Abstract
The distribution of a range of integrins, E-cadherin, and carcino-embryonic antigen (CEA) like molecules in normal human oesophageal epithelium was investigated immunohistochemically on frozen sections of endoscopic biopsy specimens. The integrin subunits alpha 2, alpha 3, alpha 6, alpha v, beta 1, and beta 5 were expressed throughout the epithelium. There was strong expression of alpha 2, alpha 3, and beta 1 subunits in the basal cell layer and for all the subunits studied the intensity of the staining decreased as cells moved towards the lumen. The heterodimer alpha v beta 3 was expressed weakly in the basal aspect of the basal cell layer only. The CEA molecules were not present in the basal cells layer but there was weak expression in the prickle cell layer and strong positivity in the mature functional layer. E-cadherin was found throughout the epithelium but was weakly expressed at the basal aspect of the basal cells layer and showed strong positivity in the prickle cell and squamous cell layers. These results indicate that cell-cell (E-cadherin, CEA) and cell-matrix (integrins) adhesion molecules show a well defined spatial pattern of immunoreactivity in the oesophageal mucosa and may play a part in the maintenance of normal tissue architecture and physiological homeostasis.
Full text
PDF
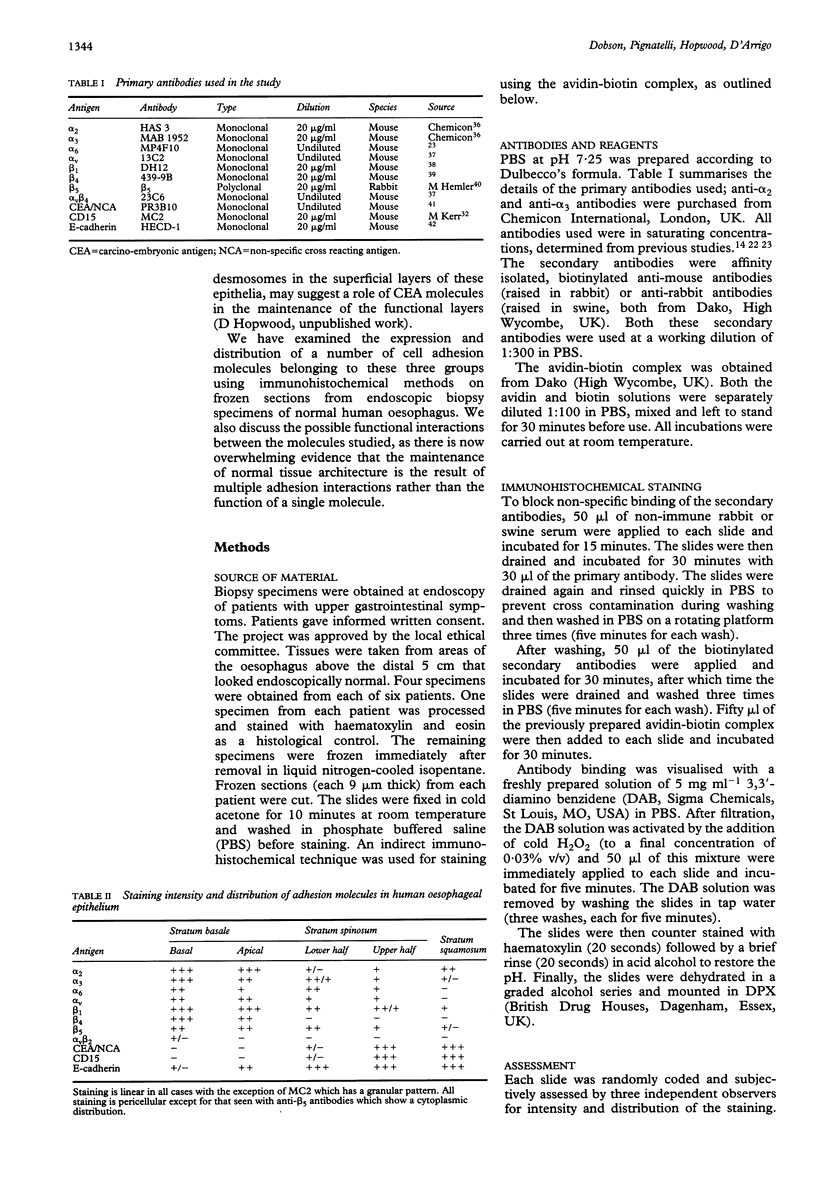


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behrens J., Birchmeier W., Goodman S. L., Imhof B. A. Dissociation of Madin-Darby canine kidney epithelial cells by the monoclonal antibody anti-arc-1: mechanistic aspects and identification of the antigen as a component related to uvomorulin. J Cell Biol. 1985 Oct;101(4):1307–1315. doi: 10.1083/jcb.101.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W. G., Wayner E. A., Bouchard T. S., Kaur P. The role of integrins alpha 2 beta 1 and alpha 3 beta 1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990 Apr;110(4):1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Warwick J., Totty N., Philp R., Helfrich M., Horton M. The osteoclast functional antigen, implicated in the regulation of bone resorption, is biochemically related to the vitronectin receptor. J Cell Biol. 1989 Oct;109(4 Pt 1):1817–1826. doi: 10.1083/jcb.109.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B., Saison M., Jaspers M., Spaepen M., Van Leuven F., Van den Berghe H., Cassiman J. J. Monoclonal antibody DH12 reacts with a cell surface and a precursor form of the beta subunit of the human fibronectin receptor. Cell Biol Int Rep. 1988 Jan;12(1):9–16. doi: 10.1016/0309-1651(88)90106-3. [DOI] [PubMed] [Google Scholar]
- De Strooper B., Van der Schueren B., Jaspers M., Saison M., Spaepen M., Van Leuven F., Van den Berghe H., Cassiman J. J. Distribution of the beta 1 subgroup of the integrins in human cells and tissues. J Histochem Cytochem. 1989 Mar;37(3):299–307. doi: 10.1177/37.3.2645360. [DOI] [PubMed] [Google Scholar]
- Dedhar S. Integrins and tumor invasion. Bioessays. 1990 Dec;12(12):583–590. doi: 10.1002/bies.950121205. [DOI] [PubMed] [Google Scholar]
