Abstract
One of the most controversial debates in evolutionary biology concerns the fitness consequences of female choice in nature. Discriminating females may benefit from high-quality territories and/or sires with high-quality genes. Here we experimentally dissociate female preferences for high-quality territories and male body size in a wild population of side-blotched lizards, Uta stansburiana. Females preferred experimentally improved territories but still chose high-quality sires for their progeny. High-quality territories were associated with earlier egg-laying dates and larger eggs. These maternal effects, evidently stimulated by high-quality territories, have been shown to enhance competitive ability and therefore the likelihood that philopatric offspring will capitalize on the direct benefits of superior territories, previously shown to promote progeny growth rate and survival (most offspring do not disperse from their natal area). Paternity analysis within clutches co-sired by two males revealed that female preferences for large males were also adaptive in terms of indirect benefits. Females used sperm from large sires to produce sons and sperm from small sires to produce daughters. Differential allocation of resources and progeny sex was adaptive and demonstrates a high degree of female control in the mating system.
Based on both field and laboratory studies, female choice is now widely accepted as an important component of sexual selection (1). However, few experiments have been carried out in a natural setting to test whether females have adaptive control over mating in the face of territorial male strategies. Numerous studies, especially with birds, have shown that strategic mate choice directly benefits females through the accumulation of resources on high-quality territories (2, 3). Females can also accrue indirect genetic benefits by choosing high-quality sires for their progeny (4). If direct and indirect benefits of mate choice are dissociated, females may face a tradeoff between the two.
Females that prefer males with high-quality territories can also gain indirect benefits through extra-pair copulations with high-quality males (5, 6). Females that engage in extra-pair copulations with high-quality males have been shown to differentially allocate energy (7, 8) or gender (9, 10) to favor progeny sired by their preferred mate. A comprehensive mate-choice assay therefore must test simultaneously for both direct and indirect components of fitness.
Side-blotched lizards provide an excellent system in which to test the adaptive significance of female preferences for direct and indirect benefits, because territory quality can be experimentally uncoupled from male quality in the mating system by moving rocks between adjacent male territories. Furthermore, progeny can be sexed at birth, allowing estimates of the sex ratio of progeny and the amount of energy invested in progeny by sex. Finally, the fitness consequences of different investments can be assessed by studying progeny survival to maturity, because females live within the territories of males, and most young do not disperse far from their natal outcropping (11–14).
Methods
Male side-blotched lizards, Uta stansburiana, exhibit one of three genetically based alternative throat colors (orange, blue, or yellow), each with an associated mating behavior (11, 12) and territorial strategy (13, 14). To study female preferences for male body size irrespective of throat color, we focus experiments here on the mate-guarding territorial strategy of blue-throated males. This study was facilitated by the fact that during the spring of 1999 and 2000, blue-throated males accounted for nearly 90% of the territorial males in our study population.
Territory Quality Manipulations.
Before the onset of mating, we experimentally uncoupled the natural association of large male body size with high-quality territories (14). Females become receptive to mating 10 days to 2 weeks before ovulation (4- to 5-mm follicular diameter), and we performed our manipulations before the point at which the first females became receptive. Territories were mapped by a single observer from daily visual censuses (mean = 7.7 and 6.9 sightings per male based on n = 381 and 654 total sightings, respectively, for males and females on two experimental plots). After initially mapping lizard territories, we removed rocks from the territories of large males and deposited them on the territories of their smaller male neighbors. Rock additions consisted of 10–40 rocks each (mean = 15.7, SD = 11.1) and provided perch heights of 0.5–1.0 m, similar to high-quality sites that occur naturally in the wild (15). We manipulated territory quality between 27 pairs of males (13 and 14 pairs of males on each of two plots). Adding rocks to a male's territory improves the quality of a male's territory by providing an increased range of microclimates available for behavioral thermoregulation. We have shown previously (14, 15) that experimentally improved territories also enhance progeny growth rate and survival. We controlled for the disruptive effects of rock movements by moving rocks within the territory boundaries of 22 males (i.e., 11 pairs) on one additional outcropping. No changes in territory quality were made on our control plot, because we did not change the total amount of rock available to each male, just the spatial location on the territory.
