Abstract
Invasions of nonindigenous species have caused ecological devastation to natural communities worldwide, yet the biological bases for invasiveness remain poorly understood. Our studies of invasive watermilfoil (Myriophyllum) populations revealed widespread polymorphisms in biparentally inherited nuclear ribosomal DNA sequences, which were not detected in populations of native North American species. Subclones of the polymorphic regions revealed the occurrence of distinct sequences matching those acquired from both nonindigenous and native North American species. Molecular data demonstrate clearly that invasive watermilfoil populations in North America have resulted from hybridization between nonindigenous and native species. These observations suggest that invasiveness in these aggressive aquatic weeds may be linked to heterosis maintained by vegetative propagation.
Thousands of nonindigenous plant species have been introduced to North America as a consequence of accidental releases or an intentional importation as comestibles, fiber, and ornamentals. Escaped introduced plants have impacted native populations significantly and now comprise ≈23% of the flora of the United States and Canada (1, 2). Although most nonindigenous plants are innocuous and persist only through intensive cultivation, nearly 15% of nonindigenous species become invasive (3) and cause severe ecological disruption of native plant communities (4). Economic costs associated with invasive plant management are enormous; they are estimated to exceed 35 billion dollars each year in the United States (1).
Researchers have successfully identified gateways of introduction, mechanisms of dispersal, and means of control for many nonindigenous plants, yet in most instances it remains unclear why certain species, benign in their native range, should suddenly become explosively invasive following their introduction into a new habitat. The manifestation of invasive characteristics in nonnative plant populations is typically explained by various hypotheses. Introductions into new “benign” environments are often assumed to provide conditions favorable for an introduced species to thrive, but experimental evidence in support of this assumption has not been forthcoming (5). Arguably, a lack of associated herbivores or other natural controls also may impart advantages to nonindigenous species over native plant species whose populations are regulated by competition and predation (4, 6).
The potential for hybridization between nonindigenous and native species has also raised concerns, due mainly to introgression, which can cause extirpation of native species through gene contamination (6–8). Another possible outcome of hybridization is heterosis, or “hybrid vigor,” which is often expressed as high vegetative vigor in some progeny (9, 10). Studies of heterosis are confined largely to yield evaluations of agricultural crops, although its significance in plant invasions has not been considered sufficiently. However, it is known that hybridization between introduced and native species can lead to novel recombinants with superior competitive phenotypes (11). Although dissolution of heterosis can occur in hybrid populations that retain sexual reproduction (12), vegetatively reproducing aquatic plants can propagate hybrid genotypes almost indefinitely (13). Therefore, the emergence of invasive, heterotic hybrid populations would not be unexpected in vegetatively prolific, perennial aquatic plants.
Some of the most severe plant infestations in North America involve several nonindigenous aquatic species in the watermilfoil genus Myriophyllum (Haloragaceae). Eurasian watermilfoil (Myriophyllum spicatum), native to Europe, Asia, and northern Africa, is now present in 45 states and three Canadian provinces (14). It is the most widely managed aquatic weed in the United States (15). Myriophyllum heterophyllum, indigenous to the eastern United States except for the northeast, recently has become invasive in the New England region, where it is not native (16). M. heterophyllum has never been implicated in hybridization, but M. spicatum has been suspect because of the discovery of plants intermediate morphologically between it and the native Myriophyllum sibiricum (17) and also the ability of both species to hybridize in vitro (18). Yet, hybridization has never been implicated as a factor in the genesis of invasive watermilfoil populations.
