 is activated by death-inducing signaling complex. Cleavage of Bid by caspase 8 yields two fragments (p15 and p7) (8). An exposed glycine residue at the C terminus of Bid (p15) undergoes N-myristoylation (9), and the resulting C-terminal fragment of Bid (p15) is translocated to the mitochondrial outer membrane, where it binds to Bax or to Bak (4, 10). These interactions induce the release of cytochrome c from the mitochondria, and the release of cytochrome c leads to formation of a complex that contains cytochrome c, Apaf1, and caspase 9 in the cytoplasm. This complex activates caspase 9, and active caspase 9 cleaves the downstream effectors caspase 3 and caspase 7 (11).
In a previous study (9), GFP was fused to the C terminus of Bid and revealed the translocation of Bid-GFP to the mitochondria from the cytosol. However, this system could not directly observe the cleavage of Bid and thus provided limited information for monitoring the activation of caspase 8, in addition to the translocation of Bid, in individual living cells.
Recently, it has been demonstrated that it is possible to fuse two proteins that fluoresce at different wavelengths and to monitor fluorescence resonance energy transfer (FRET) (12–14) and to detect proteolytic activity (15), just like the detection of intracellular cleavage of nucleic acids by FRET (16). The first successful example of this strategy was the genetic construction of tandem blue fluorescent protein–GFP molecules connected by a linker sequence that could be cleaved by a particular protease (17). FRET occurred when the two proteins that fluoresce at different wavelengths (donor and acceptor) were linked together and energy could be transferred directly from the donor to the acceptor. When the acceptor and the donor were separated by proteolytic cleavage of the linker, no FRET was recorded. Thus, FRET is a powerful tool for investigations of molecular events in living cells (12–19).
In our recent work, Bid was identified as one of the proapoptotic genes by using our functional gene discovery system that was based on randomized hybrid-ribozyme libraries (20–22). To observe directly the activation of the identified Bid by various apoptosis inducers and detect activity of caspase 8 in apoptotic cells, we constructed a fusion protein by connecting yellow fluorescent protein (YFP) and cyan fluorescent protein (CFP) to the N terminus and the C terminus of Bid, respectively. Using our system, we obtained in vivo imaging of activation of caspase 8 in a single cell that enabled us to demonstrate the absence of significant amyloid-β peptide (Aβ)- or tunicamycin (Tm)-mediated activation of caspase 8 in individual living cells.
Materials and Methods
Cloning of cDNA for Bid and Construction of an Expression Vector for YFP-Bid-CFP.
The gene for enhanced YFP (CLONTECH) was amplified by PCR and inserted into the BamHI and HindIII site of pcDNA 3 (Invitrogen) to generate pYFP. The gene for enhanced CFP (CLONTECH) was also amplified by PCR and inserted into the XhoI site of pYFP to generate pFRET. The pFRET-linker vector (encoding YFP-linker-CFP) encoded three additional glycine residues at the BamHI/EcoRI site of pFRET.
The full-length gene for Bid was amplified from a human liver cDNA library in plasmid (Takara, Kyoto) with cBid up (5′-CAGGCCATGGACTGTGAGGTCAACA-3′) and cBid down (5′-TCAGTCCATCCCATTTCTGGCTAAG-3′) as primers. The PCR product was cloned into pGEM-T (Promega), and the integrity of the cloned cDNA was confirmed by sequencing. A truncated form of Bid was amplified with BidFRET-F (5′-CGGGATCCGAGTGCATCACAAA-3′) and BidFRET-R (5′-GCGAATTCATTTCTGGCTAAGC-3′) as primers from cloned Bid in pGEM-T. The PCR product was digested with BamHI and EcoRI and subcloned at the BamHI/EcoRI site of pFRET to generate pFRET-Bid. The truncated form of Bid was inserted into the pYFP vector by using the same restriction enzymes. A methionine-added short form of Bid was amplified with Hind-Met-fBid-5′ (5′-AAGCTTATGGAGTGCATCACAAACCTACT-3′), because the truncated form of Bid did not have a start codon, and BidFRET-r as primers from cloned Bid in pGEM-T. The PCR product of Bid was inserted into the HindIII/EcoRI site of pFRET to generate pBid-CFP.
Culture and Transfection of Cells.
