Abstract
IL-6 is a multifunctional cytokine involved in regulation of differentiation, antibody production, and growth of certain types of tumor cells. Its excessive production plays a major role in pathogenesis of multiple myeloma and postmenopausal osteoporosis. In the course of a screening program aimed at IL-6 inhibitor from microbial products, we found madindoline A (MDL-A) and madindoline B, which have a fuloindoline structure with diketocyclopentene bound to the methyl group. MDL-A has no cytotoxic activities. It inhibited only activities of both IL-6 and IL-11 without affecting the IL-6-specific signal transduction cascade, JAK2/STAT3. In a dose-dependent manner [3H]MDL-A binds to gp130, which is a signal transducing 130-kDa glycoprotein, but formation of the trimeric complex IL-6/IL-6 receptor/gp130 was not inhibited, suggesting that MDL-A suppresses dimerization of trimeric complexes. Not only did MDL-A markedly inhibit IL-6- and IL-11-induced osteoclastogenesis in vitro, but it also inhibited IL-6-stimulated serum amyloid A production and bone resorption in an experimental model of postmenopausal osteoporosis in vivo by a different mechanism from that of 17β-estradiol. Here we show that MDL-A has a highly selective inhibitory effect on IL-6 and IL-11 activities by inhibiting a gp130 activity while suppressing bone loss in ovariectomized mice. MDL-A is anticipated as a lead compound for treatment of hormone-dependent postmenopausal osteoporosis, which has no serious side effects, and as a new mechanism of action, gp130 blocking.
It is well known that cytokines not only contribute to homeostasis via immune responses and biological defense, but they also are involved in cancer, inflammation, allergies, and autoimmune diseases (1). IL-6 is a multifunctional cytokine involved in control of antibody production, T cell activation, hematopoiesis and acute responses, and uncontrolled IL-6 activity causing various serious diseases. It has been reported that excess IL-6 production is closely associated with cancer cachexia (2), Castleman's disease (3), rheumatoid arthritis (4), hypercalcemia (5), and multiple myeloma (6). No effective therapeutic drugs for these diseases have been developed, but a low molecular weight compound should be developed for therapeutic use that modulates function of this cytokine by a new action mechanism. The IL-6 receptor (IL-6R) system consists of two components: a ligand-binding 80-kDa glycoprotein chain (IL-6R) and a signal-transducing glycoprotein 130 (gp130). IL-6 induces gp130 homodimerization after binding to the specific receptor, which leads to activation of the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signal transduction pathway (7). Accordingly, substances that inhibit this cascade can be used as an IL-6-specific inhibitor.
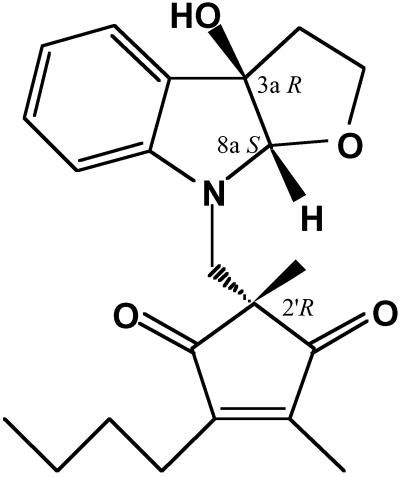
We have found some useful active substances among microbial metabolites such as macrosphelides (8, 9) and cell adhesion inhibitors. Investigation of IL-6 inhibitors uncovered compounds having a fuloindoline structure with diketocyclopentene bound to the methyl group (10) named madindoline A (MDL-A) and madindoline B; they were stereoisomers at the C2′ position (11) (Fig. 1). The IC50 values of MDL-A against IL-6 activity are 5-fold less than those of madindoline B. In this study, we examined effects of MDL-A for various cytokines' activity and discovered that MDL-A specifically acted for cytokines of gp130 homodimer types such as IL-6 and IL-11. Furthermore, having investigated its action for receptor and signal transduction cascade, we analyzed the mechanism of action and estimated gp130 dimerization antagonism by MDL-A. This compound is a specific low-molecular and nonpeptide inhibitor for IL-6 and IL-11 activities. Inhibition of bone resorption by blocking gp130 is a mechanism of action that may yield a significant area of study in the field of bone metabolism in the future.
Fig 1.
Structures of MDL-A.
Materials and Methods
Animals and Drugs.
Four-week-old female ddY mice, 6-week-old male C3H/HeJ mice, and 6-week-old C3H/HeN mice were purchased from Charles River Breeding Laboratories. Recombinant human IL-2 (rhIL-2), recombinant murine IL-3 (rmIL-3), rhIL-4, rhIL-8, recombinant murine tumor necrosis factor (TNF) α, recombinant human soluble IL-6R, and rhIL-11 were purchased from Sigma. rhIL-6, recombinant human nerve growth factor, 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3] (carcitriol), and leukemia inhibitory factor (LIF) were purchased from Upstate Biotechnology (Lake Placid, NY), Toyo Soda (Tokyo), Wako Biochemicals (Osaka), and Upstate Biotechnology, respectively. MH-60 cells and chimera receptor Baf3/granulocyte colony-stimulating factor receptor (GCSFR)-gp130 cells were kindly supplied by T. Hirano, Osaka University, Osaka. CTLL-2 cells were gifts from M. Ishizuka, Microbial Institute, Numadzu, Japan. Baf3 cells, L929 cells, U937 cells, and PC-12 cells were kindly provided by the Japanese Cancer Research Resources Bank, Tokyo.
Cytokine Specificity.
CTLL-2 cells, Baf3 cells, and MH-60 cells were maintained in suspension in RPMI 1640 medium supplemented with 10% FCS containing 1 ng/ml rmIL-2, 1 ng/ml rmIL-3, and 0.5 ng/ml rhIL-6, respectively. To estimate selectivity of MDL-A, either CTLL-2 cells, Baf3 cells, MH60 cells, or L929 cells (0.2–0.5 × 104 cells) were incubated with MDL-A (0.5–70 μM) at 37°C in 5% CO2 in the presence of 10 ng/ml rhIL-2, 5 ng/ml rhIL-3, 2 ng/ml rhIL-6 or 2 units/ml recombinant human TNF-α, respectively. After 72 h of incubation, cell growth was measured by the tetrazolium salt [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide, MTT] method (12).
