Abstract
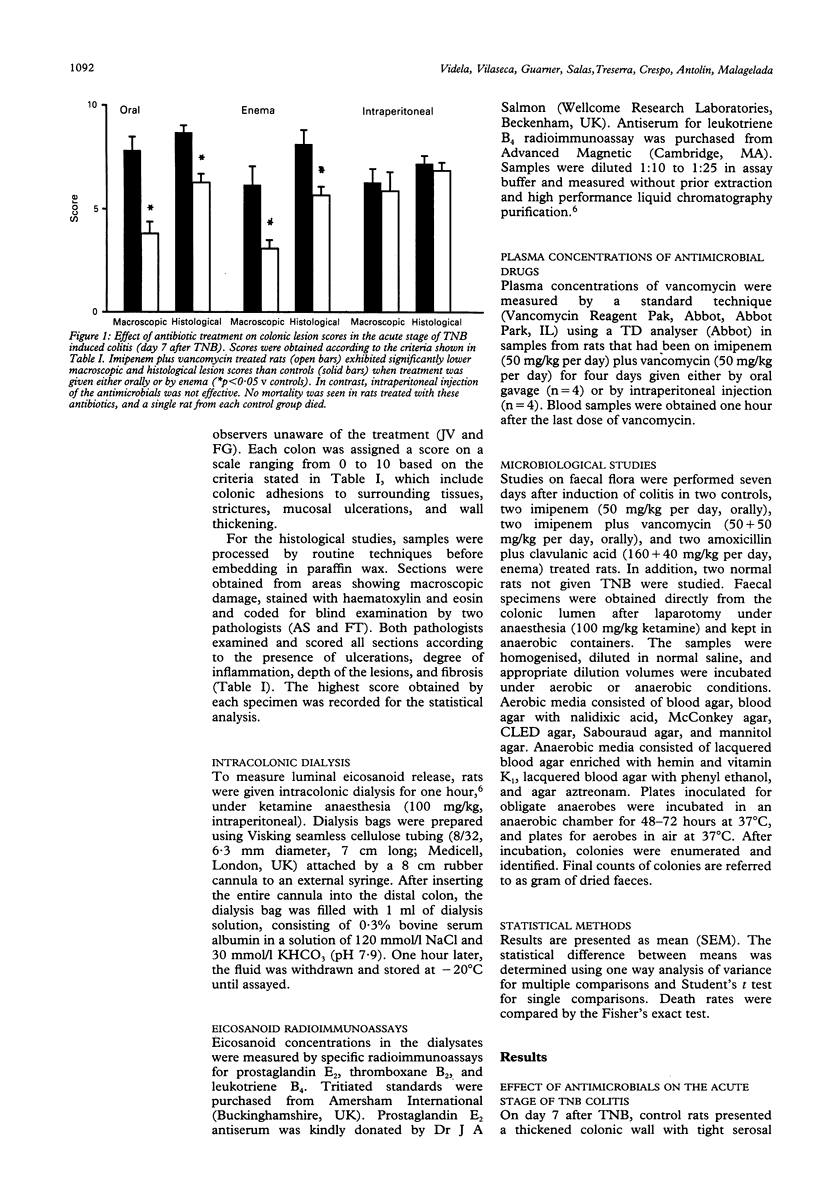
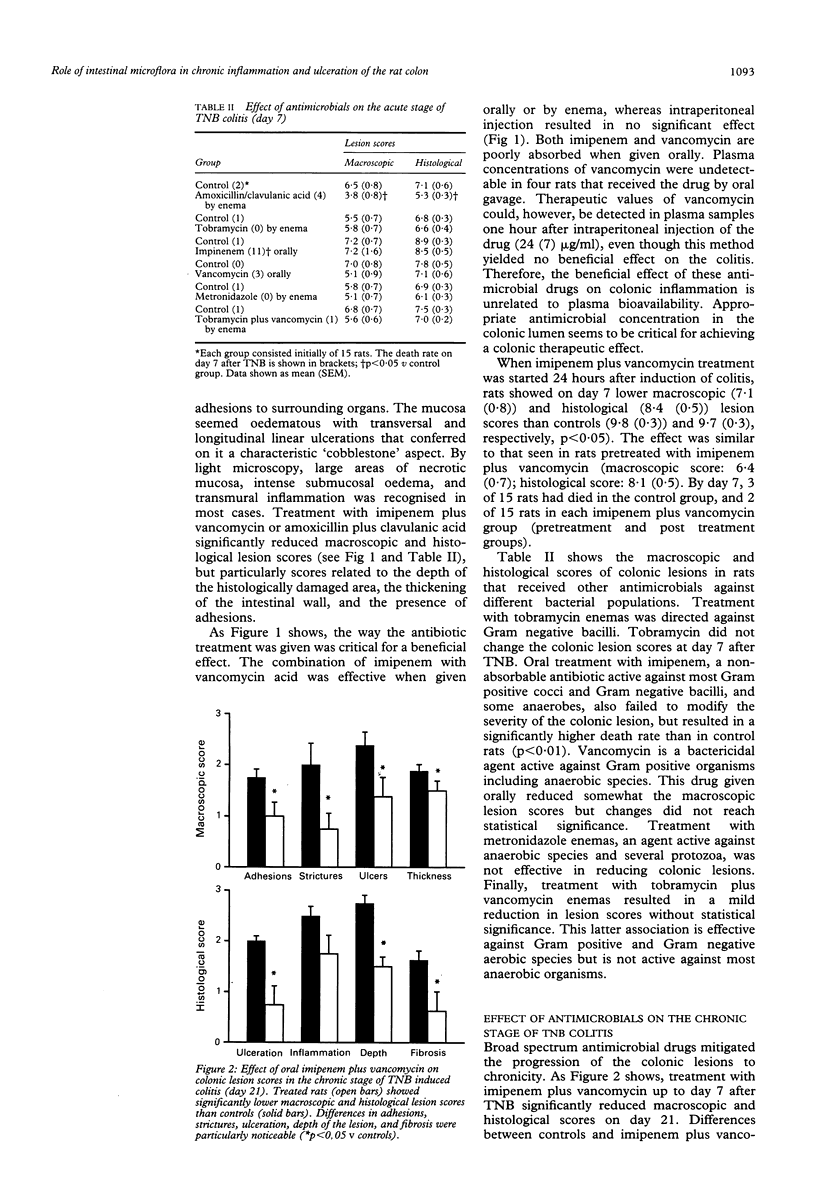
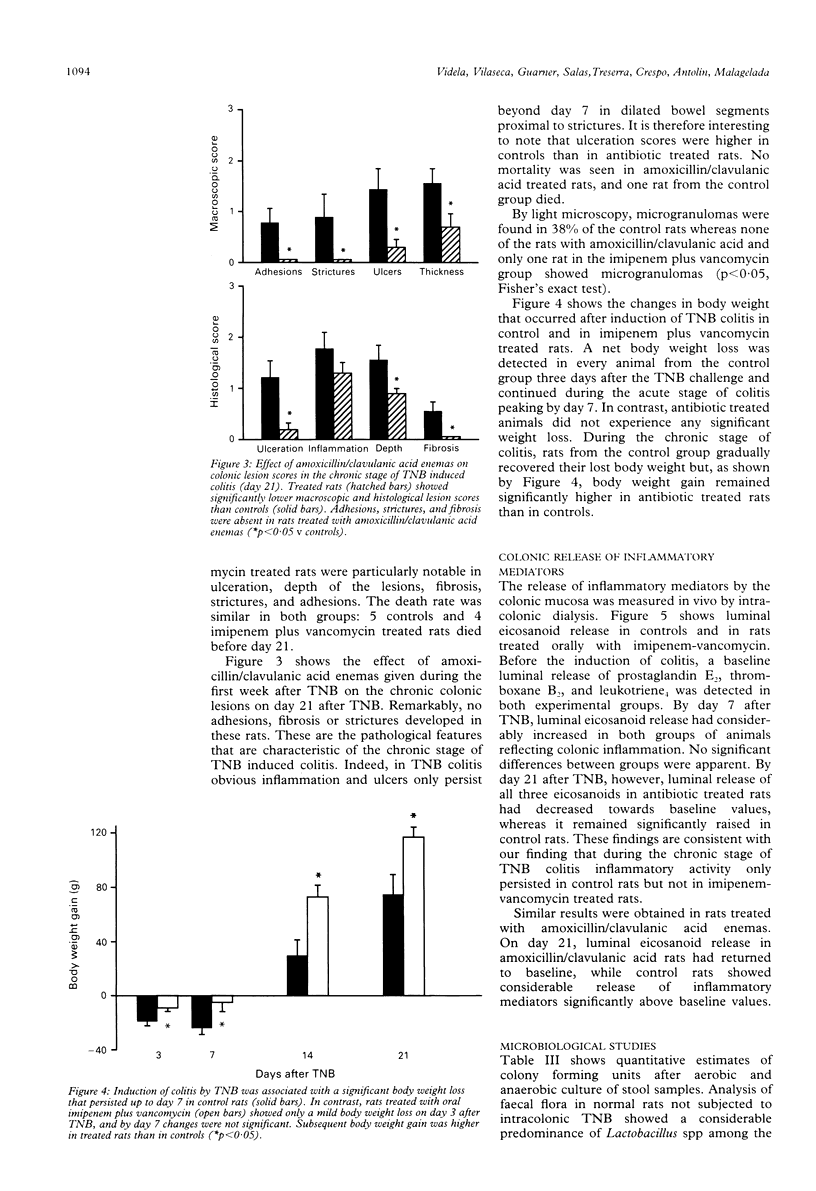
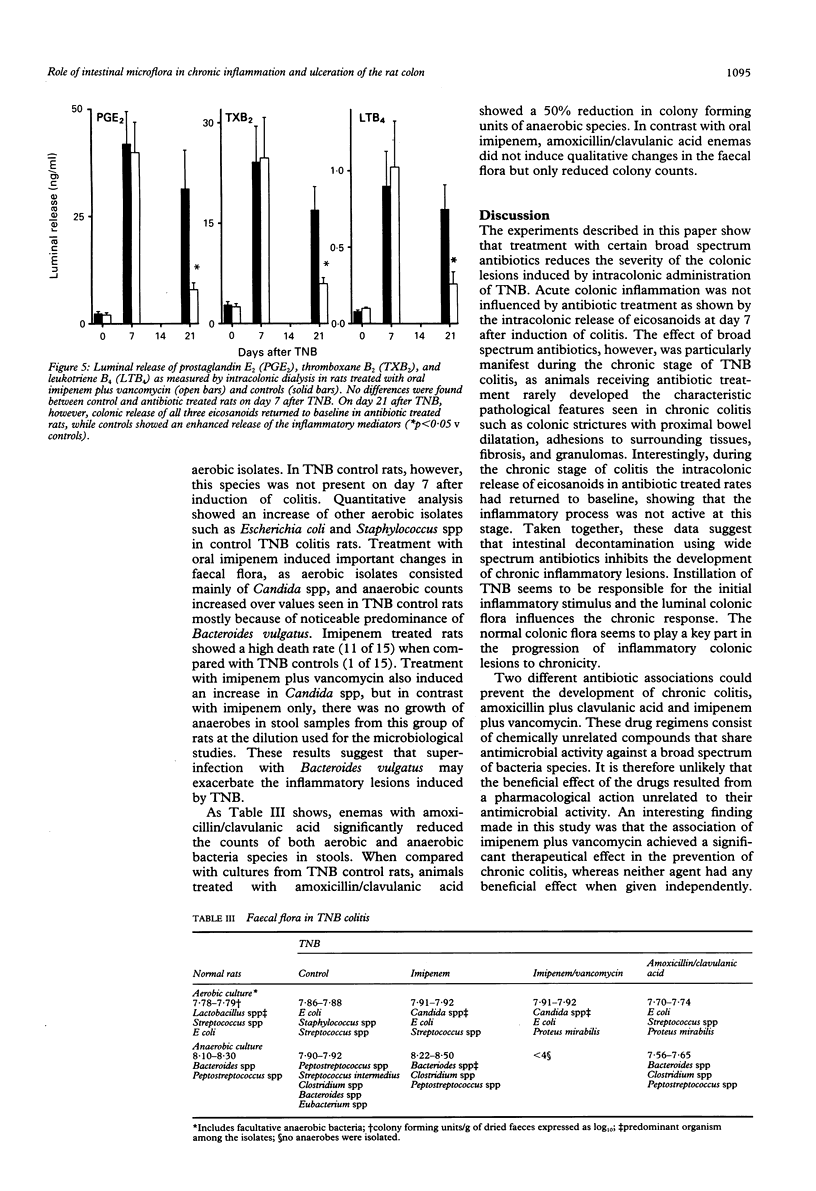
Bacteria and their products stimulate inflammatory responses. The effects of different antimicrobial regimens (amoxicillin/clavulanic acid, tobramycin, imipenem, vancomycin, metronidazole) were investigated on the course of experimental colitis induced by trinitrobenzenesulphonic acid (TNB) in the rat. On day 7 and 21 after the induction of colitis, matched groups of control and antibiotic treated rats were subjected to colonic dialysis to measure eicosanoid release, and killed for morphological assessment of the colonic lesions (macro and microscopic scores). Stool samples were cultured. Selective antibiotic treatment against Gram positive, Gram negative or anaerobic bacteria had no effect on colonic lesion scores. By contrast, certain broad spectrum antibiotics (amoxicillin/clavulanic acid or the association of imipenem plus vancomycin) significantly reduced macro and microscopic scores. Rats receiving these antibiotics did not develop chronic colitis as shown by the virtual absence of colonic strictures, adhesions, fibrosis, and granulomas. On day 21 after TNB, the intracolonic release of prostaglandin E2, thromboxane B2, and leukotriene B4 was significantly