COS7 cells and NIH 3T3 cells were grown in DMEM (Sigma) supplemented with 10% FCS (GIBCO/BRL). Before transfection, cells were divided into aliquots of 1 × 106 cells per 10-cm dish or 1 × 105 cells per 2-cm glass-bottomed dish. After 16–24 h, cells were transfected with pFRET-Bid by using the PolyFect transfection reagent (Qiagen, Chatsworth, CA) according to the manufacturer's protocol. SK-N-SH cells were grown in MEM-α (GIBCO/BRL) supplemented with 10% FCS (GIBCO/BRL). SK-N-SH cells were stably transfected with plasmids by using Lipofectamine 2000 (GIBCO/BRL) according to the manufacturer's protocol. After transfections, cells were selected by exposure to Geneticin (Sigma) and stable cell lines were cloned.
Acquisition of Fluorescent Images of Each Cell Line.
Before cells were treated with drugs, the cells expressing fusion proteins transiently or stably were cultured on the glass bottom dish (IWAKI, Tokyo) for 40 or 24 h, respectively. Cells were observed by using conventional fluorescence microscopy, Olympus IX-50. To observe the in vivo FRET effect, cells were excited by using an excitation filter (440 ± 10 nm) plus a dichroic mirror of 455 nm. The emission images of YFP (545 ± 17.5 nm) and CFP (480 ± 15 nm) were recorded by using a computer-controlled cooled charge-coupled device camera (ARGAS-20, Hamamatsu Photonics). The digital fluorescence images were then processed by using nih image software. Contrast was altered in gray scale image in the same range. Then, these images were colored by photoshop software (Adobe Systems, Mountain View, CA). Images were background subtracted. When images were compared, the contrast stretching and color scale were identical, such that comparable brightness in each image corresponded to comparable signal above background.
Cleavage of YFP-Bid-CFP by Recombinant Caspase 8 in Vitro.
COS7 cells transfected with pFRET-Bid were collected and resuspended in sonication buffer that contained 20 mM Hepes (pH 7.5), 10 mM KCl, 2.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, and 1 mM DTT at 4°C. After sonication, the homogenate was centrifuged at 10,000 × g for 10 min, and the supernatant was used for assays of cleavage by recombinant human caspase 8 (Oncogene Science) at 25°C.
Optical Measurements.
After incubation with caspase 8, reaction mixtures (total volume, 600 μl) were transferred to the cuvette of a spectrofluorometer (FP-750, Jasco, Tokyo). Fluorescence emission spectra were recorded after excitation at 433 nm. The extracts of parental cells were used as a control.
Immunoblotting.
Cell extracts that had been incubated with caspase 8 were separated by SDS/PAGE (10% polyacrylamide), and proteins were then transferred to a Clear Blot Membrane-P (ATTO, Tokyo) by electrotransfer. The blots were probed with anti-GFP polyclonal Ab and then with peroxidase-conjugated secondary antibodies (Amersham Pharmacia). Immunoreactions were detected with the ECL Plus Western blotting system (Amersham Pharmacia).
Induction of Apoptosis and Imaging of Living Cells.
NIH 3T3 cells that had been transfected with pFRET-Bid were grown in 35-mm glass-bottom dishes for 24 h. Cells were then treated with tumor necrosis factor-α (0.5 ng/ml; Sigma) and cycloheximide (1 μg/ml; Sigma) (23) and examined 3 h later by fluorescence microscopy. Mitochondria were identified with 100 nM MitoTracker (Molecular Probes).
Lines of SK-N-SH cells that had been stably transfected with pFRET-linker or pFRET-Bid were treated with anti-Fas Ab (1 μg/ml; Medical and Biological Laboratories, Nagoya, Japan) and cycloheximide (1 μg/ml; Sigma; ref. 24), Tm (2 μg/ml; Sigma; refs. 25 and 26), or Aβ (25–35 aa; 40 μM; Biochem; refs. 27–29). Anti-Fas antibody-treated cells were examined by fluorescence microscopy 6 h later, and other treated cells were examined after a 12-h incubation.
Results and Discussion
Engineering of the Identified Bid by the Gene Discovery System to Furnish Sensor Functions.
