Abstract
Propolis, a honeybee product, has gained popularity as a food and alternative medicine. Its constituents have been shown to exert pharmacological (anticancer, antimicrobial and anti-inflammatory) effects. We investigated whether Brazilian green propolis exerts neuroprotective effects in the retina in vitro and/or in vivo. In vitro, retinal damage was induced by 24 h hydrogen peroxide (H2O2) exposure, and cell viability was measured by Hoechst 33342 and YO-PRO-1 staining or by a resazurin–reduction assay. Propolis inhibited the neurotoxicity and apoptosis induced in cultured retinal ganglion cells (RGC-5, a rat ganglion cell line transformed using E1A virus) by 24 h H2O2 exposure. Propolis also inhibited the neurotoxicity induced in RGC-5 cultures by staurosporine. Regarding the possible underlying mechanism, in pig retina homogenates propolis protected against oxidative stress (lipid peroxidation), as also did trolox (water-soluble vitamin E). In mice in vivo, propolis (100 mg kg−1; intraperitoneally administered four times) reduced the retinal damage (decrease in retinal ganglion cells and in thickness of inner plexiform layer) induced by intravitreal in vivo N-methyl-d-aspartate injection. These findings indicate that Brazilian green propolis has neuroprotective effects against retinal damage both in vitro and in vivo, and that a propolis-induced inhibition of oxidative stress may be partly responsible for these neuroprotective effects.
Keywords: apoptosis, lipid peroxidation, NMDA, retinal damage, retinal ganglion cell
Introduction
Retinal ganglion cell (RGC) death is a common feature of many ophthalmic disorders such as glaucoma, optic neuropathies and various retinovascular diseases (diabetic retinopathy and retinal vein occlusions). RGC death may occur via a variety of mechanisms involving, for example, oxidative stress (1), excitatory amino acids (2), nitric oxide (3) and apoptosis (4). Although therapies already exist for treating glaucoma, and clinical trials of treatments for diabetic retinopathy are in progress, treatments sufficiently efficacious to halt or even reverse the effect of ocular diseases are urgently needed. Hence, RGC death and/or optic nerve loss represent excellent neuroprotective targets for drug discovery (5).
Propolis, a resinous substance made from the sprouts and bark of various plants by honeybees, is a traditional medicine with a long history in Eastern Europe and Brazil. It is currently used as a health food and in the treatment of various ailments in Japan, Brazil, USA and Europe (6,7). It has been reported to have a wide range of biological activities such as antibacterial (8,9), anti-inflammatory (10), antioxidative (11,12) and/or tumoricidal (13,14) activities. Baccharis dracunculifolia DC (Asteraceae), a plant native to Brazil, is the most important botanical source of Southeastern Brazilian propolis, which is known as green propolis because of its color (15–19). In recent years, many studies have been made of green propolis because of its characteristic chemical composition and biological activities (20,21). Recently, we reported that Brazilian green propolis has neuroprotective effects both against in vitro neurotoxicity in cell cultures and against in vivo ischemic neuronal damage in mice (22). However, to our knowledge, no examination of the effects of Brazilian green propolis has been carried out using RGC cultures or in vivo models of retinal damage.
The purpose of the present study was to examine the effects of Brazilian green propolis on retinal damage both in vitro and in vivo. To that end, we studied its effects on hydrogen peroxide (H2O2)- and staurosporin-induced neurotoxicity in RGC-5 cultures and on in vivo N-methyl-d-aspartate (NMDA)-induced retinal damage in mice. In addition, we examined the effects on lipid peroxidation in the homogenates of pig retina.
Methods
Materials
Drugs and sources were as follows: Dulbecco's modified Eagle's medium (DMEM), resazurin and trolox (a derivative of α-tocopherol, water-soluble Vitamin E) were purchased from Sigma–Aldrich (St Louis, MO, USA). Staurosporine was from Trevingen (Gaithersburg, MD, USA). Isoflurane was from Nissan Kagaku (Tokyo, Japan), whereas fetal bovine serum (FBS) from Valeant (Costa Mesa, CA, USA). H2O2 was from Wako (Osaka, Japan). Brazilian green propolis (Brazil, Minas Gerais state) was extracted either with 95% ethanol at room temperature (PE) or with water at 50°C (PW) to yield the extract used. The plant of origin of Brazilian green propolis is B. dracunculifolia (16). The main constituents of the propolis PE and PW extracts are shown in Table 1. The water extract was used in both the in vitro and in vivo studies, whereas the ethanol extract was used only in the in vitro study. Hoechst 33342 and YO-PRO-1 were from Molecular Probes (Eugene, OR, USA).