At the end of the breeding season (May) we captured near-term gravid females from experimental plots and brought them into the laboratory to lay their eggs. We incubated eggs under standard conditions until hatching (16). Progeny were sexed at hatching (males have enlarged postanal scales), weighed, and assigned a unique toe clip that permanently identifies individuals. To examine potential direct benefits, we tested for the possible adaptive significance of territory quality to females. We calculated the mean egg-laying dates for females on high- and low-quality territories. We also calculated the mean egg mass of sons and daughters that were produced by females on high- and low-quality territories. Partitioning our analyses between sons and daughters is biologically relevant, because females differentially allocate resources according to the sex of their progeny (see below).
Adaptive Significance of Female Preference in Nature.
We also tested for the adaptive significance of female preference for male body size. Female side-blotched lizards are extremely promiscuous, and clutches of eggs show high rates of multiple paternity (81%; ref. 12), allowing us to compare attributes of progeny that are due to differences in sire quality. We compared sex, condition, and survival of progeny from 20 clutches in which two different males co-sired a female's clutch of eggs. Sample sizes for this test differ from the above field experiment, because analyses include data from all clutches with multiple sires irrespective of territory quality. Comparing half-sibs within clutches is a powerful method that allows us to account for differences among progeny due to sire quality alone, holding variation due to female quality constant.
We measured progeny condition using a classic reptilian estimate of body condition (the residuals about the mass/snout-vent-length regression line) in our analyses (17). We estimated offspring survival by recapturing all progeny from our experimental plots during the Fall after hatchling release. Lizards that were not captured during this period were considered to have died. In addition, we censused lizards on all rock outcroppings surrounding our experimental plots out to a distance of 1 km (13), which allowed us to measure mortality and record any dispersal events that may have occurred as a result of territory manipulations. None were recorded.
DNA Paternity.
We used nine microsatellite markers to assign paternity. We extracted genomic DNA from toe tissue by overnight incubation at 55°C in 500 μl of 5% Chelex (Bio-Rad) and 2 μl of Proteinase K solution (20 mg/ml) and centrifuged and diluted it 1:10. We amplified the nine microsatellite loci from this extract via PCR (12) and assessed length polymorphism with fluorescently labeled forward primers (12) on an automated DNA sequencer (ABI 373). We assigned paternity with the program KINSHIP (18), which uses maximum likelihood and incorporates exclusionary criteria. Results using KINSHIP and other paternity-assignment software are similar (12), and we report only the results from KINSHIP. We accepted a male as a hatchling's sire if the likelihood of paternity was significantly different from that expected for unrelated males (P < 0.05). To estimate paternity we used detailed territory maps (above). The site was subdivided into neighborhoods separated by unsuitable adult habitat. We searched for putative sires within the female's neighborhood and assigned as sire the male with the highest significant likelihood value. We also screened male genotypes in a female's adjacent and immediate neighborhoods. Males from this larger area were assigned as sire only if their likelihood values were higher than those for males in the female's immediate neighborhood (12).
Statistical Methods.
Parametric tests were appropriate for all analyses unless otherwise noted. To account for heteroscedasticity in the distribution of male reproductive success, we used the Behren's Fisher F test to measure differences in male fitness. Differences in the production of sons and daughters within a clutch were estimated with a multivariate ANOVA (Pilai-Trace) in which the numbers of sons and daughters were entered simultaneously as dependent variables against the independent factor “sire body size” (19). We computed directional selection gradients (20) on mass (β1) and condition (β2) for sons and daughters and compared the difference between the progeny sexes with analysis of covariance (21). Fitness surfaces of progeny survival to maturity were visualized with a multivariate cubic spline (22), and we verified the significance of differences in selection gradients on mass and condition (e.g., results of the analysis of covariance) between sons and daughters with a delete-one jackknife (23).