Tandem repeats [e.g., nuclear ribosomal DNA (nrDNA)] are ideally suited for studying hybridization events because concerted evolution results in sequences conserved within species and divergent between species (19, 20). Because of biparental inheritance of these markers, recent hybrids initially possess both divergent parental genotypes, as evidenced by DNA sequence polymorphisms. In the absence of sexual reproduction, concerted evolutionary homogenization of sequences by interchromosomal crossing-over or gene conversion during chromosome pairing at meiosis would not be expected. Consequently, hybrids reproducing strictly vegetatively should retain copies of both divergent sequences for prolonged periods. In such instances, isolation of individual DNA sequences by molecular cloning can reveal the paternal origin of a hybridization event when sequences matching each parental species are recovered. As an adjunct, maternally inherited regions, e.g., chloroplast DNA (cpDNA) in most plants, can distinguish the maternal component of a particular hybrid cross.
We evaluated both nrDNA (internal transcribed spacer, ITS) and cpDNA (matK gene, trnK intron) sequences to study hybridization in watermilfoil populations which reproduce primarily vegetatively and display both invasive and noninvasive characteristics. This project is part of a broader ongoing phylogenetic study of the genus Myriophyllum that includes all North American species and many species from other parts of the world. This comprehensive sampling of taxa allowed us to pinpoint unequivocally the parentage of suspected hybrid watermilfoil plants collected in North America.
Materials and Methods
Taxon Sampling.
Multiple populations of parental species and putative hybrids were sampled over a wide geographic range (see Table 1, which is published as supporting information on the PNAS web site, www.pnas.org): M. spicatum (n = 9); M. sibiricum (n = 12); M. spicatum × M. sibiricum (n = 3); M. heterophyllum (n = 9); Myriophyllum pinnatum (n = 5); M. heterophyllum × M. pinnatum (n = 8). Parental species were not observed at sites where hybrid taxa were collected, unless otherwise indicated (Table 1). Species were distinguished by using traditional taxonomic characters; suspected hybrid plants were indicated initially by anomalous phenotypes that combined characteristics of different species. Several closely related North American and Australian species were included for comparative purposes (Table 1). Haloragis erecta was used as the outgroup for Myriophyllum based on our preliminary phylogenetic studies.
DNA Isolation, Subcloning, and Sequencing.
Total DNAs were extracted from either NaCl-cetyltrimethylammonium bromide preserved (21) or fresh leaf material by using a miniprep procedure (22). Double-stranded DNAs were amplified by using PCR to amplify the ITS-1, ITS-2, and 5.8S region of nuclear ribosomal DNA using the ITS-4 and ITS-5 primers (23). The cpDNA trnK introns and matK coding region were amplified by using the primers trnK-3914F and trnK-2R (24). PCR products were cloned into plasmids by using a TOPO TA cloning kit (Invitrogen). At least 10 clones were screened through amplification of PCR product of entire ITS from each individual that showed polymorphic sites in ITS and from one representative for each of the four proposed parental taxa. A subset of five clones was sequenced for entire ITS subsequently from each individual exhibiting polymorphic sites and a subset of 10 clones for each parental species. Cycle sequencing of ITS used combinations of the ITS-2, ITS-3, and ITS-4 (23) primers. Cycle sequencing of only the ITS-2 region was performed for the majority of M. spicatum and M. sibiricum specimens because of the ample variable sites (6) found in this region to differentiate the taxa, although entire ITS sequences were obtained from all of the cloned hybrids. The 5′-trnK intron was sequenced by using trnK-3914F and a newly developed Haloragaceae specific primer, “360F” (5′-TATGAATGTGTAGAAGAAG). The matK-900F (5′-TGATGAATAAGTGGAAATA) primer was used additionally to sequence M. spicatum and M. sibiricum. Sequences were obtained by using an ABI 3100 DNA automated sequencer (Applied Biosystems).
Phylogenetic Analyses.