higher in control than in antibiotic treated rats. Control stool cultures showed abundant colony forming units of both aerobic and anaerobic bacteria. Amoxicillin/clavulanic acid and imipenem plus vancomycin induced appreciable reductions in luminal bacteria. In conclusion, certain broad spectrum antibiotics prevent chronic colitis. The normal colonic flora seems to play an important pathogenetic part in the progression of inflammatory colonic lesions to chronicity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allgayer H., Deschryver K., Stenson W. F. Treatment with 16,16'-dimethyl prostaglandin E2 before and after induction of colitis with trinitrobenzenesulfonic acid in rats decreases inflammation. Gastroenterology. 1989 May;96(5 Pt 1):1290–1300. doi: 10.1016/s0016-5085(89)80016-2. [DOI] [PubMed] [Google Scholar]
- Angelico M., De Sanctis S. C., Gandin C., Alvaro D. Spontaneous formation of pigmentary precipitates in bile salt-depleted rat bile and its prevention by micelle-forming bile salts. Gastroenterology. 1990 Feb;98(2):444–453. doi: 10.1016/0016-5085(90)90837-q. [DOI] [PubMed] [Google Scholar]
- Boughton-Smith N. K., Wallace J. L., Morris G. P., Whittle B. J. The effect of anti-inflammatory drugs on eicosanoid formation in a chronic model of inflammatory bowel disease in the rat. Br J Pharmacol. 