Results
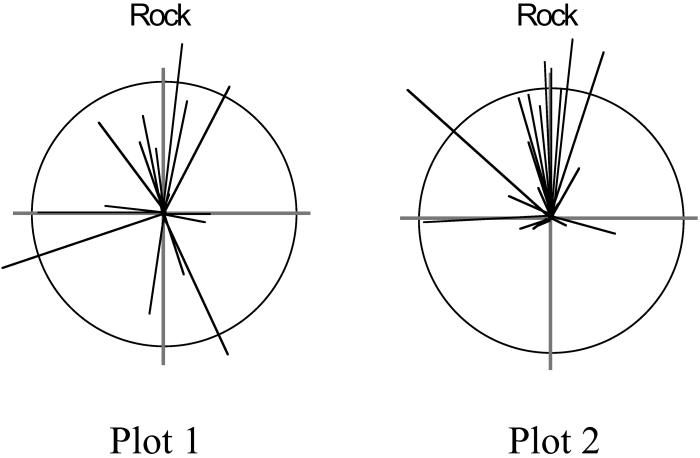
Females exhibited strong preference for high-quality territories after our manipulations, and we saw a significant redistribution of females onto rock additions. Of the 51 females on experimental plots, 37 moved onto improved territories and none moved onto reduced quality territories (Rayleigh's U test, P < 0.0001 and P < 0.005 on plots 1 and 2, respectively; Fig. 1; ref. 24). In contrast to females, none of the 54 blue-throated males moved in response to our manipulations, even though males with improved territories were smaller in body size than males on reduced quality territories. We did not measure any significant changes in territory distribution (e.g., territory size or the position of the territory centroid) resulting from our control manipulations [repeated-measures ANOVA, treatment × repeat, F(1,11) = 0.60, P = 0.47].
Fig 1.
Distribution of female movements in response to territory manipulations. Each vector represents one female. The angle circumscribed by each vector from 0° (Rock) describes the deviation of a female's observed path compared with the path predicted if she were to move directly toward rock additions. Vector length is given by the ratio of the distance a female moved relative to the expected distance (circle with radius one). A female that moved directly onto an experimental rock addition would appear as a vector pointing 0° and touching the unit circle.
Manipulations of the thermal environment associated with rock piles uncoupled the direct and indirect components of female choice by reversing the outcome of male–male competition, because moving rocks between territories allowed small males to control the best territories. Strong female preference for rock piles and female movement onto high-quality territories forced females to associate with small males that were not their original choice of mate. However, the potential cost of associating with small males may have been ameliorated by direct benefits associated with high-quality territories.
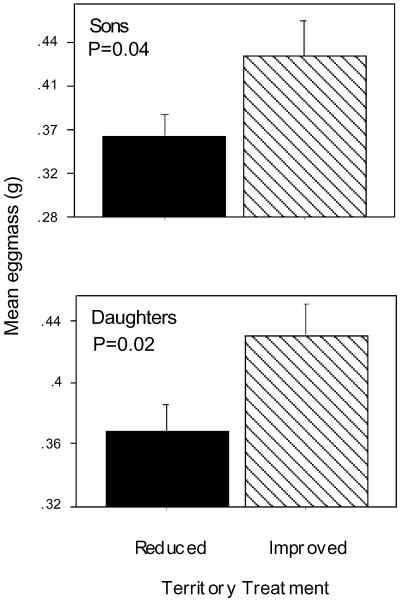
Females on high-quality territories laid their eggs an average of 5.7 days earlier than females on low-quality territories [ANOVA, F(1,13) = 5.36, P = 0.03], thereby increasing the likelihood that they would produce additional clutches of eggs (25) and their progeny would compete successfully and survive better than progeny that were laid later in the season (26). Females on high-quality territories also produced larger eggs for both sons and daughters than did females on low-quality territories [analysis of covariance for sons egg mass, F(1,11) = 4.97, P = 0.04, covariate for mother's mass P = 0.09; for daughters egg mass, F(1,11) = 7.25, P = 0.02, covariate for mother's mass P = 0.14; Fig. 2]. Laying dates and egg-size effects were not simply due to differences in female size, because there was no difference in postlaying mass between females on experimentally improved and reduced quality territories [ANOVA F(1,18), F = 2.07, P = 0.17]. Moreover, egg size has been shown previously to be one of the most important components of female fitness (27) and to be under strong selection. Better endowed offspring that are laid early and come from relatively large eggs are more likely to benefit from high-quality territories, because most young do not disperse from their natal outcropping (11–14) and high-quality territories are known to enhance progeny growth rate and survival (14, 15). Thus, high-quality territories significantly improved fitness, and female preference for good sites seems to be adaptive. However, egg-size adjustment is clearly under the control of the female and thus subject to an adaptive maternal effect (28). Definitively ruling out maternal effects in the analysis of life-history traits will require further investigation (see Discussion).