Three nrDNA ITS data sets were analyzed. One data set contained sequences from Myriophyllum species excluding all hybrids. Two data sets were constructed emphasizing each proposed hybrid (including cloned sequences) and its parental species. Analyses of M. spicatum × M. sibiricum hybrids and their parent species were limited to ITS-2 data. Sequences were aligned manually and analyzed for polymorphisms and/or variable sites by using SEQUENCHER 4.1.2 (Gene Codes, Ann Arbor, MI) and MACCLADE 4 (25). Parsimony analyses were performed with PAUP 4.0B8 (26) by using heuristic searches with random taxon addition sequences and tree bisection-reconnection with unordered, equally weighted characters. Indels were treated as missing data. Standard measures of homoplasy such as consistency index (CI), retention index (RI), and level of internal support (bootstrap values) were calculated with PAUP 4.0b8. Bootstrap analyses were conducted by using 500 replicate heuristic searches as above.
Results
The ITS data matrix (excluding hybrids) contained 724 bp for 25 taxa. Three sites were removed because of alignment ambiguities. There were 195 variable characters (126 parsimony-informative). Seven point mutations differed between M. pinnatum and M. heterophyllum. Two indels (both in ITS-1) and 12 point mutations differed between M. spicatum and M. sibiricum across all accessions sampled. The trnK + matK region of cpDNA (2,474 bp) contained 14 point mutations differing between M. heterophyllum and M. pinnatum and two point mutations differing between M. spicatum and M. sibiricum in all accessions sampled.
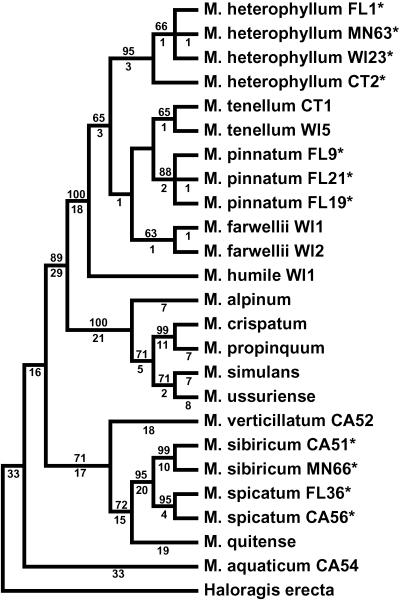
Parsimony analysis of ITS data for all Myriophyllum species sampled resulted in a single most parsimonious tree of 317 steps. In all cases, multiple accessions of the parental species were well supported as monophyletic and were distinct based on these molecular markers (Fig. 1).
Fig. 1.
Single most parsimonious tree (317 steps) of Myriophyllum species constructed from sequence data of entire nrITS (CI: 0.808; RI: 0.903). Numbers above branches are bootstrap values; those below are branch lengths. Characters after species names refer to states (U.S.) and accession numbers for collection localities. *, parental species of hybrid taxa.
Seven watermilfoil populations sampled in Connecticut and one in Florida displayed DNA polymorphisms at all seven nucleotide sites that varied between M. heterophyllum and M. pinnatum. Three invasive watermilfoil populations sampled from Wisconsin and Minnesota displayed DNA polymorphisms at all six sites in the ITS-2 region that varied between M. spicatum and M. sibiricum. The ITS-1 region could not be sequenced accurately for hybrid populations because the combination of different length sequences (due to indels in one parent) yielded polymorphisms at all sites beyond the first indel.
By using cloning techniques, only a single allele was recovered when clones from the parental species were sequenced. However, two distinct alleles (each corresponding to a parental species) were recovered from putative hybrids (Figs. 2 and 3) in all but one population. Hybrids from population CT13 contained a distinct M. pinnatum sequence, but in lieu of pure M. heterophyllum sequence, the clones examined possessed sequences with mixtures of diagnostic characters from both parental species. Other minor variations were observed. A few alleles showed unique substitutions at one or more sites (Figs. 2 and 3), and two accessions contained sequences that combined parental characters in addition to possessing copies of each individual parental sequence.
