Abstract
Ecological communities are open to the immigration of individuals and are variable through time. In open habitats immigration may permit populations of a species to persist locally even though local biotic and abiotic processes tend to exclude such “sink” populations. A general model for a sink population reveals that autocorrelated environmental variation can dramatically inflate local abundance and that such populations display a characteristic “outbreak” pattern. An experimental protist microcosm exhibits these predicted effects. Because the many ecological and environmental processes that set the rate of exclusion are typically autocorrelated, these theoretical and empirical results have broad implications for our understanding of community structure and highlight a previously unsuspected potential effect of anthropogenic climate change.
An abiding theme in ecology is that local communities are restricted subsets of regional species pools (1). Because local communities are assembled by immigration and depleted by extinction, community structure may arise from patterns in the success and failure of population growth after immigration, driven either by interspecific interactions with resident community members, abiotic fluctuations in habitat quality through time, or some combination of the two (2). A growing body of theory and evidence suggests that spatial flows of individuals among habitats are essential for maintaining the long-term integrity of populations and communities (3, 4). One consequence of the movement of individuals into habitats that are either intrinsically low in quality or have strong resident competitors or predators is the creation of population “sinks” (5, 6). In a sink habitat, in the absence of immigration, populations suffer extinction because local births are insufficient to balance local mortality. The continued presence of such otherwise excluded species within a local assemblage depends on recurrent immigration from sources, a form of the “rescue effect” (7). But if habitat quality and hence local recruitment vary because of fluctuations in biotic or abiotic environmental variables, then sink populations may experience periods of net positive local growth. The local abundance of species in such habitats should thus reflect the interplay of immigration and temporal fluctuations within the habitat.
One general property of environmental variation of particular importance for ecological dynamics is its autocorrelation structure, which defines the statistical dependency of successive values in a time series. In contrast to random fluctuations where successive values are statistically independent, autocorrelated fluctuations have a serial dependency. This property of environmental variability has been the focus of much recent study because (i) the environmental fluctuations of many physical variables (such as temperature and rainfall) are positively autocorrelated (8–10) and (ii) fluctuations of natural populations, which are often large in magnitude (11), are also often positively autocorrelated (12–15). Theoretical studies have demonstrated that extinction probabilities (16, 17) and various aspects of population (18–20) and community dynamics [e.g., coexistence of competing species (21)] are sensitive to the autocorrelation structure of environmental fluctuations (22). But most of this theoretical work has ignored the potential interplay of temporal and spatial heterogeneity. There has also been little experimental exploration of the population dynamic consequences of the autocorrelation structure of environments (23, 24). In this article we show theoretically and experimentally that in an open sink population autocorrelated fluctuations in habitat quality can increase average population size, even though such fluctuations enhance extinction risk in closed populations. This effect can be quantitatively large in magnitude. We suggest that the “inflationary” effect of autocorrelated variation on population size in sinks has important implications for community ecology, because it permits temporal variability to greatly magnify the impact of regional processes on local population abundance and hence potentially on local assemblage structure. Moreover, an appreciation of this effect has conservation implications for understanding the potential impacts of anthropogenic climate change, which will involve shifts in variance and autocorrelation as well as mean values of climatic variables.
Theory
We consider the dynamics of a population in a sink habitat where it faces deterministic extinction without the rescue of recurrent immigration. A general model for such a population with continuous, overlapping generations is
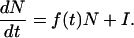 |
Here, N is population density, f(t) the local per-capita growth rate at time t, and I the constant immigration rate; f(t) can be a function of abiotic factors (e.g., temperature) as well as biotic factors (e.g., predator abundance). A species may experience temporal variation in the environment either directly or indirectly through variation in the abundance or actions of other species. We focus on the ability of a species to invade successfully, and so we concentrate on environmental conditions experienced when its density is low. If f(t) varies, so will realized population size, generating a time series of abundances, N(t). Useful properties of model 1 can be derived by using time averaging (25). Formally, over a period T the time average of X(t) is
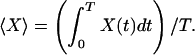 |
In a cyclic environment, T is the cycle period; if the environment is aperiodic but variation is bounded, one takes T as indefinitely large. If N is positive and bounded away from zero, and T is large, then approximately 〈dN/dt〉 = 0, and 〈(dN/dt)/N〉 = 0; thus time-averaged population growth and per-capita growth both equal zero (25). If I = 0, model 1 implies N(T) = N(0)exp(〈f〉T), where 〈f〉 is the average rate of population growth over T. If 〈f〉 is negative as T gets large (as is true by definition in a sink habitat with I = 0), N asymptotically approaches 0. In a constant environment with I > 0 and f(t) = f < 0, equilibrial population size is N* = I/|f|, where |f| is the absolute value of f. Thus, the equilibrial abundance of a sink population can be large if its rate of exclusion from the local environment is low.
