Abstract
Evidence from many systems has shown that stem cells are maintained in “niches” or specific regulatory microenvironments formed by stromal cells. The question of how stem cells are maintained in their niches is important, and further studies will lead to a better understanding of stem cell regulation and enhance the future use of stem cells in regenerative medicine. Here we show that cadherin-mediated cell adhesion is required for anchoring somatic stem cells (SSCs) to their niches in the Drosophila ovary. DE-cadherin and Armadillo/β-catenin accumulate in the junctions between SSCs and their neighboring cells, inner germarial sheath cells. Removal of DE-cadherin from SSCs results in stem cell loss in the adult ovary. Furthermore, the cadherin-mediated adhesion is also important for maintaining SSCs in their niches before adulthood. This study provides further support that SSCs are located in a niche formed by their neighboring cells. We have previously shown that DE-cadherin-mediated cell adhesion is essential for anchoring germ-line stem cells to their niches in the Drosophila ovary. This study further implicates cadherin-mediated cell adhesion as a general mechanism for anchoring stem cells to their niches in a variety of systems.
Stem cells are defined by their ability to self-renew and to continuously generate differentiated cells. They have been directly or indirectly shown to exist in many adult tissues, and are responsible for generating differentiated cells that replace lost cells throughout an organism's lifetime (1–3). The molecular mechanisms controlling stem cell function in vivo are crucial to the future use of stem cells in regenerative medicine and also in understanding the aging process, tumor formation, and degenerative diseases (4–6). Likely, one of the important niche functions is to control stem cell behavior (i.e., self-renewal, proliferation, and differentiation) through secreted growth factors and cell–cell contacts. Among the top priorities in stem cell research is to further define the structures and functions of different stem cell niches and to reveal the molecular mechanisms involved in communication between stem cells and their niches.
Two stem cell types, somatic stem cells (SSCs) and germ-line stem cells (GSCs) that exist in the Drosophila ovary represent an excellent system in which to study adult stem cells at the cellular and molecular levels in vivo in the adult Drosophila ovary (7, 8). At the anterior end of each ovariole of an ovary, or germarium (Fig. 1A), are two or three GSCs whose progeny eventually develop into mature oocytes. Recently, GSCs have been shown to be located in a niche and are directly regulated by their niche cells (9–14). Two or three SSCs reside in the middle of the germarium, likely representing a model system in which to study epithelial stem cells in adult tissues (15, 16). They divide and generate a population of mitotically active follicle progenitor cells that reside in the middle of germarium. Like stem cells in other systems, these progenitors continuously proliferate in the egg chambers of stage 1 to stage 6, and generate several types of differentiated cells that cover egg chambers. These differentiated somatic follicle cell types have characteristics similar to epithelial cells in mammals, such as basal-lateral and basal-apical polarities. Recent research indicates that hedgehog (hh), produced primarily by cap cells located a few cells away from SSCs, directly regulates SSC maintenance and proliferation, suggesting that SSCs are located in a niche (16, 17). Hyperactivation of hh signaling by means of overexpressing hh or by removing negative regulators causes the overproduction of somatic follicle cells and increases the number of SSCs. The reduction of hh signaling by removing the functions of hh or downstream positive regulators results in rapid SSC loss.
Fig 1.
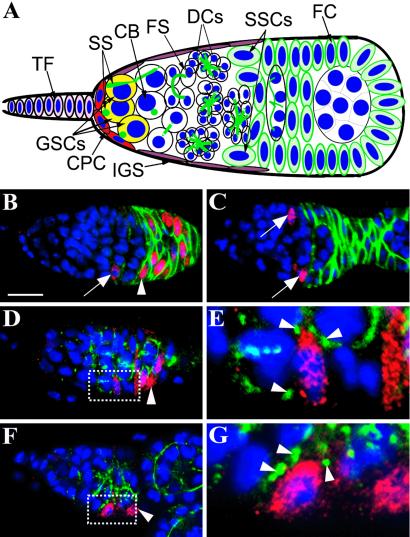
DE-cadherin and Arm in the interface between SSCs and IGS cells. (A) A cross-section diagram of a Drosophila germarium. (B) A confocal section of a germarium containing a marked lacZ+ SSC labeled for LacZ (red), Fas3 (green), and nuclei (blue). One marked lacZ+ SSC (arrow) is evident in the middle of germarium by its low Fas3 expression and the production of LacZ-positive follicle cells (one indicated by arrowhead). (C) A germarium containing two marked lacZ+ IGS cells labeled for LacZ (red), Fas3 (green), and nuclei (blue). Two lacZ+ IGS cells (arrows) with low levels of Fas3 expression are apparent, but LacZ-positive follicle cells are not present in the ovariole. (D and E) A germarium carrying a marked lacZ+ SSC labeled for DE-cadherin (green), LacZ (red), and nuclei (blue). (D) The SSC is identified by its location in the middle of the germarium and capacity to generate LacZ-positive follicle cells (one indicated by arrowhead). (E) The enlarged area (dotted rectangle shown in D) showing the existence of DE-cadherin-positive foci (arrowheads) between a marked lacZ+ SSC and neighboring IGS cells. (F and G) A germarium carrying a marked lacZ+ SSC labeled for Arm (green), LacZ (red), and nuclei (blue). (F) The SSC is identified by its location in the middle of the germarium and capacity to generate LacZ-positive follicle cells (one indicated by arrowhead). (G) The enlarged area (dotted rectangle shown in F) showing the existence of Arm-positive foci (arrowheads) between a marked SSC and neighboring IGS cells. CB, cystoblast; CPC, cap cell; DCs, developing cysts; FC, follicle cell; FS, fusome; SS = spectrosomes; TF = terminal filament cell. (Bars in B and C represent 10 μm.)