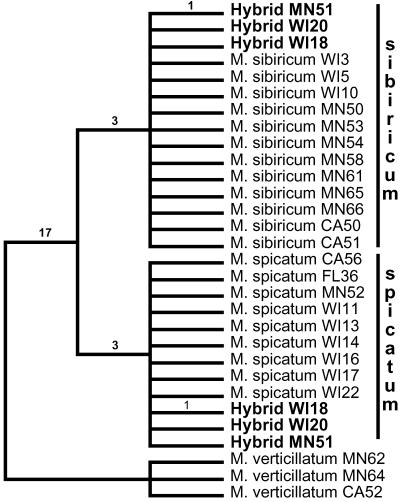
Fig. 2.
Single most parsimonious tree (72 steps) of a subset of Myriophyllum species illustrating M. heterophyllum × M. pinnatum hybrids (including sequence clones from hybrid populations in bold) constructed from entire nrITS sequence data (CI: 0.972; RI: 0.986). Numbers above branches refer to branch lengths. Characters after species names refer to states (U.S.) and accession numbers for collection localities.
Fig. 3.
Single most parsimonious tree (25 steps) of a subset of Myriophyllum species illustrating evidence of M. spicatum × M. sibiricum hybrids (including sequence clones from hybrid populations in bold) constructed from nrITS-2 sequence data (CI: 1.0; RI: 1.0). Numbers above branches refer to branch lengths. Characters after species names refer to states (U.S.) and accession numbers for collection localities.
Phylogenetic analysis of M. heterophyllum × M. pinnatum hybrid data resulted in a single most parsimonious tree of 72 steps (Fig. 2). There were 67 variable characters of which 15 were parsimony-informative. Phylogenetic analysis of M. spicatum × M. sibiricum hybrid data resulted in a single most parsimonious tree of 25 steps (Fig. 3). There were 24 variable characters of which 22 were parsimony-informative. In all cases, sequences cloned from the hybrids formed clades with each of their parental taxa (except for CT13, as discussed above). In the case of M. heterophyllum × M. pinnatum hybrids, an additional point mutation in the M. heterophyllum sequence copy was shared by six populations. Given the extent of among-population variation in ITS observed for M. heterophyllum, this result was not surprising (Fig. 2) and may indicate a common parental population from which these hybrids originated. Autapomorphies that defined the branch lengths of all terminal taxa were free from homoplasy.
All eight populations of putative M. heterophyllum × M. pinnatum hybrids showed maternal inheritance of the plastid genome through M. heterophyllum, as evidenced by cpDNA 5′-trnK intron sequence data. Two of three populations of putative M. spicatum × M. sibiricum hybrids (MN51, WI20) showed maternal inheritance of the plastid genome of M. spicatum, whereas one (WI18) showed maternal inheritance through M. sibiricum, as indicated by cpDNA matK sequence data.
Discussion
Molecular data strongly support the separation of M. heterophyllum and M. pinnatum (Fig. 1; bootstrap = 88–95%) when multiple populations were surveyed over a wide geographical range. The molecular divergence of these morphologically similar species provided a robust set of markers (seven variable nucleotides) that easily differentiated these taxa. M. spicatum and M. sibiricum are sister species phylogenetically (Fig. 1), yet they showed an even higher degree of molecular divergence (12 variable nucleotides and 2 indels) that likewise distinguished them readily. The pattern of distinctive nucleotide divergence between parental species made elucidation of hybrids straightforward upon recovery of individuals possessing copies of each unique parental sequence, thus confirming their hybrid origin.
As predicted, genomic DNA sequences of suspected hybrids revealed polymorphisms at the variable sites. Sequences of DNA cloned from putative hybrids confirmed that genomic DNA polymorphisms occurred, because each individual contained copies of the ITS region derived from each of the proposed parental species, which decisively supported their hybrid origin. Furthermore, hybrids verified in this way were noticeably aggressive (forming dense, monospecific stands) and were found primarily in localities where plants were of local management concern, confirming that invasive populations comprised hybrid plants. The retention of the divergent tandemly repeated sequences without their homogenization indicates that hybrids propagate primarily vegetatively. The geographical extent of invasive hybrid watermilfoil populations in North America should be pursued by additional sampling.