Fig 2.
Females gain direct benefits from residing on high-quality territories. Females on high-quality territories produce a larger mean egg mass for both sons (Upper) and daughters (Lower) compared with females on low-quality territories. The histogram bars show the mean difference for all females (+1 SE).
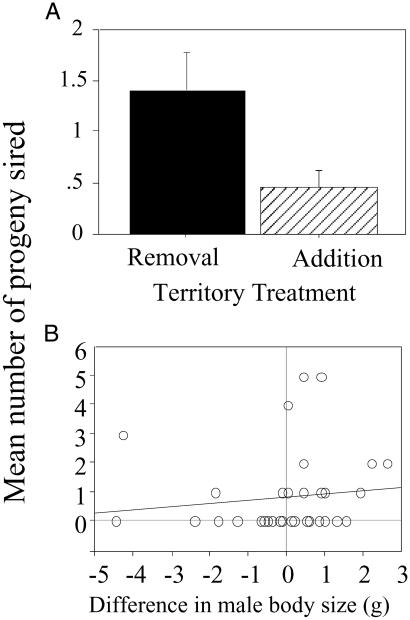
Paternity analyses revealed a 4-fold difference in overall reproductive success between small males on experimentally improved territories and large males on experimentally reduced quality territories. Large males on reduced quality territories had significantly higher reproductive success than their smaller male neighbors on improved territories [Behren's Fisher F(1,32) = 4.94, P < 0.04; Fig. 3], although larger males no longer controlled the female's preferred territory.
Fig 3.
(A) Differences in reproductive success (number of progeny sired) between males on experimentally improved and reduced quality territories. Males on reduced quality territories sired more than four times as many progeny as males on experimentally improved territories despite the fact that most females moved onto improved territories (see Fig. 1). The histogram bars show the mean (+1 SE). (B) Differences in reproductive success among males are attributable to the relative difference in male body size on adjacent territories. Males of equal body size are indicated by the vertical line at zero. Relatively larger males sired significantly more progeny than relatively smaller males (Spearman rank test, ρ = 0.36, P < 0.03).
The high incidence of multiple paternity in side-blotched lizard clutches (81%; ref. 12) allowed us to estimate the indirect fitness consequences of mating with small versus large males for those females that mated with two or more sires. We measured a significant effect of sire body size on progeny fitness. Progeny sired by the larger of the two males were themselves larger (paired t test, t = 3.76, df = 10, P < 0.004) and in better condition at hatching (ref. 17; paired t test, t = 3.30, df = 10, P < 0.008) than progeny sired by the smaller of the two males. Moreover, females were more likely to produce sons with eggs fertilized by the larger male and daughters with eggs fertilized by the smaller male [multivariate, F(1,36) = 3.94, P < 0.03; ref. 19].
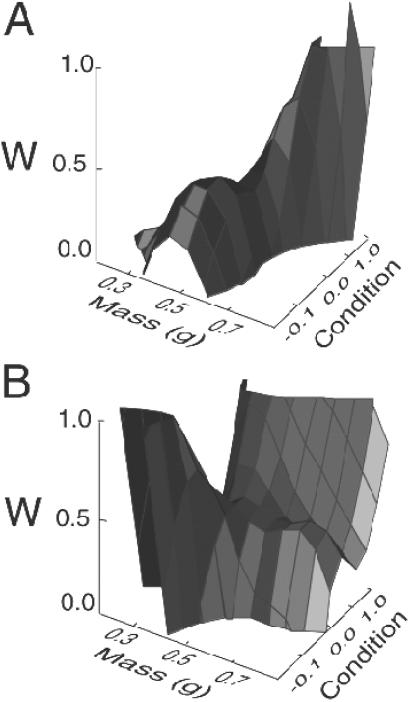
Analysis of covariance indicates that the difference in selection differentials acting on survival of sons versus daughters (Δβi = βi,son − βi,daughter) was significant for both hatchling mass [Δβmass = 0.743 ± 0.374, t = 2.290 based on a delete-one jackknife (23), P < 0.03] and hatchling condition [Δβcondition = −0.999 ± 0.333, t = −3.075 (23), P < 0.005]. The form of selection on the traits hatchling mass and hatchling condition is complex (Fig. 4), reflecting both stabilizing and disruptive selection that is typical for this species (28). Nevertheless, the analysis of covariance indicates a net directional selection gradient for larger sons and smaller daughters [e.g., a difference in optima for sons versus daughters (27); Fig. 4]. Allocating resources to produce large sons and small daughters thus was favored by selection.