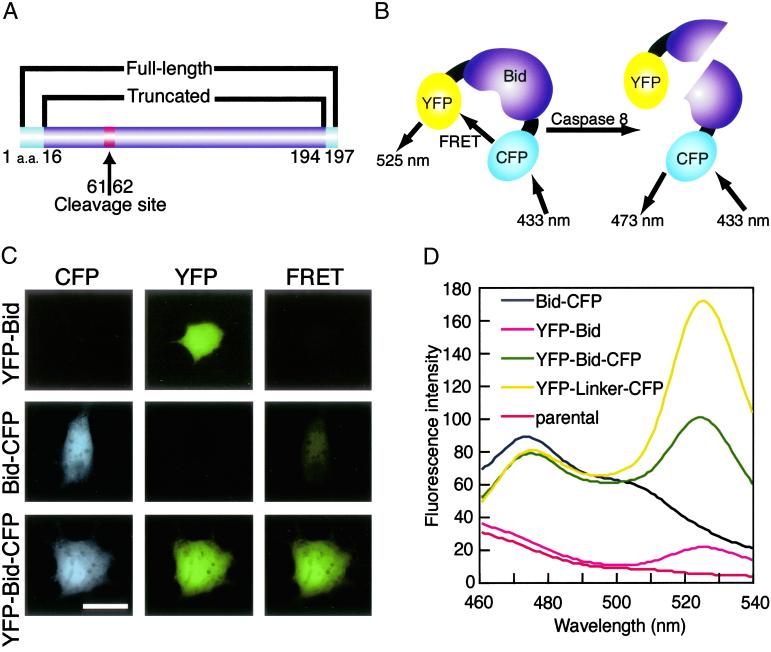
To directly observe the activation of Bid that was previously identified as one of the proapoptotic genes by using our randomized hybrid-ribozyme libraries (20–22), we connected YFP and CFP to the N and C termini of Bid, respectively, through a spacer that contained three glycine residues in each case (Fig. 1). With YFP and CFP, the maximum distance over which resonance energy transfer can occur is limited to 100 Å, and furthermore, the efficiency of energy transfer is extremely sensitive to the distance between the donor (CFP) and the acceptor (YFP). To bring the donor within an acceptable distance of the acceptor, we deleted amino acids from both termini of Bid (Fig. 1A), according to information from the crystal structure of Bid (30, 31), hoping that the resultant chimeric protein would be functional in vivo. The resultant YFP-Bid-CFP fusion protein allowed the direct transfer of resonance energy from CFP to YFP (Fig. 1D). The chimeric protein retained the sequence that is cleaved by caspase 8 and the myristoylation site (9). We anticipated that when the chimeric Bid was cleaved by caspase 8, FRET would no longer occur.
Fig 1.
(A) Schematic representation of the domain structure of CFP-Bid-YFP. YFP and CFP were connected to Bid (residues 16–194) by flexible spacers that consisted of three glycine residues each to generate CFP-Bid-YFP. The arrow between residues 61 and 62 indicates the site of cleavage by caspase 8. (B) In our FRET system, excitation of CFP at 433 nm should result in emission at 473 nm if no YFP is in close proximity. In the YFP-Bid-CFP, energy should be transferred from excited CFP to YFP, with resulting emission at 525 nm. When the fusion protein is cleaved by caspase 8, energy can no longer be transferred from excited CFP to YFP. (C) Fluorescence images of COS7 cells that expressed the indicated fusion proteins (y axis). Images were acquired by using FRET, CFP, and YFP filters (x axis). (Bar = 20 μm.) (D) Fluorescence spectra of extracts of cells that expressed various fusion proteins. The amount of total protein for each sample was adjusted to 1.4 mg/ml. The extracts of cells expressing YFP-Bid (pink line), Bid-CFP (black Line), YFP-Bid-CFP (green line), YFP-linker-CFP (yellow line), and parental cells (red line) were excited at 433 nm and emission spectra were recorded and normalized at 450 nm.
We attempted to visualize changes in FRET directly by conventional fluorescence microscopy. When COS7 cells that expressed YFP-Bid were excited with blue light (460–490 nm) and emission was monitored in the green range (510–550 nm), signals were easily detected (Fig. 1C Top Center). These cells did not emit fluorescence when excited at 400–440 nm (Fig. 1C Top Left and Right). When COS7 cells that expressed Bid-CFP were excited at 400–440 nm, the emitted fluorescence could be observed in the blue range (Fig. 1C Middle Left). In this case, no significant FRET was observed (Fig. 1C Middle Right), and 460–490 nm of blue light could not excite the Bid-CFP protein (Fig. 1C Middle Center). By contrast, when cells that expressed YFP-Bid-CFP were excited at 460–490 nm or at 400–440 nm, we detected fluorescence because of YFP, CFP, and FRET (Fig. 1C Bottom). It should be mentioned that, in all imaging experiments including these of Figs. 4 and 5, the same exposures were used for both real and control FRET images.