1988 May;94(1):65–72. doi: 10.1111/j.1476-5381.1988.tb11500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham M. B., Volkmer C., Tso P., Yamada T. Metabolism of trinitrobenzene sulfonic acid by the rat colon produces reactive oxygen species. Gastroenterology. 1991 Aug;101(2):540–547. doi: 10.1016/0016-5085(91)90036-k. [DOI] [PubMed] [Google Scholar]
- Kent T. H., Cardelli R. M., Stamler F. W. Small intestinal ulcers and intestinal flora in rats given indomethacin. Am J Pathol. 1969 Feb;54(2):237–249. [PMC free article] [PubMed] [Google Scholar]
- Little J. R., Eisen H. N. Preparation and characterization of antibodies specific for the 2,4,6-trinitrophenyl group. Biochemistry. 1966 Nov;5(11):3385–3395. doi: 10.1021/bi00875a001. [DOI] [PubMed] [Google Scholar]
- Morris G. P., Beck P. L., Herridge M. S., Depew W. T., Szewczuk M. R., Wallace J. L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989 Mar;96(3):795–803. [PubMed] [Google Scholar]
- Okayasu I., Hatakeyama S., Yamada M., Ohkusa T., Inagaki Y., Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990 Mar;98(3):694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Bartlett J. G. Bacteriological studies of experimental ulcerative colitis. Am J Clin Nutr. 1979 Jan;32(1):258–265. doi: 10.1093/ajcn/32.1.258. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Cisneros R. L., Bronson R. T. Enhancement of experimental ulcerative colitis by immunization with Bacteroides vulgatus. Infect Immun. 1983 Nov;42(2):783–788. doi: 10.1128/iai.42.2.783-788.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderdonk A. B., Hermos J. A., Dzink J. L., Bartlett J. G. Protective effect of metronidazole in experimental ulcerative colitis. Gastroenterology. 1978 Mar;74(3):521–526. [PubMed] [Google Scholar]
- Onderdonk A. B., Steeves R. M., Cisneros R. L., Bronson R. T. Adoptive transfer of immune enhancement of experimental ulcerative colitis. Infect Immun. 1984 Oct;46(1):64–67. doi: 10.1128/iai.46.1.64-67.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons L., Droy-Lefaix M. T., Bueno L. Leukotriene D4 participates in colonic transit disturbances induced by intracolonic administration of trinitrobenzene sulfonic acid in rats. Gastroenterology. 1992 Jan;102(1):149–156. doi: 10.1016/0016-5085(92)91794-5. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D., Simon P. L., Schwartz L. W., Griswold D. E., Fondacaro J. D., Wasserman M. A. Inflammatory mediators of experimental colitis in rats. Gastroenterology. 1989 Aug;97(2):326–337. doi: 10.1016/0016-5085(89)90068-1. [DOI] [PubMed] [Google Scholar]
- Robert A., Asano T. Resistance of germfree rats to indomethacin-induced intestinal lesions. Prostaglandins. 1977 Aug;14(2):333–341. doi: 10.1016/0090-6980(77)90178-2. [DOI] [PubMed] [Google Scholar]