In mice and humans, hyperactivation of the hh pathway, either by mutations or overexpression, causes over-proliferation of skin cells, resulting in the formation of skin tumors (18–20). These studies, along with those from Drosophila, suggest that mechanisms regulating epithelial cells may be conserved from Drosophila to humans. Thus, SSCs in the Drosophila ovary could represent an effective system in which to study epithelial stem cell self-renewal, proliferation, and differentiation. Although SSCs lack distinctive morphology and unique molecular markers, SSCs in the Drosophila ovary can still be studied in great detail by using a cell-marking system to monitor their activities. SSCs mutant for a given gene can be effectively marked genetically, and their behavior can be studied throughout a long period so that the effect of any gene on stem cell regulation can be assessed (16, 17).
Cadherin gene family members, encoding Ca2+-dependent transmembrane adhesion molecules, mediate interactions between different cell types in a variety of organisms through homophilic interactions (21). DE-cadherin, encoded by shotgun (shg), is required for positioning oocytes in egg chambers and for border cell migration in the Drosophila ovary (22–25). Our recent research demonstrates that DE-cadherin is required for anchoring GSCs in their niche (26). Here we report that DE-cadherin is also required for maintaining SSCs in their niche in the adult Drosophila ovary. DE-cadherin-mediated cell adhesion also requires β-catenin, encoded by armadillo (arm) in Drosophila (27). We further show that DE-cadherin-mediated cell adhesion plays an essential role in SSC maintenance before adulthood. This work further suggests that cadherin-mediated cell adhesion may represent a general mechanism by which adult stem cells are anchored to their niches in different systems.
Materials and Methods
Drosophila Stocks and Genetics.
The following fly stocks were used in this study and are described either in Flybase or as specified: X-15-29, X-15-33, MKRS hs-FLP (15, 28); FLP recombination target (FRT)42D shgR69 (24), FRT42D shg10469 (26), and FRT42D. All Drosophila stocks were maintained at room temperature on standard cornmeal/molasses/agar media.
Generating lacZ-Positive Clones to Mark SSCs.
We generated mitotic clones to mark SSCs according to the published procedures (15). One- or two-day-old females of the genotype X-15-29/X-15-33; MKRS hs-FLP/+ were heat-shocked twice in a 37°C water bath for 1 h separated by an interval of 8 h. Flies were transferred daily to fresh, yeasted food to maintain optimal conditions for oogenesis. The SSC clones were identified by the expression of the lacZ gene as detected by antibody staining.
Generating Mutant shg SSC Clones.
Clones of mutant cells were generated by FLP-mediated mitotic recombination, as described (11). To generate the stocks for stem cell clonal analysis, FRT42D, FRT42D shg10469/CyO and FRT42D shgR69/CyO males were mated with virgin females yellow (yw) hs-FLP; FRT42D armadillo–lacZ, respectively. One- or two-day-old adult non-CyO females carrying an armadillo–lacZ transgene in trans to the mutant-bearing chromosome were heat-shocked six times at 37°C for 1 h separated by intervals of 8–12 h. The females were transferred to fresh food every day at room temperature, and ovaries were removed 1, 2, or 3 weeks after the last heat-shock treatment and then further processed for antibody staining. To determine stem cell maintenance, the percentages of the ovarioles carrying a marked SSC clone at different time points were calculated by dividing the number of germaria carrying marked follicles by the number of total germaria examined.
Generating Mutant shg Pre-Somatic Stem Cells to Test SSC Establishment Before Adulthood.
To generate the stocks for marked preSSCs, FRT42D +, FRT42D shg10469/CyO, and FRT42D shgR69/CyO males were mated with virgin females yw hs-FLP; FRT42D armadillo–lacZ, respectively. The culture tubes containing the progeny from these crosses that had not reached the late third-instar stage were heat shocked twice for 1 h at an interval of 6 h. One- or two-day-old adult non-CyO females carrying an armadillo–lacZ transgene in trans to the mutant-bearing chromosome were dissected and processed for immunostaining. To determine the effect of shg mutations on SSC establishment before adulthood, the percentages of the ovarioles carrying a marked SSC clone for a given genotype were calculated by dividing the number of germaria carrying marked SSCs by the number of total germaria examined.