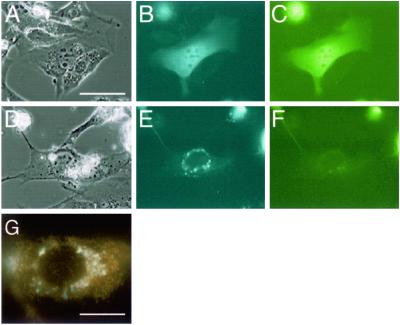
Fig 4.
Subcellular localization of YFP-Bid-CFP. NIH 3T3 cells were transfected with pFRET-Bid and incubated for 52 h. Cells on D–G were exposed to tumor necrosis factor-α plus cycloheximide for 3 h. Controls were not exposed to these agents (A–C). After incubation, cells were observed by fluorescence microscopy. Phase-contrast images (A and D), images of CFP fluorescence (B and E), and images of YFP fluorescence (C and F). (Bar = 20 μm in A–F.) The subcellular localization of truncated Bid (cyan fluorescence) and mitochondria (orange fluorescence) was assessed by fluorescence microscopy (G). (Bar = 10 μm.)
Fig 5.
Fluorescence images of YFP-Bid-CFP and YFP-linker-CFP in cells treated with various inducers of apoptosis. (A) SK-N-SH cells were stably transfected with pFRET-Bid or pFRET-linker, and images were recorded under phase-contrast conditions (phase) and with FRET, CFP, and YFP filters. Linker refers to YFP-linker-CFP and Bid refers to YFP-Bid-CFP. YFP-linker-CFP and YFP-Bid-CFP diffused uniformly throughout the cells. (Bar = 20 μm.) (B) Cells were induced to undergo apoptosis by anti-Fas Ab (Fas), Tm, or Aβ. After treatment, images were recorded as indicated above. Apoptosis-induced cells were also observed under higher (B) and lower (C) magnification. (Bar = 10 μm.)
Fig. 1D shows the fluorescence spectrum of extracts from each cell line as excited in a fluorometer at 433 nm. Lysates of cells that expressed YFP-Bid-CFP and YFP-linker-CFP (described in Materials and Methods) yielded a peak at 525 nm, which corresponded to FRET. In contrast, lysates of cells that expressed YFP-Bid did not yield a similar peak at 525 nm, and the emission spectra of Bid-CFP was observed to peak at 473 nm. Lysates of parental cells showed no peak when excited at 433 nm. These observations indicated the efficient transfer of energy between the donor (CFP) and the acceptor (YFP) within the fusion protein.
Confirmation of the Engineered YFP-Bid-CFP to Act as a Substrate for Caspase 8 in Vitro.
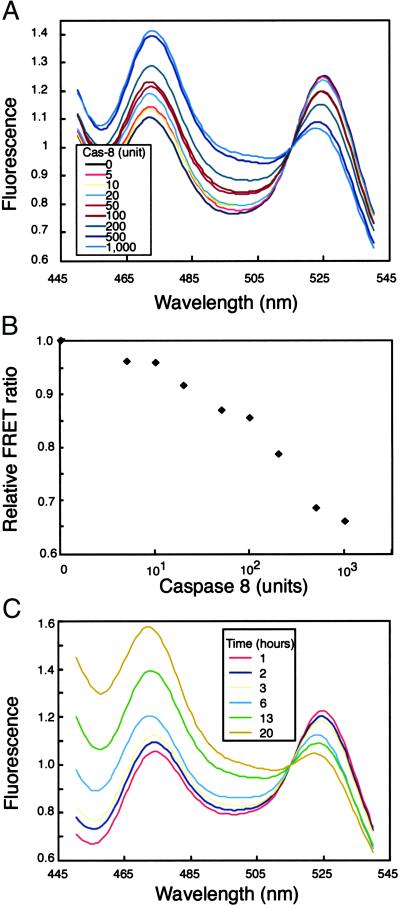
We examined whether the chimeric protein YFP-Bid-CFP could still serve as a substrate for caspase 8 in vitro and also be fully functional in vivo because, to allow FRET, we had truncated Bid at both ends. We recorded the emission spectra of an extract of COS7 cells that expressed YFP-Bid-CFP before and after incubating aliquots of the cell extract with increasing amounts of caspase 8 for 1–22 h (Fig. 2). The efficiency of FRET clearly depended on the amount of caspase 8 (Fig. 2A) and the duration of incubation (Fig. 2C). Fluorescence because of FRET (peak at 525 nm) decreased steadily with increasing concentration of caspase 8 and reached a minimum at 1,000 units (Fig. 2A, light blue line).