Chimera Receptor Assay.
Chimera receptor Baf3/GCSFR-gp130 cells were maintained in suspension in RPMI 1640 medium supplemented with 10% FCS containing 10 ng/ml GCSF at 37°C in 5% CO2. In the presence of 50 ng/ml GCSF, Baf3/GCSFR-gp130 cells (1 × 104 cells/200 μl per well) were incubated with some agents. After 72 h of incubation, cell growth was measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) method.
Differentiation of M1 Cells to Macrophage-Like Cells.
M1 cells (5 × 105 cells/250 μl per well) were seeded and incubated in the presence of 50 ng/ml IL-6 or 5 ng/ml LIF at 37°C in 5% CO2 for 72 h. Then, 1 × 109 opsonized fluorescent beads were added, and cells were incubated at 37°C in 5% CO2 for 24 h. Nonreacted beads were washed out and phagocytosis was measured by a flow cytometer (EPICS ELITE, Beckman Coulter) equipped with a 488-nm argon laser.
Preparation of [3H]-(+)-MDL-A.
At room temperature, [3H]2O (37 GBq/g, 0.5 ml) was added to a solution of (+)-MDL-A (4.2 mg, 0.011 mmol) in t-BuOH (0.3 ml). Then, t-BuOK (45 mg, 0.40 mmol) was added. The solution was stirred for 20 h at room temperature and the reaction was stopped with saturated aqueous NH4Cl (5.0 ml). The mixture was then extracted with CHCl3 (3 × 7 ml) and combined extracts were dried over Na2SO4, filtered, and concentrated. Further purification of [3H]-(+)-MDL-A mixture was conducted by using an HPLC (PEGASIL-B ODS 20φ × 250-mm column; mobile phase, 50% CH3CN/H2O; flow rate, 9.0 ml/min; detection, UV at 210 nm). Under these conditions, [3H]-(+)-MDL-A (4.2 mg, 442.8 MBq/mmol) was obtained as light-yellow crystals.
Immunoprecipitation and Autoradiography.
U266 cells (1 × 107 cells/ml) were incubated with 1 μg/ml rhIL-6 in the presence or absence of MDL-A (30 and 100 μM, respectively) for 15 min at 37°C. Cells were then lysed in 0.2 ml of digitonin extraction buffer [containing 1% digitonin, 10 mM triethanolamine (pH 7.8), 0.15 M NaCl, 10 mM iodoacetamide, and 1 mM PMSF] at 4°C. The IL-6-reacted complexes were precipitated by rabbit anti-hIL-6R antibody (Santa Cruz Biotechnology) and protein A-Sepharose. Immunoprecipitated samples were divided into two groups for Western blot and autoradiography. After washing four times with digitonin lysis buffer, for Western blot, samples were boiled for 5 min with Laemmli's sample buffer and electrophoresed on SDS/PAGE on 5–20% gradient gels and transferred to poly(vinylidene difluoride) membrane (Biocraft Laboratories, Tokyo). After blocking with Tris-buffered saline [TBS, 20 mM Tris (pH 7.6), 150 mM NaCl] containing 3% skim milk overnight, membranes were incubated with rabbit anti-hIL-6R antibody or rabbit anti-gp130 antibody (Upstate Biotechnology) and washed in TBS containing 0.05% Tween 20 (TBS-T). After incubation with goat anti-rabbit IgG, secondary antibodies coupled to alkaline phosphatase, membranes were washed five times with TBS-T and subjected to an amplified Immun Blot kit (Bio-Rad). For autoradiography, 10 μl of each sample was electrophoresed and transferred to poly(vinylidene difluoride) membrane as described above. The lanes of membrane were incubated in TBS containing 1% BSA and 1.2 kBq [3H]MDL-A for 10 min at 37°C. Membranes were washed six times with TBS containing 3% BSA and treated with EN3HANCE (NEN) according to the manufacturer's instructions. After drying, membranes were exposed to Kodak SB film.
In another experiment, U266 cells (1 × 107 cells per ml) were lysed by digitonin extraction buffer and precipitated by rabbit anti-gp130 antibody and protein A-Sepharose. Immunoprecipitated samples were divided into two groups for Western blot and autoradiography. After electrophoresing as previously noted, the transferred membrane was incubated with anti-gp130 antibody. After incubation with goat anti-rabbit IgG secondary antibodies coupled to alkaline phosphatase, membranes were washed five times with TBS-T and subjected to an amplified Immun Blot kit. For autoradiography, 10 μl of each sample was electrophoresed and transferred as described above. The lanes of the membrane were cut, and it was incubated in TBS containing 1% BSA and 1.2 kBq [3H]MDL-A in the presence or absence of unlabeled MDL-A for 10 min at 37°C. The lanes were washed six times with TBS containing 3% BSA, treated with EN3HANCE (NEN) according to the manufacturer's instructions, dried, and then exposed to Kodak SB film.
Detection of Phosphorylated STAT3.
HepG2 cells were incubated at 1 × 107 cells/ml with 1 μg/ml rhIL-6 or 1 μg/ml recombinant human LIF for 15 min at 37°C in the presence or absence of 100 μM of MDL-A, respectively. Cells were then harvested by centrifugation and lysed in ice-cold lysis buffer (1% Triton X-100/10% glycerol/150 mM NaCl/20 mM Tris⋅HCl, pH 7.4/5 mM EDTA/10 mM NaF/1 mM sodium orthovanadate/100 units/ml aprotinin/10 mM iodoacetoamide/25 μg/ml p-nitrophenyl-p′-guanidinobenzoate). Unsolubilized materials were removed by centrifugation for 15 min at 12,000 × g. The reacted complexes were precipitated by rabbit anti-STAT3 (H-190) antibody (Santa Cruz Biotechnology) and protein A-Sepharose. For the Western blot, 10 μl of samples was boiled for 5 min, electrophoresed on SDS/PAGE on 10–15% gradient gels, and transferred to an Immobilon-P membrane (Nihon Millipore, Tokyo). After blocking with TBS containing 5% BSA, the membranes were incubated with the goat antiphosphorylated STAT3 (Tyr-705) antibody (Santa Cruz Biotechnology) or the rabbit anti-STAT3 (H-190) antibody. After incubation with rabbit anti-goat IgG secondary antibodies coupled to horseradish peroxidase (Chemicon) or goat anti-rabbit IgG secondary antibodies coupled to alkaline phosphatase (Bio-Rad), the membranes were washed five times with TBS-T and subjected to an ECL detection system (Amersham Pharmacia) or an amplified Immun Blot kit, respectively.