- Sartor R. B., Cromartie W. J., Powell D. W., Schwab J. H. Granulomatous enterocolitis induced in rats by purified bacterial cell wall fragments. Gastroenterology. 1985 Sep;89(3):587–595. doi: 10.1016/0016-5085(85)90455-x. [DOI] [PubMed] [Google Scholar]
- Vilaseca J., Salas A., Guarner F., Rodriguez R., Malagelada J. R. Participation of thromboxane and other eicosanoid synthesis in the course of experimental inflammatory colitis. Gastroenterology. 1990 Feb;98(2):269–277. doi: 10.1016/0016-5085(90)90814-h. [DOI] [PubMed] [Google Scholar]
- Vilaseca J., Salas A., Guarner F., Rodríguez R., Martínez M., Malagelada J. R. Dietary fish oil reduces progression of chronic inflammatory lesions in a rat model of granulomatous colitis. Gut. 1990 May;31(5):539–544. doi: 10.1136/gut.31.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. L., MacNaughton W. K., Morris G. P., Beck P. L. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology. 1989 Jan;96(1):29–36. doi: 10.1016/0016-5085(89)90760-9. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Marshall S., Specian R. D., Grisham M. B. A comparative analysis of two models of colitis in rats. Gastroenterology. 1992 May;102(5):1524–1534. doi: 10.1016/0016-5085(92)91710-l. [DOI] [PubMed] [Google Scholar]