Table 1.
Main constituents of propolis extracts made using ethanol (PE) or water (PW)
| Main constituents | PE | PW |
|---|---|---|
| Chlorogenic acid | 3.6 | 0.76 |
| p-Coumaric acid | 3.7 | 2.5 |
| 4,5-Di-O-caffeoylquinic acid | 0.78 | 0.24 |
| 3,5-Di-O-caffeoylquinic acid | 4.9 | 2.7 |
| 3,4-Di-O-caffeoylquinic acid | 6.1 | 3.5 |
| Drupanin | 0.12 | 1.8 |
| Isosakuranetin | – | 0.54 |
| Artepillin C | 0.59 | 14.0 |
| Baccharin | 0.03 | 6.8 |
Values (content %) are expressed as mean of triplicate analyses for each sample. PE, propolis extracted with ethanol; PW, propolis extracted with water; –, not detected. Data in this table are derived from our previous report (38).
Retinal Ganglion Cell Line (RGC-5) Culture
Cultures of RGC-5 were maintained in DMEM containing 10% FBS, 100 U ml−1 penicillin (Meiji Seika Kaisha Ltd, Tokyo, Japan) and 100 µg ml−1 streptomycin (Meiji Seika Kaisha Ltd) in a humidified atmosphere of 95% air and 5% CO2 at 37°C. The RGC-5 cells were passaged by trypsinization every 3–4 days, as in our previous report (23). To examine the effect of propolis on the cell death induced by either 0.3 mM H2O2 or 200 nM staurosporine, low density of RGCs (2 × 103 cells per well) were seeded in serum-free DMEM medium with propolis. After pretreatment with both propolis extracts, PE and PW, for 1 h, H2O2 or staurosporine was added to RGC-5 cultures for 24 h.
Cell Viability—Hoechst 33342 and YO-PRO-1 Staining
The first method for assessing cell viability was a single-cell digital imaging-based method employing fluorescent staining of nuclei. Cell death was assessed on the basis of employing combination staining with fluorescent dyes [namely, Hoechst 33342 and YO-PRO-1 (Molecular Probes)], observations being made using an inverted epifluorescence microscope (Olympus). YO-PRO-1 (λex 491 nm, λem >509 nm) is a membrane-impermeant dye and is generally excluded from viable cells, whereas early-stage apoptotic and necrotic cells are YO-PRO-1-positive. At the end of the culture period, Hoechst 33342 and YO-PRO-1 dyes were added to the culture medium (at 8 and 0.1 µM, respectively) for 30 min. Images were collected using a digital camera (COOLPIX 4500). In a blind manner, a total of at least 400 cells per condition were counted using image-processing software (Image-J ver. 1.33f; National Institutes of Health, USA). Cell mortality was quantified by determining the percentage of cells that were YO-PRO-1-positive (Hoechst 33342-positive cells being taken as the total number of cells present, since Hoechst 33342 stains both live and dead cells).
Cell Viability—Resazurin-reduction Assay
The second method for assessing cell viability entailed H2O2-induced cell viability being quantified by assessing the fluorescence-intensity increase associated with the cellular reduction of resazurin to resorufin. All experiments were performed in DMEM medium at 37°C. Cell viability was assessed by culturing cells in 10% resazurin solution for 3 h at 37°C and then examining the fluorescence at 560/590 nm. This fluorescence was expressed as a percentage of that in control cells (which were in serum-free DMEM), after subtraction of background fluorescence.
Lipid Peroxidation in the Porcine Retina Homogenate
Porcine retina homogenate was prepared as previously described (24). Briefly, retinal tissues were homogenized in a glass-Teflon homogenizer in two volumes of 50 mM ice-cold Tris–HCl buffer (pH 7.4) and then stored at −80°C. The stock retinal homogenate was then diluted 10-fold with the same buffer, and 180 µl portions of the diluted homogenate were added to 10 µl of the test compound, 5µl of ferrous sulfate (Wako) at 10 mM (final concentration: 0.25 mM) and adenosine diphosphate (ADP, Sigma–Aldrich) at 160 mM (final concentration: 4 mM), and incubated at 37°C for 30 min.