Coculture of Osteoblastic Cells and Bone Marrow Cells.
Coculture of mouse calvaria cells (osteoblastic cells) and bone marrow cells was performed by the method of Atkins et al. (13) using ddY mice. Briefly, primary osteoblast-like cells (1 × 104 per well) obtained from mouse calvaria and nucleated bone marrow cells (2 × 105 per well) were cocultured in a 48-well plate with 0.4 ml of α-MEM containing 10% FCS with various cytokines. Cultures were performed in duplicate over 4 days. To determine the number of osteoclasts formed, cells were fixed and stained with tartrate-resistant acid phosphatase (TRAP) and TRAP-positive osteoclasts were counted.
IL-6-Induced Serum Amyloid A (SAA) Production in Mice.
One microgram of rhIL-6 was i.p.-injected into lipopolysaccharide (LPS)-insensitive C3H/HeJ mice; 5 ng of LPS (Escherichia coli) was also i.p.-injected into LPS-sensitive C3H/HeN mice. Mice were bled 6 h after injection. SAA level was measured with an EIA Kit (BioSource International, Camarillo, CA).
Bone Resorption in Ovariectomized (OVX) Mice.
In 4-week-old female ddY mice, dorsal skin was incised and ovaries were excised under light ether anesthesia. The sham operation group and ovariectomy group were given 5% ethanol (vehicle). OVX mice were i.p.-administered 5 μg/kg 17β-estradiol, orally with 10 mg/kg MDL-A, and orally with 60 mg/kg MDL-A every other day for 4 weeks, respectively. After 4 weeks, mice were killed, and bone mass, serum Ca2+ level, uterine weight, and serum IL-6 level were measured. Bone mass was expressed as the ratio of femoral bone weight to body weight. Serum calcium and IL-6 levels were measured with a CPT Kit (Wako Biochemicals) and ELISA (BioSource International), respectively.
All animal experiments were performed in accordance with the guidelines for the welfare of animals in experiments in The Kitasato Institute.
Action Mode Analysis.
Inhibitory mode of MDL-A for IL-6 activity was statistically analyzed by Schild plot analysis. EC50 in the dose–response curve of A alone is shown as [Ao]; EC50 in the dose–response curve of A with coexisting B is depicted as [Ax], and concentration of existing B is [Bx]. The dissociation constant of B and the receptor combined is KB. The formula for these relations can be expressed as log([Ax]/[Ao] − 1) = log[Bx] − logKB. If [Ax]/[Ao] is expressed as DR (drug ratio), using this formula, we plotted log[DR − 1] on the y axis against log[Bx] on the x axis.
Statistical Analysis.
Statistical significance of differences between the control and the experimental group was determined by Student's t test.
Results
Selectivity of MDL-A for Various Cytokines and Mode of Action.
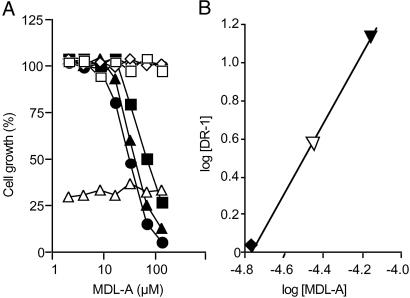
Before examination of MDL-A, suitable concentrations of various cytokines were examined. Maximum cell growth of CTLL-2 cells, Baf3 cells, and IL-6-dependent MH-60 cells was induced by 20 ng/ml rhIL-2, 5 ng/ml rmIL-3, or 2 ng/ml rhIL-6, respectively. Specificity of MDL-A for various cytokines and growth factors was investigated (Fig. 2A). Treatment with MDL-A did not change either growth of IL-2-dependent CTLL-2 cells or growth of IL-3-dependent Baf3. In addition, MDL-A did not inhibit TNF-α activity, which suppresses cell growth by 20–30% on mouse fibrosarcoma L929 cells. However, MDL-A dose-dependently suppressed IL-6-induced cell growth of IL-6-dependent MH 60 cells and caused parallel rightward shift of dose–response curves to IL-6 at concentrations of 2–8 ng/ml. In contrast, MDL-A showed no growth inhibition of IL-6-independent MH-60 cells. Parallel rightward shift of the dose–response curve to IL-6 by the addition of MDL-A indicates the competitive inhibitory mode of MDL-A to the receptor binding site. As shown in Fig. 2B, Schild plot analysis data yielded a pA2 value of 4.78 and a slope (r) of 0.99. The slope was significantly different from unity. These data clearly indicate that MDL-A is a competitive and selective antagonist in IL-6 responses, accordingly the Schild plot slope is approximately one.
Fig 2.
Selectivity of MDL-A for various cytokines and mode of action. (A) IL-2-dependent CTLL-2 cells, IL-3-dependent Baf3 cells, or TNF-sensitive L929 cells were incubated with MDL-A in the presence of rhIL-2 (□, 20 ng/ml), rmIL-3 (◊, 5 ng/ml), or TNF (▵, 2 units/ml), respectively. Similarly, IL-6-dependent MH60 cells were incubated in the presence of rhIL-6 (•, 2 ng/ml; ▴, 4 ng/ml; ▪, 8 ng/ml). IL-6-independent MH60 cells were incubated in the absence of IL-6 (○). (B) MH60 cells were incubated with various doses of rhIL-6 in the presence of MDL-A (⧫, 17.5 μM; ▿, 35 μM; ▾, 70 μM). Schild plot analysis data yielded a pA2 value of 4.78 and a slope (r) of 0.99.
Effect of MDL-A on JAK/STAT Signal Transduction of Baf3/GCSFR-gp130 Cells.