Immunohistochemistry.
The following antisera were used: monoclonal anti-Hts antibody 1B1 (1:4), monoclonal anti-Fasciclin III (Fas3) antibody 7G10 (1:4), monoclonal antibody anti-Armadillo N7A1 (1:4), Rat anti-DE-cadherin (1:100) (23) and rabbit polyclonal anti-β-galactosidase (1:300; Molecular Probes) antibodies.
Ovaries were dissected in Grace's media and fixed in PBS with 4% formaldehyde for 12–15 min, then washed with PBT (PBS and 0.2% Triton X-100) 5 times for 15 min each. The ovaries were incubated in 0.5% goat serum diluted with PBT for 1 h. Appropriate primary antibodies were added to PBS and incubated at 4°C overnight, then washed with PBT 5 times for 15 min each wash. Lastly, appropriate secondary antibodies were added and incubated overnight, then washed with PBT five times for 15 min each. After the last wash, the stained ovaries were mounted in Vectashield mounting media (Vector Laboratories). All micrographs were taken with a Leica SPII confocal microscope.
Results
DE-Cadherin and Arm Are Expressed in the Interfaces Between SSCs and Their Neighboring Cells.
To directly determine whether DE-cadherin-mediated cell adhesion is important for anchoring SSCs to niche cells, we examined the expression of DE-cadherin and Arm proteins in the SSCs of the germarium. Because no reliable markers for SSCs are available, we used the FLP-mediated FRT recombination to positively mark SSCs. This technique takes advantage of a heat-shock-inducible FLP recombinase (29) to produce an active tubulin–lacZ reporter gene by driving recombination between two complementary inactive alleles (28). In this way, mitotically active cells in the germarium, including SSCs, are labeled by lacZ expression and visualized by staining for β-galactosidase proteins. This technique also labels early follicle progenitor cells that are mitotically active in the germarium. However, because it takes 3–4 days for marked follicle progenitor cells to migrate from the germarium, all marked, transient follicle cells will completely exit the germarium within a 1-week period. Therefore, the only marked somatic follicle cells in the germarium 1 week after clone induction must be produced by marked SSCs. The ovaries from females 1 week after the last heat-shock treatment were harvested and immunolabeled for LacZ and Fasciclin III (Fas3). In some germaria, one or two marked somatic cells were observed in the middle of the germarium with marked follicle cells present in the same germarium (Fig. 1B). Discrimination between these two cell types is made possible by the expression of Fas3, which is expressed at high levels in somatic follicle cells but at much lower levels in SSCs (16). We concluded that the marked somatic cells in the middle of the germarium with low Fas3 expression were SSCs. While in other germaria, we observed lacZ-positive somatic cells located in the middle of the germarium that had low levels of Fas3 expression; however, there were no lacZ-positive follicle cells in the germarium or early egg chambers of the same ovariole (Fig. 1C). We concluded that these labeled cells are most likely inner germarial sheath (IGS) cells in the same location as SSCs. Therefore, we identified marked SSCs based on their level of Fas3 expression, location, and production of marked follicle cells.
To study the expression of DE-cadherin and Arm in SSCs, the ovaries from the treated females were immunolabeled for LacZ and DE-cadherin or for LacZ and Arm. DE-cadherin proteins accumulated at high levels on several contact sites between inner sheath cells and SSCs, and among early follicle cells at their contact sites (Fig. 1 D and E). DE-cadherin proteins also accumulated at the contact sites between germ-line cysts and IGS cells, and between germ-line cells and follicle cells. DE-cadherin-mediated cell adhesion requires Arm, the Drosophila homolog of β-catenin, which was consistently present at the contact sites between SSCs and IGS cells, between germ-line cells and follicle cells, and among follicle cells themselves (Fig. 1 F and G). The presence of DE-cadherin-mediated cell adhesion molecules between IGS cells and SSCs suggests a potential role in SSC anchorage.
DE-cadherin Is Essential for Anchoring SSCs to Their Niches.
To investigate whether DE-cadherin-mediated cell adhesion is essential for maintaining stem cells in their niche, we used FLP-mediated FRT recombination to genetically mark SSCs in the adult Drosophila ovary and to disrupt the function of shg in the marked SSC clones. Marked SSC clones were identified by the loss of expression of an arm–lacZ transgene (the fusion of the arm promoter and the bacterial lacZ gene) from follicle cells in the germarium (Fig. 2A). When this technique is used, the only lacZ− follicle cell clones present 1 week after clone induction will consist of mutant SSCs and their progeny from the last 3–4 days (8, 11). With this marking system, we can deduce the loss of marked shg SSCs due to differentiation by the disappearance of mutant lacZ− follicle cells in the germaria. Furthermore, the position in which the most recent lacZ− mutant follicle cells are found on egg chambers in the ovariole also indicates when the marked SSCs were lost.