Fig 2.
(A) Results of spectrofluorometric analysis of the cleavage of YFP-Bid-CFP in cell extracts by caspase 8. Extracts were incubated with increasing amounts of caspase 8 (Cas-8) for 22 h at 25°C, and then spectra were recorded after excitation at 433 nm. (B) The ratio of fluorescence from YFP (525 nm) to that from CFP (473 nm) was calculated for each concentration of caspase 8 in the reaction mixture. The relative FRET ratio was defined as the ratio of values of YFP fluorescence/CFP fluorescence measured before and after incubation with caspase 8. (C) Time-dependent cleavage of YFP-Bid-CFP, as demonstrated by spectrofluorometric analysis.
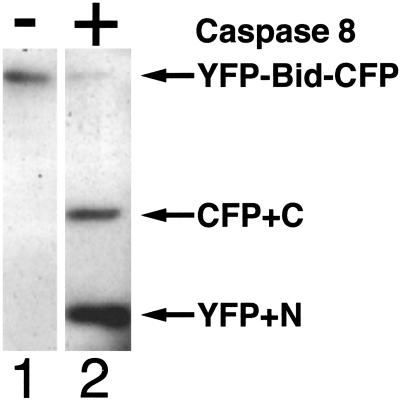
To determine the sensitivity of the fusion protein to caspase 8, we replotted the data shown in Fig. 2A, as shown in Fig. 2B, in which the relative ratio of fluorescence of YFP to that of CFP (measured at 525 and 473 nm, respectively) is plotted against the concentration of caspase 8 on a logarithmetic scale. The ratio (YFP/CFP) was normalized by reference to the ratio for that of the uncleaved fusion protein. We also confirmed that the fusion protein was cleaved by caspase 8 by Western blotting (Fig. 3). After a 13-h incubation, the level of YFP-Bid-CFP in the control lane (loaded with cell lysate without the addition of caspase 8; lane 1) remained unchanged. In contrast, when the cell lysate was incubated with 1,000 units of caspase 8 (lane 2), YFP-Bid-CFP was cleaved into two fragments with the expected mobilities. These results indicated that the fusion protein was recognized as a substrate and cleaved by caspase 8 in vitro and that FRET was very sensitive to the activity of caspase 8. Thus, YFP-Bid-CFP is a good indicator of Bid cleavage, presumably because of caspase 8 activation in vivo. Therefore, in this report, we state that the indicator is a readout of caspase activation.
Fig 3.
Western blotting analysis, showing the cleavage of YFP-Bid-CFP by caspase 8. Lane 1, control (no caspase 8) and lane 2, extract of COS 7 cells incubated with 1,000 units of caspase 8 for 22 h at 25°C. Extracts were separated by SDS/PAGE on a 10% polyacrylamide gel and subjected to Western blotting with an anti-GFP Ab. The upper arrow indicates YFP-Bid-CFP. The middle arrow indicates C-terminal Bid-CFP-fused protein, and the bottom arrow indicates YFP-N-terminal Bid-fused protein.
Monitoring of Caspase 8 Activity by YFP-Bid-CFP in a Single Cell.
Next, we examined whether the fusion protein would allow us to monitor the activity of caspase 8 in individual cells. We transfected NIH 3T3 cells with a plasmid that encoded YFP-Bid-CFP and induced apoptosis by exposing cells to tumor necrosis factor-α and cycloheximide. These agents disrupt mitochondrial membrane potential in NIH 3T3 cells (23). As shown in Fig. 4, YFP-Bid-CFP yielded diffuse fluorescent images when CFP and YFP were excited (Fig. 4 B and C) in nontreated cells. By contrast, when cell death was induced (Fig. 4 D–G), only CFP fluoresced (Fig. 4 E and F), and fluorescence was localized in mitochondria (Fig. 4G). These results indicated that, despite modifications at both ends, YFP-Bid-CFP was fully functional in vivo and acted as a sensor of activated caspase 8.