We investigated the effect of MDL-A on IL-6-specific signal transduction, JAK2/STAT3, using chimera receptor Baf3/GCSFR-gp130 cells (Table 1). GCSF-dependent cell growth was completely inhibited by pretreatment with 3 μg/ml anti-GCSFR mAb and 10 μg/ml tyrphostine AG490, a JAK2 inhibitor (14). These findings confirm that this experimental system responded to GCSF via JAK2/STAT3 signal transduction. Addition of 70 μM MDL-A did not inhibit cell growth induced by 50 ng/ml GCSF, indicating that MDL-A does not act on GCSFR or the subsequent signal transduction cascade, including activation of JAK2/STAT3, phosphorylation, and transcription processes in the intracellular domain.
Table 1.
Effect of MDL-A on JAK/STAT signal transduction of Baf/GCSFR-gp130 cells
| Agents | Growth, % |
|---|---|
| Untreated | 15.2 ± 0.5 |
| GCSF, 50 ng/ml | 100.0 |
| + MDL-A, 70 μM | 108.6 ± 6.9 |
| + GCSFR antibody, 3 μg/ml | 18.9 ± 0.2 |
| + Tyrphostin AG490, 10 μg/ml | 13.5 ± 1.8 |
Chimera receptor Baf3/GCSFR-gp130 cells (1 × 104 cells/200 μl per well) were incubated for 72 h with or without 70 μM MDL-A, 3 μg/ml anti-GCSFR antibody, or 10 μg/ml tyrophostin AG-490 in the presence of 50 ng/ml GCSF. Cell growth in presence of 50 ng/ml GCSF was taken as 100%. Values represent mean ± SE of three experiments. Statistical significance:
, P < 0.05 vs. GCSF as control.
Effect of MDL-A on IL-6- or LIF-Induced Differentiation of M1 Cells to Macrophage-Like Cells.
The effect of MDL-A on IL-6-type cytokines that share gp130 as common receptor subunit was investigated on M1 cells. As shown in Table 2, the rate of M1 cell differentiation to macrophage-like cells in the presence of 50 ng/ml IL-6 was significantly decreased to 30.4 ± 4.2% by pretreatment with 70 μM MDL-A. However, the differentiation rate caused by stimulation with 5 ng/ml LIF was not inhibited by the same concentration of MDL-A.
Table 2.
Effect of MDL-A on IL-6 or LIF-induced differentiation of M1 cells on macrophage-like cells
| Treatment
|
Population, % | |
|---|---|---|
| Nondifferentiated | Differentiated | |
| Untreated | 85.4 ± 4.6 | 11.3 ± 0.2 |
| MDL-A, 70 μM | 83.4 ± 7.7 | 15.1 ± 1.4 |
| IL-6, 50 ng/ml | 37.4 ± 5.3 | 61.4 ± 10.7 |
| + MDL-A, 70 μM | 68.6 ± 5.1 | 30.4 ± 4.2 |
| LIF (5 ng/ml) | 36.8 ± 7.1 | 63.2 ± 11.2 |
| + MDL-A, 70 μM | 40.7 ± 5.4 | 58.3 ± 9.3 |
M1 cells (5 × 105 cell/250 μl per well) were seeded and incubated with or without 70 μM MDL-A in the presence or absence of 50 ng/ml rhIL-6 or 5 ng/ml rhLIF for 72 h. Values represent mean ± SE of three experiments. Statistical significance:
, P < 0.01 vs. corresponding control.
Immunoblotting and Autoradiography of gp130 and IL-6R.
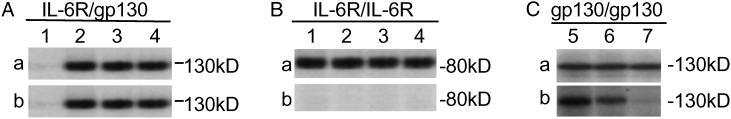
To investigate the molecular target of MDL-A on receptor subunits, IL-6R-expressing U266 cells were used in this experiment. Cell lysates were immunoprecipitated with anti-IL-6R antibody, electrophoresed, and blotted with anti-gp130 antibody. The gp130 band was observed in both MDL-A-treated cells (Fig. 3Aa, lanes 3 and 4) and MDL-A-untreated cells (Fig. 3Aa, lane 2) of cells stimulated with IL-6 (Fig. 3Aa) and the band intensities did not change by addition of an unlabeled MDL-A (Fig. 3Aa, lanes 3 and 4). In addition, clear bands were detected at the position corresponding to gp130 on autoradiography of [3H]MDL-A in Fig. 3Ab, lanes 3 and 4, showing that MDL-A does not inhibit formation of the IL-6/IL-6R/gp130 trimeric complex. By contrast, no band was observed at the position corresponding to IL-6R in autoradiography (Fig. 3Bb). Binding of MDL-A to gp130 was further examined by immunoprecipitation and blot with anti-gp130 antibody. The gp130 band was observed in Fig. 3Ca, lanes 5–7. In autoradiography, band intensity (Fig. 3Ca, lane 5) observed by incubation with [3H]MDL-A was dose-dependently decreased by incubation with an excess amount of unlabeled MDL-A (Fig. 3Cb, lanes 6 and 7). These findings demonstrate that MDL-A competitively binds to gp130, but does not inhibit formation of the IL-6/IL-6R/gp130 complex.
Fig 3.
Immunoblotting and autoradiography of gp130 and IL-6R. U266 cells (1 × 107 cells) were stimulated with or without 1 μg/ml rhIL-6 for 15 min in the presence or absence of MDL-A (30 or 100 μM, respectively). Cell lysates were immunoprecipitated with anti-IL-6R antibody, electrophoresed, and then blotted with anti-gp130 antibody (A) or anti-IL-6R antibody (B) for Western blot. In another experiment, cell lysate was also immunoprecipitated with anti-gp130 antibody, electrophoresed, and then blotted with anti-gp130 antibody (C) for Western blot. (A and B) Lanes 1–4 were incubated with 1.2 kBq [3H]MDL-A for autoradiography. (C) Lanes 5–7 were cut and incubated with 1.2 kBq [3H]MDL-A in the absence (lane 5) or presence of unlabeled MDL-A (30 μM, lane 6 or 100 μM, lane 7). a, Immunoblotting bands. b, Autoradiography in each group. Lane 1, no treatment; lane 2, 1 μg/ml rhIL-6; lane 3, 1 μg/ml rhIL-6 + 30 μM MDL-A; lane 4, 1 μg/ml rhIL-6 + 100 μM MDL-A.