Now to consider the impact of temporal variation as expressed in variation in local growth rates, we compare variable environments to constant environments with the same average rate of population decline. Applying time averaging to 1, with both sides divided by N, leads to 〈(dN/dt)/N〉 = 0 = 〈f〉 + I 〈1/N〉, which can be rewritten as Nh = (〈1/N〉)−1 = I/|〈f〉| = N* (N* is evaluated at 〈f〉). Here Nh is the harmonic mean abundance of the sink population, averaged over its fluctuations. The harmonic mean population size thus equals the equilibrium population size N* expected in a constant environment with the same average rate of decline. But the harmonic mean of a set of nonidentical positive numbers is always less than the arithmetic mean. Hence, N* < 〈N〉. In other words, temporal variation in general inflates the mean abundance of sink populations, compared with the abundance expected in constant environments with the same average rate of population decline and immigration rate.
What is the magnitude of this inflationary effect? Assume all growth rates are negative and the rate of change in the environment is sufficiently slow that the population stays near its equilibrium, so that to a good approximation N(t) = I/|f(t)|. If the range of variation in f is small, after Taylor-expanding the right side and dropping all terms after the first three, and then time averaging the resulting expression, we find
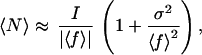 |
where σ2 is the mean-square variation (variance) in f. Hence, the inflationary effect of variation on abundance in a sink habitat increases with the magnitude of variation, and the effect is larger for species experiencing weaker exclusion (i.e., smaller |〈f〉|).
This approximation breaks down if the population does not track its equilibrium (fast environmental variation), or if there are times of positive growth. If the average growth rate is strongly negative and variation is moderate, the habitat is always a sink. But for the same magnitude of variation, if the average rate of decline is less there are likely to be periods with a positive growth rate. These periods of growth, if sustained, have a disproportionate effect on long-term average abundance. We use numerical studies of Eq. 1 with the intrinsic growth a normally distributed random process to demonstrate that these bursts of growth can have a large effect on average population size, particularly if environmental variation is autocorrelated (for details see Appendix).
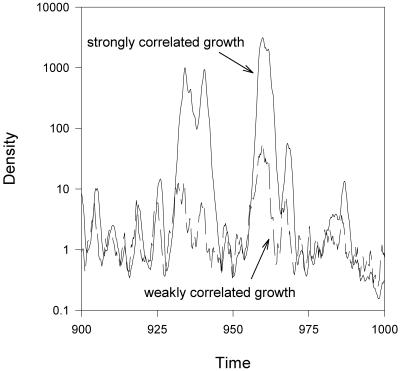
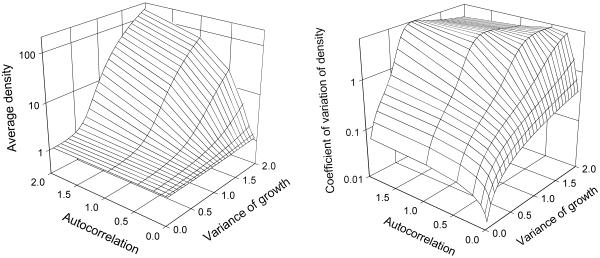
Typical examples of our results are shown in Fig. 1 as a time series of population abundance in sink environments; in essence, immigration maintains populations around a low floor, even in bad years, which then permits the population to capitalize on good years. If environmental variation (as expressed in population growth) is autocorrelated, then there can be runs of good years, which lead to population outbreaks. These outbreaks play a disproportionate role in defining mean abundance, which can be strongly inflated relative to constant conditions. Fig. 2 shows the average population density (〈N〉) and coefficient of variation in (N) as a function of the magnitude of variation in growth rates, and the strength of serial autocorrelation. In the examples shown in Figs. 1 and 2, we assume weak direct density dependence in the sink (consistent with what has been observed in some well-studied sink populations, e.g., refs. 26 and 27). Density dependence reduces population growth, particularly at higher densities, which dampens the positive response of population dynamics to environmental variation. Thus, density dependence reduces, but does not eliminate, the inflationary effect of temporal variation on abundance in a sink habitat (R.D.H., A.G., and M. Barfield, unpublished results). Comparable results are found in models that (unlike 1) explicitly incorporate demographic stochasticity (R.D.H., A.G., and M. Barfield, unpublished results).