Fig 2.
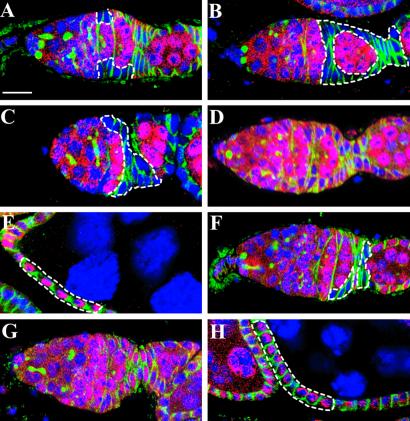
DE-cadherin anchoring SSCs in their niches. Germaria labeled for LacZ (red), Hts (green), and nuclei (blue). Marked lacZ− SSC clones or follicle cell clones are outlined. (A) A germarium carrying a wild-type SSC clone that is evident by the presence of LacZ-negative follicle cells. (B) A 3-week-old marked lacZ− wild-type SSC clone showing that all follicle cells are LacZ-negative. In this germarium, the lacZ+ SSC was lost and/or replaced by a marked lacZ− SSC. (C) A 3-week-old germarium carrying only marked lacZ− follicle cells from a shg10469 SSC clone. This has also resulted from the loss and/or replacement of a lacZ+ wild-type SSC by a marked lacZ− SSC. (D) A germarium carrying no marked lacZ− shg10469 SSCs with marked lacZ− follicle cell patches on egg chambers (E) 3 weeks after clone induction. (F) A 1-week- old shgR69 SSC clone. (G) A germarium with no shgR69 SSCs but having marked lacZ− follicle cell patches on egg chambers (H) 2 weeks after clone induction. The marked mutant follicle cells in E and H were identified by loss of expression of cytoplasmic LacZ and increased expression of nuclear LacZ because shg10469 and shgR69 still have a nuclear LacZ gene inserted in the locus capable of expressing it in late follicle cells. All of the germaria are shown at the same scale. (Bar in A represents 10 μm.)
To determine the importance of DE-cadherin-mediated adhesion in maintaining SSCs in their niches, we used two shg alleles, the deletion allele (shgR69) and a weak allele (shg10469). As a control, 46% of marked lacZ− wild-type SSCs observed during the first week after clone induction were maintained for 2 more weeks based on the presence of lacZ− follicle cell clones in the germarium (Fig. 2 A and B, and Table 1), suggesting that the half-life of SSC is about 3 weeks. This finding is consistent with previous results (15). Three weeks after clone induction, marked lacZ− follicle cell patches derived from marked lacZ− wild-type SSCs were still frequently observed. In some germaria, only marked lacZ− follicle cells were present, suggesting that non-clone (lacZ+) SSCs were lost and/or replaced by marked lacZ− SSCs (Fig. 2B). In contrast, 8.9% of marked lacZ− shg10469 SSCs observed during the first week were maintained for 2 more weeks (Fig. 2C and Table 1), suggesting that shg10469 mutant SSCs were severely destabilized. Most of the mutant SSC clones were lost during the initial 2-week period (Fig. 2 D and E). Mutant shg10469 oocytes were always localized to the posterior end of the egg chambers, indicating that homozygous shg10469 germ-line clones have no obvious defects in the DE-cadherin-based cell adhesion between oocytes and follicle cells (data not shown). Possibly, the shg10469 mutation induced by a P element primarily affects the expression of DE-cadherin in somatic cells but not in germ-line cells of the Drosophila ovary. As shown (24, 25), we observed that shgR69 mutant oocytes frequently failed to localize to the posterior end of the egg chambers (data not shown). Interestingly, we were able to produce marked, mutant shgR69 SSC clones only at a much lower frequency, 13% of the control under the same conditions (Fig. 2F, Table 1). Two weeks after clone induction, there were no germaria that carried mutant shgR69 SSC clones, but some ovarioles had mutant follicle cell patches on egg chambers (Fig. 2 G and H, Table 1). Results from our mutant clonal analysis clearly demonstrate that shg is essential for maintaining SSCs in their niche.
Table 1.
DE-cadherin is required for keeping SSCs in their niches
| Genotypes | 1 week, % | 2 weeks, % | 3 weeks, % |
|---|---|---|---|
| WT | 54.8 (104) | 42.2 (225) | 25.3 (217) |
| shg10469 | 21.3 (141) | 1.9 (216) | 1.9 (263) |
| shgR69 | 7.2 (250) | 0.3 (286) | 0.0 (360) |
Numbers indicate the percentage of germaria carrying a marked SSC at a given time point = the number of germaria carrying a marked SSC/total germaria examined ×100%. Numbers in parentheses indicate total germaria examined for each genotype at that time point.