Phosphorylation of STAT3.
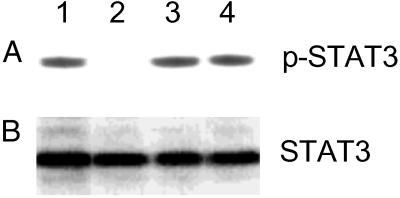
We also examined the effect of MDL-A on tyrosine phosphorylation of STAT3 in HepG2 cells (Fig. 4). LIF-stimulated tyrosine phosphorylation of STAT3 was not suppressed by treatment with 100 μM MDL-A (Fig. 4, lanes 3 and 4). By contrast, a marked reduction in intensity of the phosphorylated band in IL-6 stimulation was observed by treatment with 100 μM MDL-A (Fig. 4, lanes 1 and 2).
Fig 4.
Phosphorylation of STAT3. HepG2 cells (1 × 107 cells/ml) were incubated with 1 μg/ml rhIL-6 or 1 μg/ml recombinant human LIF for 15 min in the presence or absence of 100 μM MDL-A, and then lysed. After immunoprecipitation by anti-STAT3 antibody, samples were subjected to Western blot using anti-phosphorylated STAT3 (p-STAT3) antibody (A) or anti-STAT3 antibody (B). Lane 1, 1 μg/ml rhIL-6 (positive control); lane 2, 1 μg/ml rhIL-6 + 100 μM MDL-A; lane 3, 1 μg/ml recombinant human LIF; lane 4, 1 μg/ml recombinant human LIF + 100 μM MDL-A.
Effect of MDL-A on Osteoclast Formation in Vitro.
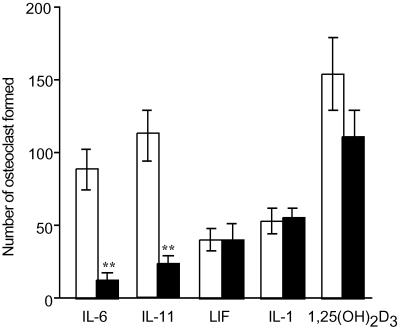
We investigated efficacy of MDL-A on osteoclast formation and gp130 dimerization by IL-6-type cytokines (Fig. 5). In coculture of mouse carvalia osteoblast cells and bone marrow cells, IL-6, IL-11, LIF, IL-1, and 1α,25(OH)2D3 (as positive control) were induced in marked tartrate-resistant acid phosphatase- positive multinuclear cells (osteoclasts). Treatment with MDL-A did not affect LIF-induced (heterodimer type of gp130), IL-1-induced (cAMP-mediated type), or 1α,25(OH)2D3-induced osteoclast formation. By contrast, IL-6- and IL-11- (homodimer types of gp130)-induced osteoclast formation was significantly suppressed by MDL-A at 30 μM.
Fig 5.
Effect of MDL-A on osteoclast formation in vitro. Osteoblast-like cells (1 × 104 per well) obtained from mouse calvaria and nucleated bone marrow cells (2 × 105 per well) were cocultured in a 48-well plate with 0.4 ml of α-MEM containing 10% FCS with 20 ng/ml rhIL-6 (containing 50 ng/ml rhIL-6R), 10 ng/ml rhIL-11, 100 ng/ml recombinant human LIF, 50 ng/ml rhIL-1, or 100 nM 1α,25(OH)2D3 in the presence (filled columns) or absence (open columns) of 30 μM MDL-A. Cultures were performed in duplicate over 4 days. Data are expressed as mean ± SEM (n = 3–4). Statistical significance: **, P < 0.01 vs. corresponding control.
Efffect of MDL-A on IL-6-Induced SAA Production in Mice.
Furthermore, we examined in vivo the effect of MDL-A on the secretion of serum amyloid (Table 3), which is a mouse acute-phase protein synthesized by IL-6 in inflammation. SAA increase occurring after i.p. injection of IL-6 (1 μg per mouse) in LPS-insensitive C3H/HeJ mice was inhibited by MDL-A in a dose-dependent manner; but MDL-A did not suppress an increase in SAA stimulated by LPS (E. coli, 5 ng per mouse) in LPS-sensitive C3H/HeN mice, confirming that MDL-A selectively inhibits action of IL-6 in vivo.
Table 3.
Effect of MDL-A on IL-6-induced SAA in mice
| Mouse | Treatment | SAA, ng/ml |
|---|---|---|
| C3H/HeJ | Saline | 4.5 ± 0.7 |
| IL-6, 1 μg/mouse | 49.1 ± 4.1 | |
| + MDL-A, 10 mg/kg | 34.7 ± 3.1 | |
| + MDL-A, 60 mg/kg | 16.3 ± 2.3 | |
| C3H/HeN | Saline | 26.2 ± 4.7 |
| LPS, 5 ng/mouse | 538.7 ± 77.1 | |
| + MDL-A, 10 mg/kg | 637.7 ± 64.6 | |
| + MDL-A, 60 mg/kg | 785.3 ± 91.5 |
One microgram of rhIL-6 was i.p. injected into LPS-insensitive C3H/HeJ mice. Five nanograms of LPS (E. coli) was also i.p. injected into LPS-sensitive C3H/HeN mice. MDL-A (10 or 60 mg/kg) was orally administrated 1 h before injection of rhIL-6. Mice were bled 6 h after injection. Statistical significance:
, P < 0.05;
, P < 0.01 vs. IL-6 as control (n = 7).
Effect of MDL-A on Bone Resorption in OVX Mice.