Fig 1.
Examples of time series of population size in a variable sink environment, using model 1, with f(t) = r(t) − dN(t). In these examples, the mean intrinsic growth 〈 r 〉 = −1, the immigration rate I = 1, and the strength of density dependence d = 0.001, so in a constant environment N* = 0.999. Examples are shown for two different magnitudes of serial autocorrelation [the SD of r(t) is σr = 1.5]; the autocorrelation time constant = 0.25 in the weakly correlated environment and 2 in the strongly correlated environment. Because we assume weak density dependence in the sink environment, during runs of favorable years the population can outbreak to high densities. See Appendix for technical details and definitions of parameters.
Fig 2.
(Left) Surface plot of time-averaged population size in variable sink environments, as a function of the magnitude of variation (right horizontal axis) and the strength of autocorrelation, tc (left horizontal axis). Other parameters are the same as in Fig. 1. The vertical axis is density on a log scale. (Right) Surface plot of the coefficient of variation (N) as a function of magnitude of variation and the strength of autocorrelation.
Experiment
We used laboratory populations of the bactivorous ciliate protozoan Paramecium tetraurelia to make empirical models of source–sink dynamics to assess the potential validity of the above theory. Source and sink “habitats” (microcosms) were created by using semicontinuous cultures in which density-independent mortality was set by dilution. Variable growth rates were attained by fluctuating temperature.
Materials and Methods
Protist Microcosms.
All populations of P. tetraurelia were maintained with standard protozoan culture methods (28). Replicate microcosms consisted of 100 ml of nutrient medium [made from autoclaved and filtered pond water to every liter of which a single (0.70 g) Carolina Biological Supply protozoan pellet was added] contained within 175-ml glass bottles, covered with an aluminum foil lid. Once autoclaved, the 100 ml of nutrient media was inoculated with three species of bacteria (Serratia marcescens, Bacillus subtilis, and Bacillus cereus) and placed at 18°C in dark, temperature-controlled incubators to allow the development of the bacterial populations. Forty-eight hours later, population growth of P. tetraurelia was initiated by pipetting 1 ml of high-density (≈1,200 individuals per ml) stock culture medium into each microcosm.
Autocorrelated Stochastic Temperature Fluctuations.
To create variable environments, we used a first-order autoregressive model of environmental temperature fluctuations. This homogeneous Markov chain model of environmental variability yields a variance spectrum that can be adjusted from red to white to blue (21). (We use the terms red and white as terms of convenience to denote autocorrelated and uncorrelated fluctuation treatments, respectively, because of the color of the variance spectrum associated with such series.) The temperature experienced by the sink populations at any one time was in one of two states: 4°C or 18°C. The transition between temperatures was governed by the following transition matrix:
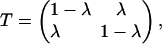 |
where 0 < λ < 1 and λ is the probability of a change in state. We assume a stationary environmental process. When 0 < λ < 0.5, the fluctuations are autocorrelated, and when λ = 0.5, the fluctuations are uncorrelated (the case where 0.5 < λ < 1 creates negatively correlated fluctuations, which typically do not characterize environmental fluctuations and so are not considered further).