DE-Cadherin-Mediated Signaling Does Not Play an Important Role in the Proliferation of Somatic Follicle Cells.
DE-cadherin-mediated cell adhesion is often involved in regulating other intercellular signaling events in many different systems (21). It has been shown that signaling from anterior somatic cells is important for SSC division (16, 17). It is very difficult to measure the division rates of SSCs because their immediate progeny cannot be effectively quantified. Instead, we studied whether DE-cadherin-mediated adhesion is important for the proliferation of somatic follicle cells by comparing the sizes of marked lacZ− wild-type and shg mutant follicle cell clones in the germaria and egg chambers. To ensure that the removal of DE-cadherin disrupts its accumulation between follicle cells and germ-line cells, we immunolabeled the ovaries for LacZ and DE-cadherin or for LacZ and Arm one week after heat-shock treatment. As expected, the removal of DE-cadherin from follicle cells sufficiently disrupted its accumulation between germ cells and follicle cells (Fig. 3 A and B). The sizes of shgR69 mutant follicle cell clones were similar to that of marked wild-type follicle clones (compare Fig. 3 A and B with Fig. 2A). To further confirm whether the accumulation of Arm in the junctions is also affected, we examined its expression in shgR69 mutant follicle clones. As shown (24, 25), the accumulation of Arm in the junctions between mutant follicle cells and germ cells in the germarium was disrupted (Fig. 3C). Sometimes, these shgR69 mutant follicle cells failed to integrate into the follicle cell epithelium of egg chambers and accumulated between egg chambers (Fig. 3D). Although shg mutant follicles have obvious defects in interacting with other cells, there were no obvious defects in follicle cell proliferation.
Fig 3.
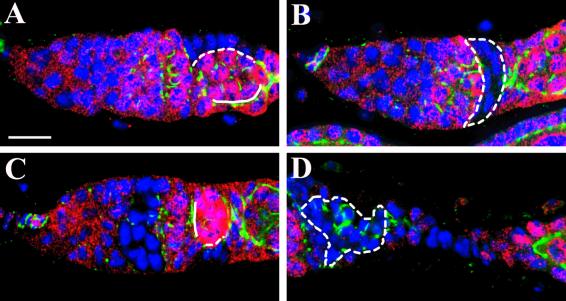
Diminished accumulation of DE-cadherin and Arm in the junctions between germ cells and mutant shg follicle cells after removal of a functional shg gene from SSCs. (A and B) One-week-old germaria labeled for LacZ (red), DE-cadherin (green), and nuclei (blue). Both panels illustrate the interface (dotted line) between lacZ− mutant shgR69 follicle cells and germ cells, and the interface (solid line) between lacZ+ wild-type follicle cells and germ cells. The removal of DE-cadherin from follicle cells can disrupt its accumulation in the interface between follicle cells and germ cells. (C) One-week-old germarium labeled for LacZ (red), Arm (green), and nuclei (blue). The dotted line indicates the interface between lacZ− mutant shgR69 follicle cells and germ cells, and the solid line indicates the interface between lacZ+ wild-type follicle cells and germ cells. The removal of DE-cadherin from follicle cells disrupts Arm accumulation in the interface between follicle cells and germ cells. (D) Part of an ovariole labeled for LacZ (red), Arm (green), and nuclei (blue) showing a patch of lacZ− shgR69 mutant follicles (outlined) in the region between a germarium and a stage-2 egg chamber. All of the micrographs are shown at the same scale. (Bar in A represents 6.7 μm.)
DE-Cadherin-Mediated Cell Adhesion Is Also Required for Anchoring SSCs During Development.
We have previously demonstrated that DE-cadherin-mediated cell adhesion is required for recruiting GSCs and keeping GSCs to their niches before adulthood (26). It is possible that this adhesion process is also required for SSC recruitment and anchorage to their niches before adulthood. The establishment of SSC identity occurs during pupation, later than the late-third-instar stage in which GSCs start to establish (26). However, no molecular markers or reliable positional information are available for identifying SSC precursors and SSCs themselves during this early developmental process. Therefore, we could not effectively determine the role of DE-cadherin in SSC recruitment, but rather focus on investigating whether DE-cadherin plays any role in maintaining SSCs before adulthood. To determine the role of DE-cadherin in maintaining SSCs before adulthood, we randomly removed DE-cadherin from the mitotically active preSSC population by using the FLP-mediated FRT recombination technique. Randomly marked lacZ− wild-type (as a control), mutant shg10469 and shgR69 preSSC populations generated during the late-third-instar stage were allowed to go through normal ovarian developmental processes and to be further integrated into individual ovarioles. To assay the ability of these labeled preSSCs to be maintained in their niches, we examined the presence of marked lacZ− follicle cells in 1- to 2-day-old germaria. Even in the control, we expected that only a fraction of the marked lacZ− preSSCs would be incorporated into the germarium and become SSCs; however, if DE-cadherin-mediated cell adhesion was important for interactions between SSCs and their niche cells before adulthood, we expected that mutant shg preSSCs would have a lower efficiency of maintenance in their niches as compared with wild-type clones.