We further investigated the effect of MDL-A on bone resorption by using OVX mice, an experimental model of postmenopausal osteoporosis in vivo. As noted in Table 4, oral administration of MDL-A at 60 mg/kg per day significantly suppressed decrease in bone mass and increase in serum Ca2+ level after ovariectomy; it was comparable to that obtained by positive control 17β-estradiol. By contrast, 17β-estradiol suppressed decrease in uterus and increase in serum IL-6 level, whereas MDL-A did not change them. These findings clearly indicate that MDL-A inhibits bone resorption by a mechanism that is completely different from that of 17β-estradiol.
Table 4.
Effect of MDL-A on bone resorption in OVX mice
| Treatment | Bone ratio | Ca2+, mg/dl | Uterus, mg | IL-6, pg/ml |
|---|---|---|---|---|
| Sham operation (normal) | 2.09 ± 0.25 | 10.4 ± 1.2 | 182.3 ± 47.2 | 26.7 ± 7.4 |
| Ovariectomy (control) | 1.42 ± 0.32 | 15.4 ± 2.4 | 38.3 ± 7.6 | 289.4 ± 97.6 |
| + 17β-estradiol, 5 μg/kg, ip | 1.96 ± 0.25 | 12.5 ± 2.1 | 223.1 ± 67.8 | 62.5 ± 10.4 |
| + MDL-A, 10 mg/kg, po | 1.56 ± 0.25 | 12.7 ± 3.4 | 28.4 ± 1.3 | 402.5 ± 104.2 |
| + MDL-A, 60 mg/kg, po | 1.92 ± 0.22 | 11.3 ± 1.1 | 32.7 ± 5.4 | 418.5 ± 96.9 |
MDL-A or 17β-estradiol was orally or i.p. administered, respectively, to OVX female mice every other day for 4 weeks. Bone mass was expressed as the ratio of femoral bone weight to body weight.
, P < 0.05;
, P < 0.01 compared with ovariectomy (control) group (n = 5), respectively.
Discussion
We confirmed the possibility of controlling activity and production of IL-6 selectively and in low concentration by development of high molecular bio-compounds such as various IL-6 variants (15–17) or humanized antibody (18, 19). Consequently, for clinical application, development of a low molecular antagonist is anticipated because of superiority in oral absorbency, antigenicity, and so forth.
A shared signal-transducing receptor subunit can be recruited by different cytokines and, depending on the ligand, activated via homodimerization or heterodimerization with other cytokine receptors. Three shared-receptor subunits have been cloned so far: IL-2-type cytokines (the γ-chain commonly used by IL-2, IL-4, IL-7, IL-9, and IL-15); IL-3-type cytokines (the β-chain common to IL-3, IL-5, and granulocyte-macrophage colony-stimulating factor); and IL-6-type cytokines (gp130, which is shared by IL-6, IL-11, LIF, ciliary neurotrophic factor, oncostatin M, and cardiotrophin). In our experiments, MDL-A did not suppress cell proliferation, differentiation, expression, or chemotaxis of any tested cytokines and growth factors except IL-6. Our results clearly indicate that MDL-A does not affect either the γ-chain common to IL-2-type cytokines or the β-chain common to IL-3-type cytokines; also, MDL-A is a small molecule showing highly selective inhibitory and noncytotoxic activities.
Receptors of IL-6-type cytokines sharing gp130 as a common subunit are divided distinctly into two types: homodimerization and heterodimerization. IL-6 (20) and IL-11 (21) require homodimerization of gp130, and LIF (22), oncostatin M (22), cardiotrophin (23), and ciliary neurotrophic factor (24) require heterodimerization of a specific cytokine receptor and gp130. Therefore, the mechanism of action of MDL-A can be predicted from differential effects of MDL-A on these two types of IL-6-type cytokines. In studies of osteoclastogenesis (Fig. 5) and differentiation to macrophage-like cells (Table 2) by IL-6 or LIF, MDL-A inhibited only IL-6-induced activities, but not LIF. IL-6 and LIF are IL-6-type cytokines sharing gp130 as the common subunit, but LIF forms a heterodimer consisting of LIF receptor and gp130 (25), whereas IL-6 forms a homodimer of gp130 during signal transduction (20). These findings suggest that MDL-A inhibits IL-6 action by inhibiting gp130 homodimer formation. Therefore, control of gp130 is expected to be more effective than blockage of cytokine receptors for developing a useful drug.
IL-6 has three topological binding sites (sites I, II, and III) (26–28), whereas gp130 has two binding sites (sites 1 and 2) (29, 30). IL-6 binds to IL-6R via its site I and then to gp130 site 2 (the first gp130) via site II, forming a trimeric IL-6/IL-6R/gp130 complex (26). The trimeric complex then induces homodimerization of gp130 and forms a hexameric complex, activating the JAK/STAT signal transduction cascade (27). It was proposed recently that the IL-6 site III role, which was previously unknown, is to bind to gp130 site 2 (the second gp130) in a trimeric complex and contribute to stabilization of the hexameric receptor complex (28). Our finding that MDL-A binds to gp130 and inhibits actions of IL-6 without inhibiting formation of trimeric complex is consistent with and supports this hypothesis. Therefore, it seems most likely that the MDL-A mechanism of action involves binding to gp130 site 2, which is the site for IL-6 site III, and inhibiting gp130 homodimerization, resulting in inhibition of IL-6 and IL-11 activities. Although site III of IL-6, IL-11, and LIF is hypothesized to bind to the Ig-like domain of gp130 (31), the precise mechanism has not been elucidated.
We also examined the effect of MDL-A on IL-6- and LIF-induced tyrosine phosphorylation of STAT3 in HepG2 cells. A marked reduction in intensity of the band phosphorylated by IL-6, but not by LIF, was observed by treatment with 100 μM MDL-A in this study. Furthermore, MDL-A did not affect JAK2/STAT3 signal transduction in experiments using Baf/GCSFR-gp130 chimera cells. These results clearly indicate that MDL-A suppresses the IL-6-induced tyrosine phosphorylation of STAT3 by inhibiting the activation of gp130.