Role of Aβ in Alzheimer's Disease.
Using the fusion protein with donor and acceptor moieties, we examined other cell-death systems, focusing on the etiology of Alzheimer's disease, a neurodegenerative disorder characterized by neuritic plaques and neurofibrillary tangles in various areas of the brain (32). Aβ, a peptide of 39–43 aa, is a major constituent of senile plaques. It is an extensively studied toxic fragment of the integral membrane protein known as amyloid precursor protein (32). Aβ induces the death of primary cultured neurons via the activation of c-Jun and the secretion of the Fas ligand, with the apoptotic cascade proceeding in a JNK-dependent manner (27). Specifically, the secreted Fas ligand is believed to activate caspase 8 and induces a cascade of events that leads to cell death. Furthermore, in Alzheimer's disease, there appears to be a strong relationship between stress at the level of the endoplasmic reticulum (ER) and neuronal death (25, 26, 33). Recently, it has been reported that caspase 12 is localized to the ER and activated by ER stress, and that caspase 12-deficient cortical neurons are defective in apoptosis induced by Aβ (25). Therefore, caspase 12 appears to mediate an ER-specific apoptosis pathway and may contribute to Aβ neurotoxicity.
Real-Time Detection of Activation of Bid and Its Subsequent Translocation to Mitochondria.
To examine apoptotic signaling in individual nerve cells by fluorescence microscopy, we investigated the effects of three inducers of apoptosis: Aβ, Tm, which induces ER stress (25, 26), and an anti-Fas Ab, which works as a Fas ligand, on SK-N-SH human neuroblastoma cells. We isolated stable clones that expressed YFP-linker-CFP or YFP-Bid-CFP proteins (Fig. 5A). The cells were then treated with the inducers of apoptosis mentioned above. As shown in Fig. 5B, we observed translocation of Bid to mitochondria only in cells treated with the anti-Fas Ab (arrows). Aβ-treated cells exhibited the standard features of apoptosis, but the localization of fluorescent proteins in most cells (>94%) was reproducibly the same as in nontreated cells (see Table 1 and similar images in Fig. 5C of more cells that were taken independently from those shown in Fig. 5B). These cells rarely exhibited fluorescent proteins localized to mitochondria. Similarly, we could not detect localization of any fusion proteins to mitochondria in tunicamycin-treated cells (Table 1, Fig. 5 B and C Bottom).
Table 1.
The percentage of apoptotic cells that presented mitochondrial localization of cleaved Bid
| Protein
|
Apoptosis inducers | ||
|---|---|---|---|
| Fas | Tm | Aβ | |
| YFP-linker-CFP | 0 | 0 | 0 |
| YFP-Bid-CFP | 94 (±7.0) | 2 (±0.3) | 3 (±2.5) |
SK-N-SH cell lines expressing YFP-linker-CFP or YFP-Bid-CFP were stimulated by each of cell death inducers, anti-Fas antibody (Fas), Tm, or Aβ. The concentration of each inducer is described in Materials and Methods. The number of apoptotic cells was counted after stimulation. We observed >100 dead cells in each case and calculated standard deviation (performing this experiment more than three times).
When SK-N-SH cells were treated with the anti-Fas Ab, a known inducer of caspase 8-dependent apoptosis, the fused Bid protein was cleaved and fluorescence was emitted mainly from the CFP (indicated by arrows in Fig. 5 B and C Top and Table 1), with the concomitant disappearance of FRET. Moreover, the C-terminal region of the fused protein was translocated to mitochondria, as we had expected. Our results indicate that SK-N-SH cells are sensitive to the anti-Fas Ab and that caspase 8 can be activated in these cells via the death-inducing signaling complex. The negative results obtained with tunicamycin support an earlier report that activation of caspase 8 is not necessary for induction of apoptosis by this drug (34).
Evidence Against Significant Involvement of Aβ in the Activation of Caspase 8: Controversy Might Originate from Real-Time Measurements in a Single Cell vs. Measurements in Bulk Cells.