One exception was encountered in population CT13, which possessed chimeric sequences containing nucleotide substitutions diagnostic of both M. pinnatum and M. heterophyllum. In this instance, we presume homogenization of the ITS region of M. heterophyllum has been initiated through concerted evolution (19).
Phylogenetic analysis was not used to evaluate DNA sequences obtained from the chloroplast genome, which is assumed to be inherited maternally in Myriophyllum. Although organelle inheritance has not been studied previously in Haloragaceae, biparental inheritance of cpDNA is uncommon in angiosperms, and cpDNA is inherited maternally in Crassulaceae (27), the closest sister family to Haloragaceae (28). In our study, examination of cpDNA sequence data showed consistent nucleotide differences between the parental taxa (14 substitutions differing between M. heterophyllum/M. pinnatum; two between M. spicatum/M. sibiricum). All putative hybrid populations were monomorphic at these variable sites, indicating that hybrids shared cpDNA sequences with only one parental species, thus indicating uniparental inheritance.
Both M. heterophyllum and M. heterophyllum × M. pinnatum hybrids are nonindigenous to New England. M. heterophyllum is nonaggressive in other parts of the country where it is native, in some cases to the extent of being categorized as an imperiled species (29). Similarly, our field observations indicate that New England populations of pure M. heterophyllum rarely exhibited invasive characteristics, whereas the hybrids always did. In Moodus Reservoir, Connecticut, where M. heterophyllum and M. heterophyllum × M. pinnatum hybrids coexist in a single lake, the former grows along the shoreline scattered among several native aquatic species, whereas the latter forms dense monospecific mats in deeper water. Thus, it seems that New England potentially comprises both benign M. heterophyllum populations and invasive hybrid populations. Because we have yet to locate any authentic records of M. pinnatum in New England, we conclude that hybridization of M. heterophyllum and M. pinnatum occurred outside of the region originally. We confirmed that hybrids occur within the native range of sympatry, though not, in this case, within the same body of water as the parental species (e.g., FL1, Highlands County, Florida).
Although hybridization of M. spicatum and M. sibiricum had been suspected (17), its occurrence in natural populations has never been documented. Yet, these closely related species have been confused taxonomically for decades as a result of inconsistent morphological differences, mainly quantitative characters, used in their delimitation. Differences in leaf-segment number have been used primarily to differentiate these species. The pinnately compound leaves of M. spicatum typically have 14–21 segment pairs; those of M. sibiricum have only 6–11 pairs (30). However, many specimens collected in the field show overlap in leaf-segment number, which makes confident identification virtually impossible by using this trait. We attribute these differences to hybrids that are intermediate morphologically for leaf-segment number. Once we distinguished parental species and hybrids by using molecular markers, we observed that leaf-segment characteristics of the parental species no longer showed overlap.
Recognition of hybridity in invasive watermilfoil populations raises new issues regarding management strategies currently adopted for controlling infestations. In some cases, hybrids are known to deter herbivory better that their parental species (31–33). Recent studies have shown that biological control in the form of an aquatic weevil (Euhrychiopsis lecontei) is effective in reducing M. spicatum populations (34, 35), whereas the weevils have little effect on the native M. sibiricum (36, 37). These beetles damage M. spicatum by consuming shoot apical tissue, and late larval instars burrow into stems to deposit pupae, a phenomenon not found in M. sibiricum (37–39). Yet, some populations of M. spicatum are reportedly resistant to the beetle (40, 41). Of significance is that plants from one resistant population (Lake Beulah, Wisconsin) showed very low susceptibility to the weevils, with pupal forms nearly absent from apical meristems (40). Our analysis of plants from this same population (WI20) confirmed that they are hybrids, an indication that some hybrid milfoils may show resistance to phytophagous beetles while also displaying high vegetative vigor.