Our studies showed that MDL-A dose-dependently suppressed osteoclastogenesis induced by both IL-6 and IL-11 in vitro. Papadopoulos et al. (32) and Lin et al. (33) reported that IL-6 and IL-6R increase because of hormone imbalance after menopause and induce osteoporosis. Thus, we investigated the effect of MDL-A on bone loss by using OVX mice, an experimental model of postmenopausal osteoporosis in vivo. Oral administration of MDL-A at 60 mg/kg per day significantly suppressed decrease in bone mass and increase in serum Ca2+ level after ovariectomy; the effect was comparable to that obtained by positive control 17β-estradiol. In contrast, 17β-estradiol suppressed decrease in uterus weight and increase in serum IL-6 level, whereas MDL-A did not change them. These findings clearly indicate that MDL-A inhibits bone loss by a mechanism completely different from that of 17β-estradiol.
In the course of research for a new therapeutic drug for osteoporosis, hormone replacement therapy (HRT) was developed; its efficacy has been widely recognized in clinical studies (34). However, side effects including vaginal bleeding and increased risk of endometrial and breast cancer continue to limit its acceptability. Raloxifen (35), developed as a selective estrogen receptor modulator (SERM) (36), suppresses the reduction of bone density caused by postmenopausal osteoporosis without the risks in incidence of virginal bleeding and hormone-dependent cancer. However, the detailed action mechanism of SERM on selectivity in tissue remains unclear. Because MDL-A showing no estrogen agonist inhibits bone loss by an apparently different mechanism from that of HRT, it is considered that MDL-A will be a useful drug for hormone-dependent osteoporosis treatment without HRT-like side effects.
With gradual clarification of the molecular control mechanism in osteoclast differentiation, osteoclast differentiation factor (ODF)/receptor activation of NFκB ligand (RANKL), which facilitates differentiation of osteoclasts, and the osteoclastogenesis-inhibitory factor/osteoprotegerin, which inhibits action of ODF/RANKL, have been reported (37). IL-6 acts on osteoblasts to express ODF/RANKL, whereas estrogen inhibits IL-6 gene expression and controls bone resorption (38). Therefore, inhibition of IL-6 and IL-11 actions has been thought to inhibit bone resorption and suppress osteoporosis.
Indeed, MDL-A inhibited osteoclastogenesis in vitro and suppressed bone resorption in OVX mice in vivo, demonstrating that the gp130 inhibitor, MDL-A, is useful for the treatment of osteoporosis by controlling differentiation to osteoclasts. For development of new drugs to treat and prevent other refractory diseases known to involve IL-6 as well as osteoporosis, MDL-A is expected to serve as a lead compound.
Acknowledgments
We thank Dr. H. Tomoda for his helpful suggestions regarding this manuscript. This work was supported in part by a grant-in-aid for scientific research from the Ministry of Education, Science, Sports, and Culture in Japan. Financial support from the Japan Keirin Association is also gratefully acknowledged.
Abbreviations
MDL-A, madindoline A
IL-6R, IL-6 receptor
gp130, glycoprotein 130
rhIL, recombinant human IL
rmIL, recombinant murine IL
TNF, tumor necrosis factor
1α,25(OH)2D3, 1α,25-dihydroxyvitamin D3
LIF, leukemia inhibitory factor
JAK, Janus kinase
STAT, signal transducer and activator of transcription
GCSF, granulocyte colony-stimulating factor
GCSFR, GCSF receptor
TBS, Tris-buffered saline
LPS, lipopolysaccharide
OVX, ovariectomized
SAA, serum amyloid A
References
- 1.Kishimoto T., Akira, S. & Taga, T. (1992) Science 258, 593-597. [DOI] [PubMed] [Google Scholar]
- 2.Strassmann G., Masui, Y., Chizzonite, R. & Fong, M. (1993) J. Immunol. 150, 2341-2345. [PubMed] [Google Scholar]
- 3.Yoshizaki K., Matsuda, T., Nishimoto, N., Kuritani, T., Taeho, L., Aozasa, K., Nakahata, T., Kawai, H., Tagoh, H., Komori, T., et al. (1989) Blood 74, 1360-1367. [PubMed] [Google Scholar]
- 4.Takagi N., Mihara, M., Moriya, Y., Nishimoto, N., Yoshizaki, K., Kishimoto, T., Takeda, Y. & Ohsugi, Y. (1998) Arthritis Rheum. 41, 2117-2121. [DOI] [PubMed] [Google Scholar]
- 5.de la Mata J., Uy, H. L., Guise, T. A., Story, B., Boyce, B. F., Mundy, G. R. & Roodman, G. D. (1995) J. Clin. Invest. 95, 2846-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X. G., Bataille, R., Jourdan, M., Saeland, S., Banchereau, J., Mannoni, P. & Klein, B. (1990) Blood 76, 2599-2605. [PubMed] [Google Scholar]
- 7.Lutticken C., Wegenka, U. M., Yuan, J., Buschmann, J., Schindler, C., Ziemiecki, A., Harpur, A. G., Wilks, A. F., Yasukawa, K., Taga, T., et al. (1994) Science 263, 89-92. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi M., Kim, Y. P., Hiraoka, H., Natori, M., Takamatsu, S., Kawakubo, T., Masuma, R., Komiyama, K. & Omura, S. (1995) J. Antibiot. (Tokyo) 48, 1435-1439. [DOI] [PubMed] [Google Scholar]