Fig 4.
(A) Probability of survival as a function of hatchling mass and condition for sons. (B) Probability of survival as a function of hatchling mass and condition for daughters. Fitness surfaces for hatchling mass and condition of male and female progeny were fitted with a multivariate cubic-spline regression (22). The difference in directional selection gradients on sons versus daughters (see Discussion) favored females that could simultaneously produce large sons and small daughters. That is, the ridge of high fitness for sons occurs at larger values of hatchling mass than does the ridge for daughters. The complex disruptive surface for daughters is not an artifact of our plotting technique but reflects correlational selection on two alternative female morphs, which are discussed elsewhere (27).
Discussion
Although a few studies have demonstrated female preference for both direct and indirect benefits (29), the fitness consequences of such preferences have remained one of the most controversial topics in studies of sexual selection (4). We have shown that female side-blotched lizards prefer high-quality territories and that this preference significantly enhances female fitness. We further demonstrated a strong female preference for large male body size. Two lines of evidence suggest that preference for large males is also adaptive. First, progeny sired by large males are larger and in better condition than progeny sired by smaller males. Second, females bias the sex of their offspring by producing sons with eggs fertilized by larger sires, and daughters with eggs fertilized by smaller sires (30–32). This result differs from other results concerning sex-ratio modulation, because sex ratio per se is not biased toward one sex. Rather, females produce even sex ratios within a clutch but the production of sons and daughters depends on the phenotype of the sire.
A relative increase in the production of sons further supports the hypothesis that females demonstrate a preference for large males, because females should experience higher fitness gains from high-quality sons than daughters (10). Marginal gains in progeny fitness differ as a function of investment between sons and daughters, and thus females should be under selection to invest differently in male and female progeny. Thus females that mate with high-quality males can gain indirect (i.e., genetic) benefits by making sons. Furthermore, by selectively fertilizing progeny with sperm from different sires, the female can produce both sons and daughters with high fitness. That is, large and small males can both confer benefits, but the benefits depend on female control of progeny sex. Fitness benefits to progeny from sires of different body sizes represent indirect benefits accrued to females as a consequence of mate choice. Although we do not present data on the production of grand offspring, we have shown previously that large males are more likely to secure high-quality territories (15) that are attractive to females and that small female progeny survive well in low-density years (27).
An alternative interpretation of our data is that differences among offspring represent effects due to sire quality. For example, mating-order effects (33), differences in sperm quality or quantity, forced copulations by larger males, or some form of a selfish element that gives male progeny an advantage at obtaining resources from the female (34) are all possible alternative interpretations of our results. Although we cannot rule out a sire effect definitively, we suggest that the female-allocation hypothesis provides a more compelling explanation for several reasons. First, natural selection favored females that produced both larger sons and smaller daughters at hatching (Fig. 4). The mutually beneficial effects on both sons and daughters suggest that differential resource allocation (e.g., a maternal effect) is adaptive to females. High survival of small female progeny is consistent also with the reproductive strategies of adult females (27) in that 1999 was a low-density year that favored small female progeny. Sinervo et al. have shown previously that two female morphs drive a population density cycle and small female progeny size is favored at low density, but large female progeny size is favored at high density (27). Previous work on the endocrine control of progeny size (25) suggests that females can alter the size of sons and daughters by modulating circulating levels of plasma corticosterone. In order for a selfish male effect to have a selective advantage, males would need to be able to anticipate a female's allocation of resources to her progeny.
Second, if ejaculate quantity or quality differed as a function of sire body size, then large males would be expected to sire the majority of progeny in a polyandrous female's clutch. However, the number of progeny sired by large and small sires within a clutch was not significantly different [ANOVA, F(1,34) = 0.63, P = 0.43]. In addition, if large males produced mostly Y sperm, then large sires should have produced sons in all reproductive episodes. We investigated this possibility by analyzing cases in which males were involved in multiple paternity bouts in two or more females. Males that produce sons with one female do not produce sons with all females [F(9,10) = 1.103, repeatability = 0.046, P = 0.44; R.C. and B.S., unpublished data]. Finally, the question of second male precedence or increased incidence of forced copulations by larger males is far too complex to address in the current study and may be the subject of future studies in this system. However, neither of these alternatives would provide a compelling explanation for the observed patterns of sex allocation by polyandrous females.