For this experiment we created two 50-day (one change in temperature per day) red series (λ = 0.2), denoted R1 (mean = 10.7°C, variance = 49.92) and R2 (mean = 12.4°C, variance = 48). A pair of white series, W1 and W2, was constructed by randomly permuting the elements in R1 and R2, respectively. The treatment pairs R1/W1 and R2/W2 were therefore matching series with each pair possessing the same number of days at 4°C and 18°C and identical mean and variance, differing only in their autocorrelation structure (the generality of the treatment effect was tested by using two different mean temperatures for the two treatment pairs). As a control for growth at 4°C, and to ensure that we had in fact created sink conditions, two constant temperature series (C1 and C2) were also established, giving a total of six fluctuation treatments.
Experimental Design.
Three replicate sink microcosms inoculated with P. tetraurelia were randomly allocated to each of the six temperature fluctuation treatments, R1, W1, R2, W2, C1 (with dilution), and C2 (without dilution).
Source populations.
Two high-density populations at carrying capacity were designated as source microcosms and were randomly assigned to each of the two main fluctuation treatment groups, R1/W1 and R2/W2, for the duration of the experiment. The source populations were maintained in the dark at 18°C as semicontinuous batch cultures; twice a week we replaced 10 ml of nutrient medium with fresh, sterile nutrient medium.
Sink populations.
Previous work established that this strain of P. tetraurelia had a positive, but extremely low, growth rate at 4°C (0.02 ± 0.01 SE cell divisions day−1) (A.G., unpublished observation). Under semicontinuous culture the populations are barely self-sustaining, and therefore these culture conditions cannot quite be considered as true sink habitats. To establish weakly excluding sink conditions during the experiment, every 2 days we removed 60 ml of the nutrient medium (dilution rate of 0.3 day−1) from each microcosm and replaced it with sterile, nonbacterized, nutrient medium. The combination of low growth rates and mortality caused by imposed dilution created sink habitats. Every day throughout the experimental period, and just after dilution, we simulated stochastic dispersal from the assigned source populations to each of the replicate sink populations by pipetting three drops (≈0.35 ml) of well mixed stock culture into each replicate microcosm. Dispersal from the source is thus independent of the fluctuations in the sink populations. Furthermore, dispersal was stochastic in the sense that, although the volume of liquid pipetted is known, the number of individuals contained within these three drops is a sample and so varied somewhat from event to event (and also because of minor fluctuations in the source populations; the number of dispersers will on average be proportional to the density of P. tetraurelia in the source populations, which will not be perfectly constant). Throughout the experiment each sink population was maintained in the dark in one of two temperature-controlled incubators, one set at a constant 4°C and the other at 18°C. Temperature fluctuations experienced by a given sink population were achieved by moving the microcosms between the two incubators in accordance with the assigned temperature series.
Population Density.
Population density was estimated every day over the 50-day duration of the experiment. The microcosms were sampled just before dilution, when conducted. The bottles were gently swirled to produce an even suspension of organisms throughout the water column, and 10 drops of the medium were placed on a Petri dish with a sterile Pasteur pipette. The drops were weighed to 0.0001 g on a Sartorious balance, and the cells were counted by using a stereomicroscope. Because of the high densities occasionally achieved by P. tetraurelia, samples sometimes required dilution before counting.
We periodically estimated the density (as above) of P. tetraurelia in each of the two source populations to verify the relative constancy of the dynamics of the source populations.
Data and Statistical Analysis.
The analysis concentrated on the comparison between treatment pairs W1/R1 and W2/R2. A strict test of the effect of environmental color requires that all other statistical properties of the temperature series (e.g., mean and variance) remain equal; here this was only the case for these matching treatment pairs. The sink control treatments allowed us to verify that low-temperature conditions, particularly with dilution, represented sink conditions.
The maximum density, arithmetic mean, and the coefficient of variation were calculated. These are the statistics that correspond to the predictions of the theoretical analysis. Differences in these summary statistics between treatment groups were tested by one-way ANOVA, and, given the a priori theoretical predictions available to us, differences between the treatment pairs W1/R1 and W2/R2 were tested by planned comparison [using the appropriate F test (29)]. The homogeneity of the variances was verified by Bartlett's test before ANOVA.
To check the existence of sink conditions we measured the growth rate rt at day t from the mean number of cell divisions of P. tetraurelia per day, calculated as follows,
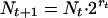 |
giving,
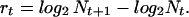 |
It should be noted that this value also incorporates immigrating individuals and therefore is not exactly equal to the intrinsic growth rate.