There exists a report that Aβ is a neurotoxic agent that induces secretion of the Fas ligand in primary cultured cells (27). In our system, although the cleavage of YFP-Bid-CFP by caspase 8 could be detected clearly on treatment of cells by Fas ligands, the corresponding activation by Aβ was barely detectable in individual cells when real-time measurements were made by fluorescence microscopy. The controversial data regarding the involvement of Aβ in the activation of caspase 8 might be reconciled by the present analysis if we assume that Aβ does not play a significant role in the cleavage of pro-caspase 8. It is important to note that we observed apoptotic events in single individual cells, whereas detection of the activation of caspase 8 from a small number of apoptotic cells via the death-inducing signaling complex-dependent pathway might have been amplified when bulk cells were used in the past. It is likely, therefore, that in SK-N-SH cells some other major pathway (e.g., ER stress) plays a critical role in Aβ-dependent cell death.
Conclusion
In summary, (i) we have developed a FRET system by using YFP-Bid-CFP, and (ii) results obtained with this system in vitro and in vivo are consistent with a model in which the activation of a mitochondria-based apoptotic pathway leads to apoptosis in NIH 3T3 cells. Finally, (iii) we have demonstrated in individual cells that an anti-Fas Ab could activate caspase 8, whereas Tm and Aβ failed to (appreciably) do so. The present real-time measurements within a single cell, in contrast to the past analyses based on bulk cells, enable us to propose that Aβ does not play a significant role in the cleavage of pro-caspase 8. Therefore, the functionally active YFP-Bid-CFP fusion protein should be a very useful tool for further analysis of various apoptotic pathways in various lines of cultured cells.
Acknowledgments
We thank Prof. Yukiko Gotoh at the University of Tokyo and Dr. Laura Nelson at the National Institute of Advanced Industrial Science and Technology for helpful comments on the manuscript. This research was supported by grants from the Ministry of Economy, Trade and Industry (METI) of Japan, a grant from the New Energy and Industrial Technology Development Organization (NEDO) of Japan, a grant from the Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN) of Japan, and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Culture (MEXT) of Japan.
Abbreviations
BH domain, Bcl-2 homology domain
YFP, yellow fluorescent protein
CFP, cyan fluorescent protein
Aβ, amyloid-β peptide
Tm, tunicamycin
FRET, fluorescence resonance energy transfer
ER, endoplasmic reticulum
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Li H. & Yuan, J. (1999) Curr. Opin. Cell Biol. 11, 261-266. [DOI] [PubMed] [Google Scholar]
- 2.Korsmeyer S. J., Wei, M. C., Saito, M., Weiler, S., Oh, K. & Schlesinger, P. H. (2000) Cell Death Differ. 7, 1166-1173. [DOI] [PubMed] [Google Scholar]
- 3.Wang K., Yin, X. M., Chao, D. T., Milliman, C. L. & Korsmeyer, S. J. (1996) Genes Dev. 10, 2859-2869. [DOI] [PubMed] [Google Scholar]
- 4.Wei M. C., Lindsten, T., Mootha, V. K., Weiler, S., Gross, A., Ashiya, M., Thompson, C. B. & Korsmeyer, S. J. (2000) Genes Dev. 14, 2060-2071. [PMC free article] [PubMed] [Google Scholar]
- 5.Gross A., Yin, X. M., Wang, K., Wei, M. C., Jockel, J., Milliman, C., Erdjument-Bromage, H., Tempst, P. & Korsmeyer, S. J. (1999) J. Biol. Chem. 274, 1156-1163. [DOI] [PubMed] [Google Scholar]
- 6.Cheng E. H., Wei, M. C., Weiler, S., Flavell, R. A., Mak, T. W., Lindsten, T. & Korsmeyer, S. J. (2001) Mol. Cell 8, 705-711. [DOI] [PubMed] [Google Scholar]
- 7.Luo X., Budihardjo, I., Zou, H., Slaughter, C. & Wang, X. (1998) Cell 94, 481-940. [DOI] [PubMed] [Google Scholar]