One of the end products of peroxidation, malondialdehyde (MDA), was assessed as an indicator of lipid peroxidation using Lipid Peroxidation Assay Kit (437634, Calbiochem, USA) in accordance with a manufacturer's protocol. Briefly, the reaction was stopped by adding 650 µl of N-methyl-2-phenylindole in acetonitrile solution (Reagent 1) and 150 µl of 12 N HCl and diluted to total volume of 1 ml with dilution buffer. The mixtures were heated for 60 min at 45°C. The absorbance was then measured at 586 nm. At the same time, MDA standard was prepared and assessed to determine the concentration of MDA in each sample. The results were represented as a percentage of vehicle-treated control.
NMDA-induced Retinal Damage
Male adult ddY mice weighing 36–43 g (Japan SLC, Hamamatsu, Japan) were kept under lighting conditions involving 12 h light: 12 h dark. Anesthesia was induced with 3.0% isoflurane and maintained with 1.5% isoflurane in 70% N2O and 30% O2 via an animal general anesthesia machine (Soft Lander; Sin-ei Industry Co. Ltd, Saitama, Japan). The body temperature was maintained at between 37.0 and 37.5°C with the aid of a heating pad and heating lamp. Retinal damage was induced by the injection (2 µl per eye) of NMDA (Sigma–Aldrich) dissolved at 20 mM in 0.01 M phosphate-buffered saline. This was injected into the vitreous body of the left eye under the above anesthesia. One drop of levofloxacin ophthalmic solution (Santen Pharmaceuticals Co. Ltd, Osaka, Japan) was applied topically to the treated eye immediately after the intravitreal injection. Seven days after the NMDA injection, eyeballs were enucleated for histological analysis.
Propolis at 100 mg kg−1 (0.1 ml/10 g) or saline was intraperitoneally administered at 48 h, 24 h and 60 min before and at 6 h after the NMDA injection. The drug was dissolved in saline, fresh solution being made daily.
Histological Analysis of Mouse Retina
In mice under anesthesia produced by an intraperitoneal injection of sodium pentobarbital (80 mg kg−1), each eye was enucleated and kept immersed for at least 24 h at 4°C in a fixative solution containing 4% paraformaldehyde. Six paraffin-embedded sections (thickness, 5 µm) cut through the optic disc of each eye were prepared in a standard manner and stained with hematoxylin and eosin. Retinal damage was evaluated as described previously (25), three sections from each eye being used for the morphometric analysis. Light-microscope images were photographed, and (i) the cell counts in the ganglion cell layer (GCL) at a distance between 375 and 625 µm from the optic disc, and (ii) the thickness of the inner plexiform layer (IPL) were measured on the photographs in a masked fashion by a single observer (Y.I.). Data from three sections (selected randomly from the six sections) were averaged for each eye, and used to evaluate the cell count in the GCL and the thickness of the IPL.
Statistical Analysis
Data are presented as means ± SEM. Statistical comparisons were made using a one-way ANOVA followed by a Student's t-test or Dunnett's test [using STAT VIEW version 5.0 (SAS Institute Inc., Cary, NC, USA)]. P < 0.05 was considered to indicate statistical significance.
Results
Propolis Inhibited H2O2-induced Apoptosis and Necrosis in RGC-5 Culture
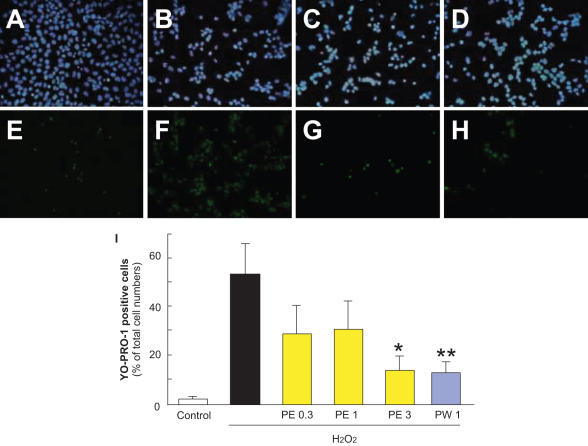
Typical photographs of Hoechst 33342 and YO-PRO-1 staining are shown in Fig. 1A–H. Hoechst 33342 stains all cells (live and dead cells), whereas YO-PRO-1 stains early-stage apoptotic and necrotic cells. Both types of propolis (extracted with ethanol or water, Fig. 1G and H, respectively) decreased (versus vehicle-treatment) the number of cells that showed YO-PRO-1 staining following treatment with H2O2.
Figure 1.