Documentation of hybridization in watermilfoil populations does not in itself demonstrate that their invasive vigor is due necessarily to heterosis. Yet, the existence of hybridization in these populations and other circumstantial evidence warrant a further assessment of this hypothesis. Hybrid vigor provides a plausible explanation for why these aquatic plants are benign within their native range, yet become invasive upon entering new territories where hybridization with related congeners is possible (42). Also, because most submersed aquatic plants like watermilfoils are prolific vegetatively (43), hybrid heterosis could be maintained indefinitely without succumbing to erosion by sexual recombination, as is indicated by our sequence data. Furthermore, different responses of phytophagous herbivores to various watermilfoil populations may reflect variations in specificity among pure species and hybrid populations, particularly if the biocontrol agents were selected originally to target the parental species. If this is true, then the implications for management programs employing biocontrol agents would be immense.
Supplementary Material
Acknowledgments
We thank T. Cochrane, D. Gerber, C. Jacono, Y. Kadono, K. Metzler, C. Morse, N. Murray, D. Perleberg, N. Ritter, D. Schmidt, and C. Welling for assistance with collections. We also thank D. L. Dilcher and two anonymous reviewers for helpful comments on this manuscript. Funding was provided by National Science Foundation Grant DEB-9806537 and awards from the New England Botanical Club Graduate Student Research Award Program and the University of Connecticut Bamford Endowment Fund.
Abbreviations
cpDNA, chloroplast DNA
ITS, internal transcribed spacer
References
- 1.Pimentel D., Lach, L., Zuniga, R. & Morrison, D. (2000) Bioscience 50, 53-65. [Google Scholar]
- 2.Scoggan H. J., (1978–79) The Flora of Canada (Canadian Museum of Nature, Ottawa).
- 3.Westbrooks R. G., (1998) Invasive Plants, Changing the Landscape of America (Federal Interagency Committee for the Management of Noxious and Exotic Weeds, Washington, DC), pp. 1–9.
- 4.Cox G. W., (1999) Alien Species in North America and Hawaii: Impacts on Natural Ecosystems (Island, Washington, DC).
- 5.Willis A. J. & Blossey, B. (1999) Biocontr. Sci. Tech. 9, 567-577. [Google Scholar]
- 6.Galatowitsch S. M., Anderson, N. O. & Ascher, P. D. (1999) Wetlands 19, 733-755. [Google Scholar]
- 7.Echelle A. A. & Echelle, A. F. (1997) Conserv. Biol. 11, 153-161. [Google Scholar]
- 8.Perry W. L., Lodge, D. M. & Feder, J. L. (2002) Syst. Biol. 51, 255-275. [DOI] [PubMed] [Google Scholar]
- 9.Barton N. H. (2001) Mol. Ecol. 10, 551-568. [DOI] [PubMed] [Google Scholar]
- 10.Briggs D. & Walters, S. M., (1984) Plant Variation and Evolution (Cambridge Univ. Press, Cambridge), pp. 134–135.
- 11.Thompson J. D. (1991) Bioscience 41, 393-401. [Google Scholar]
- 12.Keller M., Kollmann, J. & Edwards, P. J. (2000) J. Appl. Ecol. 37, 647-659. [Google Scholar]
- 13.Les D. H. & Philbrick, C. T. (1993) Aquat. Bot. 44, 181-228. [Google Scholar]
- 14.Natural Resources Conservation Service U.S. & Department of Agriculture, (2001) THE PLANTS DATABASE V.3.1 (National Data Center, Baton Rouge, LA).
- 15.Bartodziej W. & Ludlow, J. (1998) Aquatics 20, 15-18. [Google Scholar]
- 16.Les D. H. & Mehrhoff, L. J. (1999) Biol. Invas. 1, 281-300. [Google Scholar]
- 17.Patten B. C. (1954) Rhodora 56, 213-225. [Google Scholar]
- 18.Aiken S. G. (1981) Brittonia 33, 57-69. [Google Scholar]
- 19.Graur D. & Li, W.-H., (2000) Fundamentals of Molecular Evolution (Sinauer, Sunderland, MA).