Our experimental uncoupling of the association between direct and indirect benefits allowed us to take steps toward directly measuring the adaptive significance of both territory quality and male quality. The timing of our manipulations ensured that females would not have copulated before rock movement. Follicular development follows a predictable pattern of weekly growth. Females begin yolking follicles in early Spring and ovulate within 4 weeks. Females become receptive to mating 10 days to 2 weeks before ovulation (≈5-mm follicle diameter; ref. 11). We performed our rock manipulations before the first female could potentially be receptive (1- to 3-mm follicular diameter).
One limitation of this study is that our measures of direct benefits (e.g., egg size or laying date) are likely to be under maternal control. Thus, female preferences for sire quality (indirect benefits) may influence her life-history allocation decisions to individual progeny and confound the distinction between direct and indirect benefits of mate choice. We point out that this will always be the case in nature, and nothing short of artificial insemination in which the female has no direct knowledge of the sires phenotype could control for such female-allocation effects. In nature, large males typically control the highest quality territories (15), and females may, for example, assess male body size as an indicator of the thermal quality of territories (e.g., male body size as a sexually selected “thermometer”) and invest more in current reproduction and individual progeny. In support of this idea, although juvenile survival is enhanced on improved quality territories, adult survival is reduced on high-quality territories, suggesting that life-history trade-offs such as costs of reproduction (25) may influence plastic female-allocation decisions (15). Thus, the potential influence of female mate assessment followed by adaptive maternal plasticity in allocation limits our ability to fully separate direct and indirect fitness components of mate choice. This may generally be true of most systems in nature; reconciling the magnitude of indirect versus direct benefits presents a formidable challenge in evolutionary ecology.
Female side-blotched lizards are often subject to a severe disassociation of direct and indirect benefits, because the coercive mating strategies present in this species lead to one of the highest rates of multiple paternity in amniote vertebrates (11, 12). Our results demonstrate that females have a high degree of control over the consequences of multiple mating, and support the assumptions of mate-choice models that predict direct benefits of territory quality and indirect fitness from mate choice. The allocation of progeny sex as a function of sire quality suggests that some components of female choice are cryptically associated with selective sperm fertilization (35), because male side-blotched lizards are the heterogametic sex (36).
Acknowledgments
We thank A. Chaine, T. Commendant, B. Holland, B. Lyon, P. Raimondi, and J. N. Thompson for helpful comments and discussions concerning this article. We especially thank E. Svensson for etymological expertise. We thank G. Corrigan for help in the laboratory. M. J. West-Eberhard and two anonymous reviewers made especially insightful comments that greatly improved this article. Research was supported by grants from the American Museum of Natural History and the American Society of Ichthyologists and Herpetologists, and by a National Science Foundation grant (to R.C.).
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Andersson M., (1994) Sexual Selection (Princeton Univ. Press, Princeton).