Results
The population dynamics of P. tetraurelia in the sink systems were substantially altered by the presence of fluctuations in ambient temperature.
The Existence of Sink Habitat Conditions in the Experimental Treatments.
Our results show that the experimental protocol created ambient sink conditions for these microbial populations. Establishing this fact was a necessary preamble to evaluating the more specific predictions we made about inflationary impacts of autocorrelated growth conditions in sink habitats.
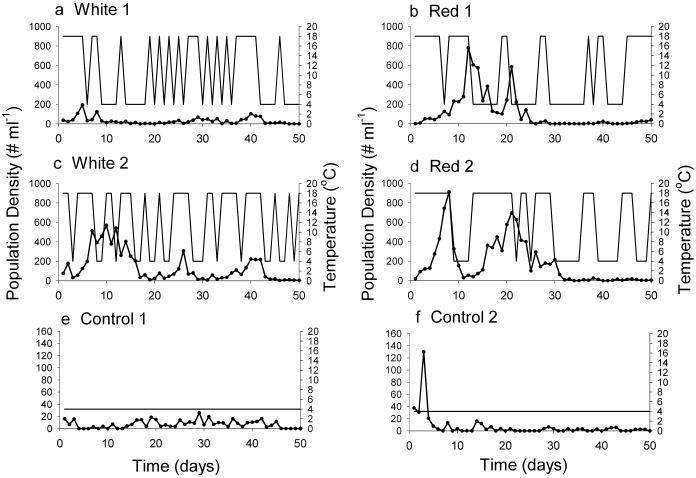
An immigration event represented on average 418 ± 114.1 (SD) individuals per ml from source 1 and 464 ± 74.1 individuals per ml from source 2 (from day 6 onward; the first 6 days were characterized by very high densities, 1,318 ± 114.2 individuals per ml). Over the period of the experiment, this finding certainly sufficed to establish sink populations, and it corresponds to our theoretical assumption in model 1 that there was a steady stream of immigrants. Both control treatments at low temperatures (with and without dilution, but with steady immigration) were characterized by consistently very low densities. Populations exposed to fluctuating temperatures periodically exhibited elevated densities (Fig. 3). Consistent with our assumption of sink conditions, the estimated mean growth rate for all treatments was negative (Table 1), although weakly so (i.e., “weak exclusion”).
Fig 3.
Experimental time series of P. tetraurelia (•, daily densities) under differing temperature regimes (continuous lines; e.g., horizontal for constant temperature treatment). a and b correspond to a replicate from each of the treatments W1 and R1 described in the text. c and d correspond to a replicate from each of the treatments W2 and R2. e and f correspond to the two constant, low-temperature sink controls.
Table 1.
Summary statistics with means for the replicate sink populations calculated over the duration of the experiment
| Treatment | Maximum density | Arithmetic mean density | CV | r |
|---|---|---|---|---|
| Nondiluted C1,1 | 72.4 | 12.8 | 97.2 | −0.10 |
| C1,2 | 70.1 56.1 | 8.4 9.5 | 102.6 95.8 | −0.08 −0.09 |
| C1,3 | 25.8 | 7.2 | 87.4 | −0.08 |
| Diluted C2,1 | 114.8 | 9.3 | 128.7 | −0.15 |
| C2,2 | 130 162.7 | 6.7 12.2 | 143.2 144.8 | −0.15 −0.16 |
| C2,3 | 243.3 | 20.6 | 162.5 | −0.17 |
| Mean 10.7°C W1,1 | 193.1 | 31.1 | 119.2 | −0.11 |
| W1,2 | 374.6 236 | 40.8 31.3 | 156.7 139.8 | −0.10 −0.10 |
| W1,3 | 140 | 22.0 | 143.5 | −0.10 |
| Mean 10.7°C R1,1 | 627.5 | 122.4 | 128.9 | −0.009 |
| R1,2 | 817.2 742 | 147.5 126.7 | 147.4 146.3 | −0.005 −0.01 |
| R1,3 | 780.7 | 111.1 | 162.4 | −0.008 |
| Mean 12.4°C W2,1 | 570 | 137.5 | 112.9 | −0.08 |
| W2,2 | 600 552 | 135.1 138.5 | 100.9 100.0 | −0.02 −0.03 |
| W2,3 | 486 | 142.8 | 86.0 | −0.04 |
| Mean 12.4°C R2,1 | 1,037 | 178.5 | 125.1 | −0.11 |
| R2,2 | 1,077 1,008 | 198.2 185.2 | 123.4 124.6 | −0.09 −0.10 |
| R2,3 | 910 | 179.5 | 125.4 | −0.10 |
CV = coefficient of variation, r = growth rate. Cij, Wij, and Rij denote, respectively, the ith control, white, and red treatments, and jth replicates.