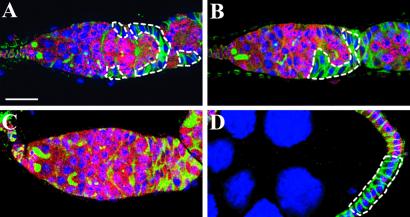
The presence of marked lacZ− SSCs was determined by the presence of marked lacZ− follicle cells in the germarium because labeled SSC precursors that failed to be maintained in niches had already moved out of the germarium during pupation. As a control, labeled, wild-type follicle patches were present in 34.3% of germaria (n = 300, Fig. 4A). The labeled shg10469 mutant follicle patches were present in 18.7% of germaria (n = 246, Fig. 4B); however, labeled follicle cell patches homozygous for the deletion allele, shgR69, were detected in only 0.8% of germaria (n = 249). We induced wild-type and mutant shgR69 clones under the same conditions (in the same experiment, 39% and 41% of ovarioles carried marked lacZ− wild-type and mutant shgR69 cysts, respectively); therefore, the difference in the percentages of germaria carrying mutant SSC clones reflects the role of DE-cadherin in the interactions between SSCs and their niche cells before adulthood. The presence of labeled follicle patches in the germarium indicates the presence or recent presence of labeled SSCs in the same ovariole. Ovarioles were observed in which mutant follicle cell clones were present in egg chambers; however, labeled follicle patches were absent in the germarium (Fig. 4 C and D), indicating that labeled SSC precursors fail to be integrated into niches as SSCs or that labeled SSCs are integrated into niches and are lost during development. Currently, we cannot effectively separate the establishment and maintenance role of DE-cadherin in SSCs; however, our results can be accounted for at least by its maintenance role before adulthood. We conclude, therefore, that disrupting the expression of DE-cadherin in preSSCs reduces their ability to be maintained in their niches during development.
Fig 4.
The requirement of DE-cadherin-mediated cell adhesion for SSC maintenance before adulthood. Ovaries of the females that developed from heat-shock-treated late third-instar larvae labeled for LacZ (red), Hts (green), and nuclei (blue). (A) A wild-type germarium showing a marked lacZ− SSC clone (outlined). (B) A germarium showing a marked lacZ− mutant shg10469 SSC clone (outlined). (C) A germarium showing no mutant shgR69 clones with a lacZ− mutant follicle clone on late egg chambers (D). All of the micrographs are shown at the same scale. (Bar in A represents 10 μm.)
Discussion
In this study, we show that DE-cadherin accumulates at high levels at the contact sites between SSCs and their neighboring IGS cells. We demonstrate that DE-cadherin is essential for holding SSCs against IGS cells and preventing SSCs from moving away from their niche and, therefore, from differentiating. Terminal filament cells/cap cells express the gene hh, known for its role in maintaining SSC identity (16, 17). Therefore, we propose that one function mediated by the DE-cadherin-based cell adhesion is to ensure that SSCs stay close to important SSC growth factors, such as Hh, to maintain their stem cell characteristics.
Different Stem Cell Types May Have Different Life Spans.
Because stem cells have the capacity to self-renew, it has been thought that individual stem cells can live as long as host organisms. Because of the difficulties in quantifying the self-renewal properties of stem cells, this important question has not been carefully tested in many stem cell systems. Fortunately, the life spans of GSCs and SSCs, can be analyzed quantitatively in the Drosophila ovary because we can mark both stem cell types and follow their activities for a long period. The differences in the limited life spans of GSCs (half-life of 4–5 weeks) and SSCs (half-life of 2–3 weeks) (refs. 11, 15, and 16, and this study) may reflect the various capacities of diverse stem cells to self-renew. It currently remains unclear what determines these self-renewal capacities for different stem cells. One possibility is that the lost stem cells are defective in their ability to receive critical niche cell signals. For Drosophila ovarian GSCs, any defect in receiving the dpp signal shortens their life span (11). For Drosophila ovarian SSCs, the failure to receive the hh signal also results in a shortened life span (16). Another hypothesis is that over time, stem cells undergo intrinsic changes that shorten their life span. For example, O-2 oligodentricyte precursor cells undergo senescence after a certain number of cell divisions due to continuous accumulation of cell-cycle inhibitors, such as p21 (30). Damage in DNA and protein machinery represent other possible mechanisms that could contribute to this stem cell instability. It will be interesting to investigate what intrinsically determines GSC and SSC life span in the Drosophila ovary.