Representative fluorescence microscopy of Hoechst 33342 (blue) and YO-PRO-1 (green) staining at 24 h after H2O2. RGC-5 were immersed in serum-free DMEM and then propolis PE (extract with ethanol) or propolis PW (extract with water) was added to the cultures. Cells, which were maintained in this condition for 24 h, were grouped as described below. (A) Non-treated cells showed normal nuclear morphology and (E) were negative for YO-PRO-1 (early-stage apoptotic and necrotic cells are YO-PRO-1-positive). (B) H2O2 (0.3 mM)-induced neurotoxicity, with cells showing condensation and fragmentation of their nuclei, including YO-PRO-1-positively stained cells (F). (C) Pretreatment with 3 µg ml−1 PE or 1 µg ml−1 PW at 1 h before H2O2 (0.3 mM)-treatment reduced both nuclear condensation (C and D, respectively) and YO-PRO-1-positive staining (G and H, respectively). A and E: control; B and F: vehicle-treatment plus H2O2; C, G: propolis PE at 3 µg ml−1 plus H2O2; D and H: propolis PW at 1 µg ml−1 plus H2O2; A–D: Hoechst 33342 staining; E–H: YO-PRP-1 staining; I: the number of cells exhibiting YO-PRO-1 fluorescence was counted, and positive cells were expressed as the percentage of YO-PRO-1-positive to Hoechst 33342-positive cells. Viable cells are Hoechst 33342-positive and YO-PRO-1-negative, whereas dead cells are Hoechst 33342-positive and YO-PRO-1-positive. Non-treated cells showed normal nuclear morphology and were negative for YO-PRO-1. H2O2-treated cells showed shrinkage and condensation of their nuclei, including YO-PRO-1-positive cells. Treatment with propolis (PE or PW) reduced both nuclei shrinkage and YO-PRO-1-positive staining. Each column represents the mean ± SEM, n = 8. *P < 0.05, **P < 0.01 versus H2O2-treatment alone.
Propolis extracted with ethanol (PE) inhibited H2O2-induced apoptosis in RGC-5 culture, the effect being significant at a concentration of 3 µg ml−1 (Fig. 1I). At 1 µg ml−1, propolis extracted with water (PW) inhibited the H2O2-induced apoptosis and necrosis as effectively as propolis extracted with ethanol (PE) did at 3 µg ml−1 (Fig. 1I).
Propolis Inhibited H2O2- or Staurosporine-induced Cell Damage in RGC-5 Culture
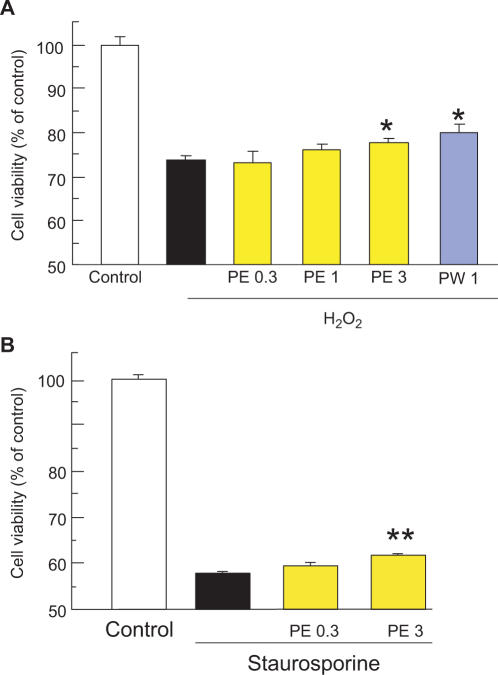
In a resazurin assay for the evaluation of cell viability, cell death was observed at 24 h after treatment with H2O2 (0.3 mm) or staurosporine (200 nM). Propolis extracted with ethanol (PE) was used at 0.3–3 µg ml−1, and it inhibited H2O2-induced cell death in a concentration-related manner, as evidenced by its effect on the increase in fluorescence intensity (Ex560/Em590 nm) associated with the reduction of resazurin to resorufin, the effect of propolis PE being significant at 3 µg ml−1 (Fig. 2A). Similarly, propolis extracted with water (PW) significantly inhibited cell death at 1 µg ml−1. In the case of staurosporine-induced cell death, propolis extracted with ethanol (PE) significantly inhibited cell death at 3 µg ml−1 (Fig. 2B). Although we tested the effect of propolis (PE and PW) alone on cell viability without any treatment such as H2O2 and staurosporine using resazurin-reduction test, there was little effect on the fluorescence intensity.