- Del Buono R., Pignatelli M., Bodmer W. F., Wright N. A. The role of the arginine-glycine-aspartic acid-directed cellular binding to type I collagen and rat mesenchymal cells in colorectal tumour differentiation. Differentiation. 1991 Mar;46(2):97–103. doi: 10.1111/j.1432-0436.1991.tb00870.x. [DOI] [PubMed] [Google Scholar]
- Griepp E. B., Dolan W. J., Robbins E. S., Sabatini D. D. Participation of plasma membrane proteins in the formation of tight junctions by cultured epithelial cells. J Cell Biol. 1983 Mar;96(3):693–702. doi: 10.1083/jcb.96.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B., Simons K. A functional assay for proteins involved in establishing an epithelial occluding barrier: identification of a uvomorulin-like polypeptide. J Cell Biol. 1986 Feb;102(2):457–468. doi: 10.1083/jcb.102.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P. A., Coates P., Lemoine N. R., Horton M. A. Characterization of integrin chains in normal and neoplastic human pancreas. J Pathol. 1991 Sep;165(1):33–41. doi: 10.1002/path.1711650107. [DOI] [PubMed] [Google Scholar]
- Hertle M. D., Kubler M. D., Leigh I. M., Watt F. M. Aberrant integrin expression during epidermal wound healing and in psoriatic epidermis. J Clin Invest. 1992 Jun;89(6):1892–1901. doi: 10.1172/JCI115794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormia M., Ylänne J., Virtanen I. Expression of integrins in human gingiva. J Dent Res. 1990 Dec;69(12):1817–1823. doi: 10.1177/00220345900690120601. [DOI] [PubMed] [Google Scholar]
- Humphries M. J. The molecular basis and specificity of integrin-ligand interactions. J Cell Sci. 1990 Dec;97(Pt 4):585–592. doi: 10.1242/jcs.97.4.585. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Lander A. D. Contact and adhesive specificities in the associations, migrations, and targeting of cells and axons. Cell. 1992 Jan 24;68(2):303–322. doi: 10.1016/0092-8674(92)90472-o. [DOI] [PubMed] [Google Scholar]
- Inoue M., Ogawa H., Miyata M., Shiozaki H., Tanizawa O. Expression of E-cadherin in normal, benign, and malignant tissues of female genital organs. Am J Clin Pathol. 1992 Jul;98(1):76–80. doi: 10.1093/ajcp/98.1.76. [DOI] [PubMed] [Google Scholar]
- Jones J., Sugiyama M., Watt F. M., Speight P. M. Integrin expression in normal, hyperplastic, dysplastic, and malignant oral epithelium. J Pathol. 1993 Feb;169(2):235–243. doi: 10.1002/path.1711690210. [DOI] [PubMed] [Google Scholar]
- Kemler R. Classical cadherins. Semin Cell Biol. 1992 Jun;3(3):149–155. doi: 10.1016/s1043-4682(10)80011-x. [DOI] [PubMed] [Google Scholar]
- Klein C. E., Steinmayer T., Mattes J. M., Kaufmann R., Weber L. Integrins of normal human epidermis: differential expression, synthesis and molecular structure. Br J Dermatol. 1990 Aug;123(2):171–178. doi: 10.1111/j.1365-2133.1990.tb01844.x. [DOI] [PubMed] [Google Scholar]
- Peltonen J., Larjava H., Jaakkola S., Gralnick H., Akiyama S. K., Yamada S. S., Yamada K. M., Uitto J. Localization of integrin receptors for fibronectin, collagen, and laminin in human skin. Variable expression in basal and squamous cell carcinomas. J Clin Invest. 1989 Dec;84(6):1916–1923. doi: 10.1172/JCI114379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli M., Cardillo M. R., Hanby A., Stamp G. W. Integrins and their accessory adhesion molecules in mammary carcinomas: loss of polarization in poorly differentiated tumors. Hum Pathol. 1992 Oct;23(10):1159–1166. doi: 10.1016/0046-8177(92)90034-z. [DOI] [PubMed] [Google Scholar]