Although stem cells in the Drosophila ovary have a limited life span, the ovary can function well beyond the life span of individual stem cells. One of the solutions is to replace lost stem cells with daughter cells of other stem cells that reside in the same or nearby niche. To repopulate an empty GSC niche in the Drosophila ovary, a daughter cell of another stem cell in the same niche occupies the same position where a lost GSC normally resides (12). Two pieces of independent evidence suggest that lost SSCs can be replaced (15, 16). Our study also supports these published results. The replaced SSCs must be derived from the progeny of other SSCs within the same germarium. It needs to be determined what mechanisms are used to replace lost SSCs in the Drosophila ovary.
Existence of an SSC Niche.
In the Drosophila ovary, GSCs have been shown unequivocally to be located in a niche (9–14). The neighboring somatic cells, namely terminal filament, cap cells, and IGS cells, form an asymmetrical niche structure in which two daughter cells of a GSC interact with different cell types and, therefore, adopt two completely different cell fates. The number of cap cells directly correlates with the number of GSCs, suggesting that cap cells are a major component of the niche. Consistent with this idea, the daughter cell that stays in contact with cap cells remains a stem cell, whereas the daughter cell that fails to contact cap cells and is located closer to IGS cells differentiates. Because they are located in the middle of the germarium where IGS cells also reside, SSCs are in close contact with a posterior group of IGS cells. After SSC division, one of the two daughter cells remains in its original position as a stem cell, whereas the other daughter cell differentiates and moves away posteriorly. This study provides evidence supporting the existence of SSC niches. The removal of DE-cadherin from SSCs prevents them from anchoring to IGS cells and, thus, results in SSC loss. It appears that close contact with IGS cells is essential for maintaining SSC identity. These stem cells directly contact underlying germ-line cysts and posterior IGS cells. Germ-line cysts are transiently present in the germarium, whereas IGS cells are permanent residents. Therefore, it is most likely that IGS cells function as a niche for SSCs. However, it remains unclear how the SSC niche is organized within one germarium.
Cadherin-Mediated Cell Adhesion Is a Common Strategy to Anchor Stem Cells to Their Niches.
Our previous research demonstrated that DE-cadherin-mediated cell adhesion is required for anchoring GSCs in the Drosophila ovary (26). Interestingly, DE-cadherin also accumulates at high levels between GSCs and hub cells in the Drosophila testis (data not shown). It has been proposed that hub cells serve as a niche for testicular GSCs (31, 32). In mouse testis, E-cadherin is expressed in both primordial germ cells and pre-Sertoli cells (33). In the adult rat testis, N-cadherin-11 is localized to peritubular cell junctions between germ cells and Sertoli cells (34). Although these cadherin molecules have not been directly tested for their role in anchoring GSCs to their niches, they could perform similar functions for GSCs in mammalian testes. It still remains to be seen if stem cells and their neighboring cells express cadherin molecules in other mammalian systems, including humans. The Drosophila DE-cadherin is closely related to all classic cadherins in mammals, including N-cadherin and E-cadherin. Therefore, we propose that stem cells in many tissues and organisms may use the cadherins or related molecules to stay in their niche. We show that DE-cadherin-mediated adhesion is involved in anchoring GSCs and SSCs to their niches. In a similar manner, cadherin or related adhesion molecules may also help anchor stem cells to their niches in other organisms, including humans. Increasing the expression of cadherin-related adhesion molecules on the surface of stem cells by different means may help stem cells find and be maintained in their niches as stem cells. Our studies provide a basis for identifying and evaluating the role of cadherin-related adhesion molecules in maintaining stem cells in their niches (ref. 26 and this study). Additionally, our results will assist future research efforts in the application of stem cells in regenerative medicine.
Acknowledgments
We thank the members of the Xie laboratory for comments on the manuscript, Ms. J. Chatfield for help in preparing the manuscript, and U. Tepass, H. Oda, the Bloomington stock center, and the Developmental Studies Hybridoma Bank for reagents. This work is supported by a Stowers Institute for Medical Research fund and National Institutes of Heath Grant 1R01 GM64428-01.
Abbreviations
IGS, inner germarial sheath
SSC, somatic stem cell
GSC, germ-line stem cell
Fas3, Fasciclin III
FRT, FLP recombination target
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Morrison S. J., Shah, N. M. & Anderson, D. J. (1997) Cell 88, 287-298. [DOI] [PubMed] [Google Scholar]
- 2.Watt F. M. & Hogan, B. L. (2000) Science 287, 1427-1430. [DOI] [PubMed] [Google Scholar]
- 3.Spradling A., Drummond-Barbosa, D. & Kai, T. (2001) Nature 414, 98-104. [DOI] [PubMed] [Google Scholar]
- 4.Donovan P. J. & Gearhart, J. (2001) Nature 414, 92-97. [DOI] [PubMed] [Google Scholar]
- 5.Reya T., Morrison, S. J., Clarke, M. F. & Weissman, I. L. (2001) Nature 414, 105-111. [DOI] [PubMed] [Google Scholar]
- 6.Temple S. (2001) Nature 414, 112-117. [DOI] [PubMed] [Google Scholar]
- 7.Lin H. (1998) Curr. Opin. Cell Biol. 10, 687-693. [DOI] [PubMed] [Google Scholar]
- 8.Xie T. & Spradling, A. C. (2001) in Stem Cell Biology, eds. Marshak, D. R., Gardner, R. L. & Gottlieb, D. (Cold Spring Harbor Lab. Press, Plainview, NY), pp. 129–148.