- 8.Li H., Zhu, H., Xu, C. J. & Yuan, J. (1998) Cell 94, 491-501. [DOI] [PubMed] [Google Scholar]
- 9.Zha J., Weiler, S., Oh, K. J., Wei, M. C. & Korsmeyer, S. J. (2000) Science 290, 1761-1765. [DOI] [PubMed] [Google Scholar]
- 10.Desagher S., Osen-Sand, A., Nichols, A., Eskes, R., Montessuit, S., Lauper, S., Maundrell, K., Antonsson, B. & Martinou, J. C. (1999) J. Cell Biol. 144, 891-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li P., Nijhawan, D., Budihardjo, I., Srinivasula, S. M., Ahmad, M., Alnemri, E. S. & Wang, X. (1997) Cell 91, 479-489. [DOI] [PubMed] [Google Scholar]
- 12.Pollok B. A. & Heim, R. (1999) Trends Cell Biol. 9, 57-60. [DOI] [PubMed] [Google Scholar]
- 13.Sorkin A., McClure, M., Huang, F. & Carter, R. (2000) Curr. Biol. 10, 1395-1398. [DOI] [PubMed] [Google Scholar]
- 14.Harpur A. G., Wouters, F. S. & Bastiaens, P. I. (2001) Nat. Biotechnol. 19, 167-169. [DOI] [PubMed] [Google Scholar]
- 15.Luo K. Q., Yu, V. C., Pu, Y. & Chang, D. C. (2001) Biochem. Biophys. Res. Commun. 283, 1054-1060. [DOI] [PubMed] [Google Scholar]
- 16.Uchiyama H., Hirano, K., Kashiwasake-Jibu, M. & Taira, K. (1996) J. Biol. Chem. 271, 380-384. [DOI] [PubMed] [Google Scholar]
- 17.Xu X., Gerard, A. L., Huang, B. C., Anderson, D. C., Payan, D. & Luo, Y. (1998) Nucleic Acids Res. 26, 2034-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ota N., Hirano, K., Warashina, M., Andrus, A., Mullah, B., Hatanaka, K. & Taira, K. (1998) Nucleic Acids Res. 26, 735-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu L., Chahroudi, A., Silvestri, G., Wernett, M. E., Kaiser, W. J., Safrit, J. T., Komoriya, A., Altman, J. D., Packard, B. Z. & Feinberg, M. B. (2002) Nat. Med. 8, 185-189. [DOI] [PubMed] [Google Scholar]
- 20.Kawasaki H., Onuki, R., Suyama, E. & Taira, K. (2002) Nat. Biotechnol. 20, 376-380. [DOI] [PubMed] [Google Scholar]
- 21.Kawasaki H. & Taira, K. (2002) EMBO Rep. 3, 443-450. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Kawasaki H. & Taira, K. (2002) Nucleic Acids Res. 30, 3609-3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khwaja A. & Tatton, L. (1999) J. Biol. Chem. 274, 36817-36823. [DOI] [PubMed] [Google Scholar]
- 24.Galvan V. & Roizman, B. (1998) Proc. Natl. Acad. Sci. USA 95, 3931-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakagawa T., Zhu, H., Morishima, N., Li, E., Xu, J., Yankner, B. A. & Yuan, J. (2000) Nature 403, 98-103. [DOI] [PubMed] [Google Scholar]
- 26.Imaizumi K., Miyoshi, K., Katayama, T., Yoneda, T., Taniguchi, M., Kudo, T. & Tohyama, M. (2001) Biochim. Biophys. Acta 1536, 85-96. [DOI] [PubMed] [Google Scholar]
- 27.Morishima Y., Gotoh, Y., Zieg, J., Barrett, T., Takano, H., Flavell, R., Davis, R. J., Shirasaki, Y. & Greenberg, M. E. (2001) J. Neurosci. 21, 7551-7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Estus S., Tucker, H. M., van Rooyen, C., Wright, S., Brigham, E. F., Wogulis, M. & Rydel, R. E. (1997) J. Neurosci. 17, 7736-7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imaizumi K., Morihara, T., Mori, Y., Katayama, T., Tsuda, M., Furuyama, T., Wanaka, A., Takeda, M. & Tohyama, M. (1999) J. Biol. Chem. 274, 7975-7981. [DOI] [PubMed] [Google Scholar]
- 30.Chou J. J., Li, H., Salvesen, G. S., Yuan, J. & Wagner, G. (1999) Cell 96, 615-624. [DOI] [PubMed] [Google Scholar]
- 31.McDonnell J. M., Fushman, D., Milliman, C. L., Korsmeyer, S. J. & Cowburn, D. (1999) Cell 96, 625-634. [DOI] [PubMed] [Google Scholar]
- 32.Yuan J. & Yankner, B. A. (2000) Nature 407, 802-809. [DOI] [PubMed] [Google Scholar]
- 33.Nakagawa T. & Yuan, J. (2000) J. Cell Biol. 150, 887-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bitko V. & Barik, S. (2001) J. Cell Biochem. 80, 441-454. [DOI] [PubMed] [Google Scholar]