- 20.Page R. D. & Holmes, E. C., (1998) Molecular Evolution: A Phylogenetic Approach (Blackwell Scientific, Oxford), pp. 76–77.
- 21.Rogstad S. H. (1992) Taxon 41, 701-708. [Google Scholar]
- 22.Doyle J. J. & Doyle, J. L. (1987) Phytochem. Bull. 19, 11-15. [Google Scholar]
- 23.White T. J., Bruns, T., Lee, S. & Taylor, J. (1990) in PCR Protocols: A Guide to Methods and Applications, eds. Innin, M. A., Gelfand, D. H., Sninsky, J. J. & White, T. J. (Academic, San Diego), pp. 315–322.
- 24.Johnson L. A. & Soltis, D. E. (1994) Syst. Bot. 19, 143-156. [Google Scholar]
- 25.Maddison D. R. & Maddison, W. P., (2000) MacClade 4: Analysis of Phylogeny and Character Evolution (Sinauer, Sunderland, MA). [DOI] [PubMed]
- 26.Swofford D. L., (1998) paup*, Phylogenetic Analysis Using Parsimony (Sinauer, Sunderland, MA), Version 4.0.
- 27.Corriveau J. L. & Coleman, A. W. (1988) Am. J. Bot. 75, 1443-1458. [Google Scholar]
- 28.Fishbein M., Hibsch-Jetter, C., Soltis, D. E. & Hufford, L. (2001) Syst. Biol. 50, 817-847. [DOI] [PubMed] [Google Scholar]
- 29.Stuckey R. L. & Roberts, M. L. (1977) Sida 7, 24-41. [Google Scholar]
- 30.Coffey B. T. & McNabb, C. D. (1974) Mich. Bot. 13, 159-165. [Google Scholar]
- 31.Floate K. D. & Whitham, T. G. (1994) Oecologia 97, 215-221. [DOI] [PubMed] [Google Scholar]
- 32.Fritz R. S., Nichols-Orians, C. M. & Brunsfeld, S. J. (1994) Oecologia 97, 106-117. [DOI] [PubMed] [Google Scholar]
- 33.Whitham T. G., Martinsen, G. D., Floate, K. D., Dungey, H. S., Potts, B. M. & Keim, P. (1999) Ecology 80, 416-428. [Google Scholar]
- 34.Creed R. P. & Sheldon, S. P. (1993) Aquat. Bot. 45, 245-256. [Google Scholar]
- 35.Creed R. P. & Sheldon, S. P. (1995) Ecol. Appl. 5, 1122-1132. [Google Scholar]
- 36.Solarz S. L. & Newman, R. M. (1996) Oecologia 106, 337-344. [DOI] [PubMed] [Google Scholar]
- 37.Creed R. P. (1998) J. Aquat. Plant Manage. 36, 16-22. [Google Scholar]
- 38.Creed R. P., Sheldon, S. P. & Cheek, D. M. (1992) J. Aquat. Plant Manage. 30, 75-76. [Google Scholar]
- 39.Sheldon S. P. & O'Brien, L. M. (1996) Entomol. News 107, 16-22. [Google Scholar]
- 40.Jester L. L., Bozek, M. A., Helsel, D. R. & Sheldon, S. P. (2000) J. Aquat. Plant Manage. 38, 88-97. [Google Scholar]
- 41.Johnson R. L., Van Dusen, P. J., Toner, J. A. & Hairston, N. G. (2000) J. Aquat. Plant Manage. 38, 78-81. [Google Scholar]
- 42.Ellstrand N. C. & Schierenbeck, K. A. (2000) Proc. Natl. Acad. Sci. USA 97, 7043-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Philbrick C. T. & Les, D. H. (1996) Bioscience 46, 813-826. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.