- 2.Emlen S. & Oring, L. (1977) Science 197, 215-222. [DOI] [PubMed] [Google Scholar]
- 3.Alatalo R. V., Lundberg, A. & Glynn, C. (1986) Nature 323, 151-153. [Google Scholar]
- 4.Moore A. J. (1994) Behav. Ecol. Sociobiol. 35, 235-241. [Google Scholar]
- 5.Ratti O., Hovi, M., Lundberg, A., Tegelstrom, H. & Alatalo, R. (1995) Behav. Ecol. Sociobiol. 37, 419-425. [Google Scholar]
- 6.Kempenaers B., Verheyen, G. R., Van der Broeck, M., Burke, T., Van Broeckhoven, C. & Dhondt, A. A. (1992) Nature 357, 494-496. [Google Scholar]
- 7.Gil D., Graves, J., Hazon, N. & Wells, A. (1999) Science 286, 126-128. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham E. J. A. & Russell, A. F. (2000) Nature 404, 74-76. [DOI] [PubMed] [Google Scholar]
- 9.Elgar M. A., Schneider, J. M. & Herberstein, M. E. (2000) Proc. R. Soc. London Ser. B 267, 2439-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clutton-Brock T. H., Albon, S. D. & Guiness, F. E. (1984) Nature 308, 358-360. [Google Scholar]
- 11.Sinervo B. & Doughty, P. (1996) Evolution (Lawrence, Kans.) 50, 1314-1327. [DOI] [PubMed] [Google Scholar]
- 12.Zamudio K. & Sinervo, B. (2000) Proc. Natl. Acad. Sci. USA 97, 1427-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinervo B., Miles, D. B., Frankino, W. A., Klukowski, M. & DeNardo, D. F. (2000) Horm. Behav. 38, 222-233. [DOI] [PubMed] [Google Scholar]
- 14.Calsbeek R., Alonzo, S. H., Zamudio, K. & Sinervo, B. (2002) Proc. R. Soc. London Ser. B 269, 157-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calsbeek R. & Sinervo, B. (2002) J. Anim. Ecol. 71, 513-523. [Google Scholar]
- 16.Sinervo B. & Adolph, S. C. (1994) Ecology 75, 776-790. [Google Scholar]
- 17.Jakob E. M., Marshall, S. D. & Uetz, E. W. (1996) Oikos 77, 61-67. [Google Scholar]
- 18.Goodnight K. F., Quellar, D. C. & Poznansky, T., (1996) GOODNIGHT (Rice Univ., Houston), Version 1.1.2 (available from the authors at http://gsoft.smu.edu/gsoft.html).
- 19.Tabachnick B. G. & Fidell, L. S., (1996) Using Multivariate Statistics (Harper Collins College, New York).
- 20.Lande R. & Arnold, S. J. (1983) Evolution (Lawrence, Kans.) 37, 1210-1226. [DOI] [PubMed] [Google Scholar]
- 21.Sinervo B., Doughty, P., Huey, R. B. & Zamudio, K. (1992) Science 258, 1927-1930. [DOI] [PubMed] [Google Scholar]
- 22.Schluter D. & Nychka, D. (1994) Am. Nat. 143, 597-616. [Google Scholar]
- 23.Mitchell-Olds T. & Shaw, R. G. (1987) Evolution (Lawrence, Kans.) 41, 1149-1161. [DOI] [PubMed] [Google Scholar]
- 24.Batchelet E., (1981) Circular Statistics in Biology (Academic, Toronto).
- 25.Sinervo B. & DeNardo, D. F. (1996) Evolution (Lawrence, Kans.) 50, 1299-1313. [DOI] [PubMed] [Google Scholar]
- 26.Svensson E. & Sinervo, B. (2000) Evolution (Lawrence, Kans.) 54, 1396-1403. [DOI] [PubMed] [Google Scholar]
- 27.Sinervo B., Svensson, E. & Comendant, T. (2000) Nature 406, 985-988. [DOI] [PubMed] [Google Scholar]
- 28.Sinervo B. (2000) in Adaptive Genetic Variation in the Wild, eds. Mousseau, T., Sinervo, B. & Endler, J. A. (Oxford Univ. Press, Oxford).
- 29.Bart J. & Earnst, S. L. (1999) Behav. Ecol. Sociobiol. 45, 355-359. [Google Scholar]
- 30.Svensson E. & Nilsson, J.-A. (1996) Proc. R. Soc. London Ser. B 263, 357-361. [Google Scholar]
- 31.Trivers R. L. & Willard, D. E. (1973) Science 179, 90-92. [DOI] [PubMed] [Google Scholar]
- 32.Olsson M., Shine, R., Gullberg, A., Madsen, T. & Tegelström, H. (1996) Nature 383, 585. [DOI] [PubMed] [Google Scholar]
- 33.Price C. S. C., Dyer, K. A. & Coyne, J. A. (1999) Nature 400, 449-452. [DOI] [PubMed] [Google Scholar]
- 34.Haig D. (1997) Proc. R. Soc. London Ser. B 264, 1657-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eberhard W. G., (1996) Monographs in Behavior and Ecology; Female Control: Sexual Selection by Cryptic Female Choice (Princeton Univ. Press, Princeton).
- 36.Reeder T. W. & Wiens, J. J. (1996) Herpetol. Monogr. 10, 43-84. [Google Scholar]