- Pignatelli M., Liu D., Nasim M. M., Stamp G. W., Hirano S., Takeichi M. Morphoregulatory activities of E-cadherin and beta-1 integrins in colorectal tumour cells. Br J Cancer. 1992 Oct;66(4):629–634. doi: 10.1038/bjc.1992.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli M., Smith M. E., Bodmer W. F. Low expression of collagen receptors in moderate and poorly differentiated colorectal adenocarcinomas. Br J Cancer. 1990 Apr;61(4):636–638. doi: 10.1038/bjc.1990.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy H., Hemler M. E. Cloning, primary structure and properties of a novel human integrin beta subunit. EMBO J. 1990 May;9(5):1561–1568. doi: 10.1002/j.1460-2075.1990.tb08275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman P. I., Bodmer W. F. Monoclonal antibodies to human colorectal epithelium: markers for differentiation and tumour characterization. Int J Cancer. 1987 Mar 15;39(3):317–328. doi: 10.1002/ijc.2910390309. [DOI] [PubMed] [Google Scholar]
- Sanders D. S., Kerr M. A., Hopwood D., Coghill G. Expression of the CD 15 antigen is a marker of cellular differentiation in cervical intra-epithelial neoplasia (CIN). J Pathol. 1988 Jul;155(3):207–212. doi: 10.1002/path.1711550305. [DOI] [PubMed] [Google Scholar]
- Sanders D. S., Kerr M. A., Hopwood D., Coghill G., Milne G. A. Expression of the 3-fucosyl N-acetyllactosamine (CD 15) antigen in normal, metaplastic, dysplastic, and neoplastic squamous epithelia. J Pathol. 1988 Mar;154(3):255–262. doi: 10.1002/path.1711540308. [DOI] [PubMed] [Google Scholar]
- Sanders D. S., Stocks S. C., Milne D. M., Milne G. A., Hopwood D., Kerr M. A. Membranous expression of carcinoembryonic antigen (CEA) in the normal cervical squamous mucosa. J Pathol. 1992 May;167(1):77–82. doi: 10.1002/path.1711670113. [DOI] [PubMed] [Google Scholar]
- Shimoyama Y., Hirohashi S., Hirano S., Noguchi M., Shimosato Y., Takeichi M., Abe O. Cadherin cell-adhesion molecules in human epithelial tissues and carcinomas. Cancer Res. 1989 Apr 15;49(8):2128–2133. [PubMed] [Google Scholar]
- Stamp G. W., Pignatelli M. Distribution of beta 1, alpha 1, alpha 2 and alpha 3 integrin chains in basal cell carcinomas. J Pathol. 1991 Apr;163(4):307–313. doi: 10.1002/path.1711630407. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Functional correlation between cell adhesive properties and some cell surface proteins. J Cell Biol. 1977 Nov;75(2 Pt 1):464–474. doi: 10.1083/jcb.75.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G., Piotrowicz R. S., Kunicki T. J. The function of multiple extracellular matrix receptors in mediating cell adhesion to extracellular matrix: preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion to fibronectin and react with platelet glycoproteins Ic-IIa. J Cell Biol. 1988 Nov;107(5):1881–1891. doi: 10.1083/jcb.107.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock M. J., Knudsen K. A. Cadherins and associated proteins. In Vivo. 1991 Sep-Oct;5(5):505–513. [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]