Differences in the summary statistics (see Table 1 and below) of these populations indicate two main types of effect: an effect of fluctuation autocorrelation and a simple effect of mean temperature.
Maximum and Arithmetic Mean Densities.
Temperature fluctuations resulted in significantly different maximum densities among treatments (F[5,12] = 60.4, P < 0.001). Maximum densities were greater under autocorrelated fluctuations, R1 > W1 (F[1,12] = 56.5, P < 0.001) and R2 > W2 (F[1,12] = 45.9, P < 0.001). In addition, there was a marked effect of the 1.7°C increase in mean temperature, with R2 and W2 replicates reaching substantially higher maximum densities than those observed in the R1 and W1 replicates (F[1,12] = 37.35, P < 0.001). No significant difference in maximum density was observed between the two sink control group types (F[1,12] = 2.29, not significant).
Mean population densities differed significantly among fluctuation treatments (F[5,12] = 159.9, P < 0.001), with greater values attained under autocorrelated fluctuations, R1 > W1 (F[1,12] = 128.3, P < 0.001) and R2 > W2 (F[1,12] = 30.6, P < 0.001).
Population Variability.
The coefficient of variation in population density varied significantly across temperature treatments (F[5,12] = 7.82, P = 0.002) and was greater under autocorrelated temperature fluctuations but was only significantly so for the 12.4°C treatment, R2 > W2 (F[1,12] = 4.75, P = 0.01) and R1 = W1 (F[1,12] = 0.35, not significant). The C2 control was more variable than the C1 control (F[1,12] = 18.43, P < 0.01), again demonstrating the effect of habitat dilution.
Discussion
We have shown theoretically that autocorrelated environments in a sink can both inflate average abundance and lead to a characteristic pattern of population outbreaks. We have also shown that both of these theoretical predictions can be observed in an experimental microcosm. Generalizations of the specific model 1 explored above are needed to corroborate the generality of the phenomena demonstrated by the experimental results. Preliminary studies suggest that the inflationary effect of autocorrelated variation on local population size in a sink habitat is robust to the incorporation of demographic stochasticity and interspecific interactions (R.D.H., A.G., and M. Barfield, unpublished work).
Inflation.
The theoretical expectation that fluctuations in growth can inflate average abundance in sink populations was clearly apparent in our experimental results. We believe that these results have important implications for our understanding of natural ecological communities. Populations excluded from local communities by processes such as competition, predation, and maladaptive habitat selection can nonetheless be present at high average abundance, if the rate of exclusion is weak and the rate of immigration is high. It is likely that the effect we have explored is particularly relevant to species excluded from local communities by interspecific interactions. The strength of interspecific interactions imposed by residents on invading species usually will vary with resident population size. Because population densities usually are spatially and temporally autocorrelated the rate of exclusion arising from interspecific interactions (e.g., competition, predation) in turn should also be autocorrelated.
We have shown that the inflationary effect of temporal variability is magnified if this variability is autocorrelated. Recently theoretical work has demonstrated that the correlation structure or variance spectrum of environmental fluctuations can strongly influence population persistence. In particular, prior theoretical studies have suggested that autocorrelated fluctuations, or variance spectra dominated by low frequencies (a “reddened” spectrum), can greatly elevate extinction probabilities (17, 18). However, none of this work has explicitly considered source–sink dynamics, in particular the impacts of temporal variation in sink environments.