- 9.Cox D. N., Chao, A., Baker, J., Chang, L., Qiao, D. & Lin, H. (1998) Genes Dev. 12, 3715-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King F. J. & Lin, H. (1999) Development (Cambridge, U.K.) 126, 1833-1844. [DOI] [PubMed] [Google Scholar]
- 11.Xie T. & Spradling, A. C. (1998) Cell 94, 251-260. [DOI] [PubMed] [Google Scholar]
- 12.Xie T. & Spradling, A. C. (2000) Science 290, 328-330. [DOI] [PubMed] [Google Scholar]
- 13.King F. J., Szakmary, A., Cox, D. N. & Lin, H. (2001) Mol. Cell 7, 497-508. [DOI] [PubMed] [Google Scholar]
- 14.Cox D. N., Chao, A. & Lin, H. (2000) Development (Cambridge, U.K.) 127, 503-514. [DOI] [PubMed] [Google Scholar]
- 15.Margolis J. & Spradling, A. (1995) Development (Cambridge, U.K.) 121, 3797-3807. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y. & Kalderon, D. (2001) Nature 410, 599-604. [DOI] [PubMed] [Google Scholar]
- 17.Forbes A. J., Lin, H., Ingham, P. W. & Spradling, A. C. (1996) Development (Cambridge, U.K.) 122, 1125-1135. [DOI] [PubMed] [Google Scholar]
- 18.Johnson R. L., Rothman, A. L., Xie, J., Goodrich, L. V., Bare, J. W., Bonifas, J. M., Quinn, A. G., Myers, R. M., Cox, D. R., Epstein, E. H., Jr. & Scott, M. P. (1996) Science 272, 1668-1671. [DOI] [PubMed] [Google Scholar]
- 19.Oro A. E., Higgins, K. M., Hu, Z., Bonifas, J. M., Epstein, E. H., Jr. & Scott, M. P. (1997) Science 276, 817-821. [DOI] [PubMed] [Google Scholar]
- 20.Fan H., Oro, A. E., Scott, M. P. & Khavari, P. A. (1997) Nat. Med. 3, 788-792. [DOI] [PubMed] [Google Scholar]
- 21.Nagafuchi A. (2001) Curr. Opin. Cell Biol. 13, 600-603. [DOI] [PubMed] [Google Scholar]
- 22.Tepass U., Gruszynski-DeFeo, E., Haag, T. A., Omatyar, L., Torok, T. & Hartenstein, V. (1996) Genes Dev. 10, 672-685. [DOI] [PubMed] [Google Scholar]
- 23.Uemura T., Oda, H., Kraut, R., Hayashi, S., Kotaoka, Y. & Takeichi, M. (1996) Genes Dev. 10, 659-671. [DOI] [PubMed] [Google Scholar]
- 24.Godt D. & Tepass, U. (1998) Nature 395, 387-391. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Reyes A. & St Johnston, D. (1998) Development (Cambridge, U.K.) 125, 3635-3644. [DOI] [PubMed] [Google Scholar]
- 26.Song X., Zhu, C. H., Doan, C. & Xie, T. (2002) Science 296, 1855-1857. [DOI] [PubMed] [Google Scholar]
- 27.Riggleman B., Wieschaus, E. & Schedl, P. (1989) Genes Dev. 3, 96-113. [DOI] [PubMed] [Google Scholar]
- 28.Harrison D. A. & Perrimon, N. (1993) Curr. Biol. 3, 424-433. [DOI] [PubMed] [Google Scholar]
- 29.Golic K. G. & Lindquist, S. (1989) Cell 59, 499-509. [DOI] [PubMed] [Google Scholar]
- 30.Durand B. & Raff, M. (2000) BioEssays 22, 64-71. [DOI] [PubMed] [Google Scholar]
- 31.Kiger A. A., Jones, D. L., Schulz, C., Rogers, M. B. & Fuller, M. T. (2001) Science 294, 2542-2545. [DOI] [PubMed] [Google Scholar]
- 32.Tulina N. & Matunis, E. (2001) Science 294, 2546-2549. [DOI] [PubMed] [Google Scholar]
- 33.Mackay S., Nicholson, C. L., Lewis, S. P. & Brittan, M. (1999) Anat. Embryol. (Berlin) 200, 91-102. [DOI] [PubMed] [Google Scholar]
- 34.Johnson K. J. & Boekelheide, K. (2002) Biol. Reprod. 66, 992-1000. [DOI] [PubMed] [Google Scholar]