- 9.Fukami A., Iijima, K., Hayashi, M., Komiyama, K. & Omura, S. (2002) Biochem. Biophys. Res. Commun. 291, 1065-1070. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi M., Kim, Y. P., Takamatsu, S., Enomoto, A., Shinose, M., Takahashi, Y., Tanaka, H., Komiyama, K. & Omura, S. (1996) J. Antibiot. (Tokyo) 49, 1091-1095. [DOI] [PubMed] [Google Scholar]
- 11.Sunazuka T., Hirose, T., Shirahata, T., Harigaya, Y., Hayashi, M., Komiyama, K., Omura, S. & Smith, I. A. B. (2000) J. Am. Chem. Soc. 122, 2122-2123. [Google Scholar]
- 12.Carmichael J., Degraff, W. G., Gazdar, A. F., Minna, J. D. & Mitchell, J. B. (1987) Cancer Res. 47, 936-942. [PubMed] [Google Scholar]
- 13.Atkins G. J., Haynes, D. R., Geary, S. M., Loric, M., Crotti, T. N. & Findlay, D. M. (2000) Bone 26, 653-661. [DOI] [PubMed] [Google Scholar]
- 14.Meydan N., Grunberger, T., Dadi, H., Shahar, M., Arpaia, E., Lapidot, Z., Leeder, J. S., Freedman, M., Cohen, A., Gazit, A., et al. (1996) Nature 379, 645-648. [DOI] [PubMed] [Google Scholar]
- 15.Savino R., Ciapponi, L., Lahm, A., Demartis, A., Cabibbo, A., Toniatti, C., Delmastro, P., Altamura, S. & Ciliberto, G. (1994) EMBO J. 13, 5863-5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sporeno E., Savino, R., Ciapponi, L., Paonessa, G., Cabibbo, A., Lahm, A., Pulkki, K., Sun, R. X., Toniatti, C., Klein, B., et al. (1996) Blood 87, 4510-4519. [PubMed] [Google Scholar]
- 17.Ward L. D., Hammacher, A., Howlett, G. J., Matthews, J. M., Fabri, L., Moritz, R. L., Nice, E. C., Weinstock, J. & Simpson, R. J. (1996) J. Biol. Chem. 271, 20138-20144. [DOI] [PubMed] [Google Scholar]
- 18.Sato K., Ohtomo, T., Hirata, Y., Saito, H., Matsuura, T., Akimoto, T., Akamatsu, K., Koishihara, Y., Ohsugi, Y. & Tsuchiya, M. (1996) Hum. Antibodies Hybridomas 7, 175-183. [PubMed] [Google Scholar]
- 19.Sato K., Tsuchiya, M., Saldanha, J., Koishihara, Y., Ohsugi, Y., Kishimoto, T. & Bendig, M. M. (1994) Mol. Immunol. 31, 371-381. [DOI] [PubMed] [Google Scholar]
- 20.Murakami M., Hibi, M., Nakagawa, N., Nakagawa, T., Yasukawa, K., Yamanishi, K., Taga, T. & Kishimoto, T. (1993) Science 260, 1808-1810. [DOI] [PubMed] [Google Scholar]
- 21.Yin T., Taga, T., Tsang, M. L., Yasukawa, K., Kishimoto, T. & Yang, Y. C. (1993) J. Immunol. 151, 2555-2561. [PubMed] [Google Scholar]
- 22.Gearing D. P., Comeau, M. R., Friend, D. J., Gimpel, S. D., Thut, C. J., McGourty, J., Brasher, K. K., King, J. A., Gillis, S., Mosley, B., et al. (1992) Science 255, 1434-1437. [DOI] [PubMed] [Google Scholar]
- 23.Pennica D., King, K. L., Shaw, K. J., Luis, E., Rullamas, J., Luoh, S. M., Darbonne, W. C., Knutzon, D. S., Yen, R., Chien, K. R., et al. (1995) Proc. Natl. Acad. Sci. USA 92, 1142-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis S., Aldrich, T. H., Stahl, N., Pan, L., Taga, T., Kishimoto, T., Ip, N. Y. & Yancopoulos, G. D. (1993) Science 260, 1805-1808. [DOI] [PubMed] [Google Scholar]
- 25.Gearing D. P., Thut, C. J., VandeBos, T., Gimpel, S. D., Delaney, P. B., King, J., Price, V., Cosman, D. & Beckmann, M. P. (1991) EMBO J. 10, 2839-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciapponi L., Graziani, R., Paonessa, G., Lahm, A., Ciliberto, G. & Savino, R. (1995) J. Biol. Chem. 270, 31249-31254. [DOI] [PubMed] [Google Scholar]
- 27.Paonessa G., Graziani, R., De Serio, A., Savino, R., Ciapponi, L., Lahm, A., Salvati, A. L., Toniatti, C. & Ciliberto, G. (1995) EMBO J. 14, 1942-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barton V. A., Hall, M. A., Hudson, K. R. & Heath, J. K. (2000) J. Biol. Chem. 275, 36197-36203. [DOI] [PubMed] [Google Scholar]
- 29.Barton V. A., Hudson, K. R. & Heath, J. K. (1999) J. Biol. Chem. 274, 5755-5761. [DOI] [PubMed] [Google Scholar]
- 30.Kurth I., Horsten, U., Pflanz, S., Dahmen, H., Kuster, A., Grotzinger, J., Heinrich, P. C. & Muller-Newen, G. (1999) J. Immunol. 162, 1480-1487. [PubMed] [Google Scholar]
- 31.Yawata H., Yasukawa, K., Natsuka, S., Murakami, M., Yamasaki, K., Hibi, M., Taga, T. & Kishimoto, T. (1993) EMBO J. 12, 1705-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papadopoulos N. G., Georganas, K., Skoutellas, V., Konstantellos, E. & Lyritis, G. P. (1997) Clin. Rheumatol. 16, 162-165. [DOI] [PubMed] [Google Scholar]
- 33.Lin S. C., Yamate, T., Taguchi, Y., Borba, V. Z., Girasole, G., O'Brien, C. A., Bellido, T., Abe, E. & Manolagas, S. C. (1997) J. Clin. Invest. 100, 1980-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christiansen C., Christensen, M. S. & Transbol, I. (1981) Lancet 1, 459-461. [DOI] [PubMed] [Google Scholar]
- 35.Hoszowski K. (1995) Pol. Tyg. Lek. 50, 41-42. [PubMed] [Google Scholar]
- 36.Burckhardt P. (1999) Schweiz. Med. Wochenschr. 129, 1926-1930. [PubMed] [Google Scholar]
- 37.Yasuda H., Shima, N., Nakagawa, N., Yamaguchi, K., Kinosaki, M., Mochizuki, S., Tomoyasu, A., Yano, K., Goto, M., Murakami, A., et al. (1998) Proc. Natl. Acad. Sci. USA 95, 3597-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Girasole G., Jilka, R. L., Passeri, G., Boswell, S., Boder, G., Williams, D. C. & Manolagas, S. C. (1992) J. Clin. Invest. 89, 883-891. [DOI] [PMC free article] [PubMed] [Google Scholar]