For many purposes, time-averaged population size is an important and useful measure. For instance, to assess the role of a species in ecosystem processes, what often matters is the size of the standing crop (e.g., amount of nitrogen on average immobilized in biomass), which is precisely the arithmetic mean for a fluctuating population. Some ecologists have argued that many species have stable source populations sustaining an array of fluctuating sink populations (30). If these fluctuating sink populations are spatially uncorrelated then the time average of abundance in a single population should tend to equal the average abundance over space among populations. So the total number of individuals sustained in such arrays of sink habitats could be large. Our results suggest that sink habitats could contribute substantially to the measured total abundance of species in fluctuating environments, and thus, given changes in connectivity (lowering immigration) or habitat degradation (lowered local growth rates), one could see large and unexpected declines in total regional abundance (4). In general, autocorrelated variation may magnify the effect of regional processes mediated by immigration, affecting which species may coexist in local communities (21).
Outbreaks.
The second theoretical prediction is that correlated environments in sink habitats can generate a characteristic pattern of population outbreaks. This kind of dynamic is well known in empirical field studies (32) and clearly characterized the dynamics we observed in the microcosms. In open communities many rare species may be important and abundant episodically and so facilitate the temporal stability of community and ecosystem processes (33, 34), even in habitats where on average these species would be excluded were it not for immigration (35).
We also note that human-induced climate change may involve not only changes in mean conditions, but also changes in the temporal correlation structure of environments (10). To our knowledge, this possibility has been entirely ignored in the literature as a potential climate change impact. Our models and experiments suggest that shifts in climate correlation structure may have important consequences for the abundance and dynamics of populations. For instance, an increased autocorrelation could lead to accentuated outbreak dynamics in the abundances of many rare species, for example, at the margins of species' ranges (where many populations may be in sink habitats), even if the average environmental conditions were not to change.
We have shown that a sink population sustained by immigration in a temporally variable environment can be both abundant on average and exhibit sporadic outbreaks to high densities. Putting these ideas together suggests that immigration from regional species pools should have the greatest impact on local species richness and community structure when (i) species are weakly excluded and (ii) local community dynamics or the abiotic environment is temporally variable and autocorrelated. Beyond the specific details of the models and our experimental system, we believe these results highlight the many surprises that await us as we try to develop a deeper understanding of ecological systems that are both extended in space and variable through time.
Acknowledgments
We thank John Lawton and the Centre for Population Biology, Imperial College, University of London, Silwood Park, Ascot, U.K., for support during early stages of this work. We also thank Michael Barfield for conducting the simulations and preparing the associated figures. R.D.H. thanks the National Science Foundation and the University of Florida Foundation for support.
Appendix
We simulated Eq. 1 with the growth rate f(t) = r(t) − dN(t), where r(t) is the intrinsic growth rate at time t, and d is the (invariant) strength of density dependence. A normally distributed random process was used for r(t), with mean r0, standard deviation σr, and exponential autocorrelation function R(τ) = σ exp(−τ/tc). In this expression, τ is the lag time and tc is a time constant (the lag time required for the autocorrelation to decay by a factor e). A fixed step size Runge–Kutta algorithm was used with time step Δt. Successive values of the intrinsic growth rate were calculated by using
exp(−τ/tc). In this expression, τ is the lag time and tc is a time constant (the lag time required for the autocorrelation to decay by a factor e). A fixed step size Runge–Kutta algorithm was used with time step Δt. Successive values of the intrinsic growth rate were calculated by using
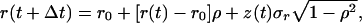 |
where ρ = exp(−Δt/tc) and z(t) is a sequence of independent, zero-mean, unit-variance normal random deviates. It can be shown that the sequence generated has a mean of r0 and standard deviation σr at every time step. The correlation coefficient between consecutive values is ρ, and that between values separated by n time steps is ρn = exp(−nΔt/tc). This is the discrete equivalent to the autocorrelation function given above.
References
- 1.Ricklefs R. E. (1987) Science 235, 167-171. [DOI] [PubMed] [Google Scholar]
- 2.Chesson P. & Huntly, N. (1997) Am. Nat. 150, 519-553. [DOI] [PubMed] [Google Scholar]
- 3.Hanski I. A., (1999) Metapopulation Ecology (Oxford Univ. Press, London).
- 4.Gonzalez A., Lawton, J. H., Gilbert, F. S., Blackburn, T. M. & Evans-Freke, I. (1998) Science 281, 2045-2047. [DOI] [PubMed] [Google Scholar]
- 5.Holt R. D. (1985) Theor. Popul. Biol. 28, 181-208. [Google Scholar]
- 6.Pulliam H. R. (1988) Am. Nat. 132, 652-661. [Google Scholar]
- 7.Brown J. H. & Kodric-Brown, A. (1977) Ecology 58, 445-449. [Google Scholar]
- 8.Mandelbrot B. B. & Wallis, J. R. (1969) Water Resourc. Res. 5, 321-339. [Google Scholar]
- 9.Steele J. H. (1985) Nature 313, 355-358. [Google Scholar]
- 10.Wigley T. M. L., Smith, R. L. & Santer, B. D. (1998) Science 282, 1676-1679. [DOI] [PubMed] [Google Scholar]
- 11.Schoener T. W. (1985) Am. Nat. 126, 633-641. [Google Scholar]
- 12.Pimm S. L. & Redfearn, A. (1988) Nature 334, 613-614. [Google Scholar]
- 13.Arino A. & Pimm, S. L. (1995) Evol. Ecol. 9, 429-443. [Google Scholar]
- 14.Gilman M. P. & Dodd, M. E. (1998) Bot. J. Linn. Soc. 126, 65-74. [Google Scholar]
- 15.Inchausti P. & Halley, J. (2001) Science 293, 655-657. [DOI] [PubMed] [Google Scholar]
- 16.Ripa J. & Lundberg, P. (1996) Proc. R. Soc. London Ser. B 263, 1751-1753. [Google Scholar]
- 17.Petchey O. L., Gonzalez, A. & Wilson, H. B. (1997) Proc. R. Soc. London Ser. B 264, 1841-1847. [Google Scholar]
- 18.Heino M. (1998) Oikos 83, 368-375. [Google Scholar]
- 19.Morales J. M. (1999) Ecol. Lett. 2, 228-232. [Google Scholar]
- 20.Roughgarden J. (1975) Am. Nat. 109, 713-736. [Google Scholar]
- 21.Caswell H. & Cohen, J. E. (1995) J. Theor. Biol. 176, 301-316. [Google Scholar]
- 22.Ripa J., Lundberg, P. & Kaitala, V. (1998) Am. Nat. 151, 256-263. [DOI] [PubMed] [Google Scholar]
- 23.Cohen A. E., Gonzalez, A., Lawton, J. H., Petchey, O. L., Wildman, D. & Cohen, J. (1998) Proc. R. Soc. London Ser. B 265, 11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petchey O. L. (2000) Proc. R. Soc. London Ser. B 267, 747-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levins R. (1979) Am. Nat. 114, 765-783. [Google Scholar]
- 26.Keddy P. A. (1981) J. Ecol. 69, 615-630. [Google Scholar]
- 27.Keddy P. A. (1982) Oecologia 52, 348-355. [DOI] [PubMed] [Google Scholar]
- 28.Lawler S. P. & Morin, P. J. (1993) Am. Nat. 141, 675-686. [DOI] [PubMed] [Google Scholar]
- 29.Sokal R. R. & Rohlf, J. F., (1995) Biometry (Freeman, New York).
- 30.Holt R. D. (2003) in Food Webs at the Landscape Scale, eds. Polis, G. A., Power, M. & Huxel, G. (Univ. of Chicago Press, Chicago), in press.
- 31.Harrison S. & Taylor, A. D. (1997) in Metapopulation Biology, eds. Hanski, I. A. & Gilpin, M. E. (Academic, San Diego), pp. 27–42.
- 32.Williamson M., (1972) The Analysis of Biological Populations (Edward Arnold, London).
- 33.Yachi S. & Loreau, M. (1999) Proc. Natl. Acad. Sci. USA 96, 1463-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker B., Kinzig, A. & Langridge, J. (1999) Ecosystems 2, 95-113. [Google Scholar]
- 35.Gonzalez A. & Chaneton, E. J. (2002) J. Anim. Ecol. 71, 594-603. [Google Scholar]