Figure 2.
Propolis reduced the retinal damage induced by H2O2 or staurosporine in RGC-5 culture. RGC-5 were immersed in serum-free DMEM, and then propolis PE (extract with ethanol) or propolis PW (extract with water) was added to the cultures. Cells were maintained in this condition for 24 h. Cell viability was assessed by immersing cells in 10% resazurin solution for 3 h at 37°C, with fluorescence being recorded at 560/590 nm. H2O2 (A) and staurosporines (B) each induced cell death. Propolis (PE or PW) inhibited the H2O2-induced cell death (A), whereas propolis PE also inhibited staurosporine-induced cell death (B). Each column represents the mean ± SEM, n = 6–8. *P < 0.05, **P < 0.01 versus H2O2-r staurosporine-treatment alone.
Propolis Reduced Lipid Peroxidation in Pig Retina Homogenate
In the lipid peroxidation study, the MDA level in the supernatant increased after 30 min incubation at 37°C, and propolis extracted with ethanol (PE) and trolox each inhibited the lipid peroxidation in a concentration-dependent manner, the effects of propolis PE and trolox being significant at concentrations of 2 µg ml−1 or more and 20 µM or more, respectively (Table 2). The IC50 values (95% confidence limits) for propolis extracted with ethanol (PE) and trolox were 13.4 (8.9–20.8) µg ml−1 and 26.1 (13.0–57.1) µM, respectively.
Table 2.
Propolis reduces lipid peroxidation in pig retina homogenate
| Treatments | TBARS (% of control) | IC50 (95% confidence limit) |
|---|---|---|
| Control | 100.0 ± 0.9 | |
| PE 0.2 µg ml−1 | 104.0 ± 4.2 | |
| PE 2 | 94.0 ± 0.8** | 13.4 (8.9–20.8) µg ml−1 |
| PE 20 | 56.9 ± 1.0** | |
| PE 200 | 1.3 ± 1.1** | |
| Control | 100.0 ± 2.9 | |
| Trolox 0.2 µM | 100.1 ± 2.3 | |
| Trolox 2 | 98.5 ± 0.2 | 26.1 (13.0–57.1) µM |
| Trolox 20 | 88.4 ± 3.9** | |
| Trolox 200 | 33.8 ± 1.7** |
TBARS, thiobarbituric acid-reactive substance; PE, propolis extracted with ethanol. Values represent the mean ± SEM of four independent experiments.
**P < 0.01 versus control (vehicle-treated group).
Propolis Reduced the Retinal Damage Induced by Intravitreal Injection of NMDA in Mice
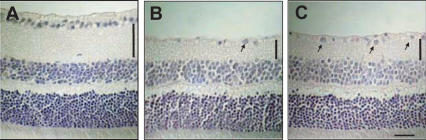
Intravitreal injection of NMDA at 40 nmol per eye decreased both the cell count in the GCL and the thickness of the IPL in the mouse retina (Table 3, Fig. 3B) versus those of the non-treated normal retina (Table 3, Fig. 3A). Treatment with propolis extracted with water (PW, 100 mg kg−1, intraperitoneally administered at 48 h, 24 h and 60 min before and at 6 h after the NMDA injection) significantly reduced the magnitude of both of the above effects of NMDA (Table 3, Fig. 3C).
Table 3.
Propolis protects against N-methyl-d-aspartate (NMDA)-induced retinal damage in mice
| Treatments | n | Retinal ganglion cells (cell number mm−1) | Inner plexiform layer (thickness, µm) |
|---|---|---|---|
| Normal | 10 | 130.7 ± 2.3** | 36.6 ± 1.3** |
| Glutamate + vehicle | 8 | 53.7 ± 2.4 | 22.7 ± 0.7 |
| Glutamate + PW | 7 | 64.0 ± 2.5** (13.4%)# | 25.2 ± 0.8** (18.0%)# |
Propolis (PW, extract with water) at 100 mg kg−1 (0.1 ml/10 g) was intraperitoneally administered at 48 h, 24 h and 60 min before and at 6 h after the NMDA injection (40 nmol per eye).
# Inhibition % = {1−[Normal − (Glutamate + PW)/Normal − (Glutamate + Vehicle)]} × 100. Values represent the mean ± SEM.
**P< 0.01 versus control (glutamate + vehicle).
Figure 3.
Hematoxylin and eosin staining of retinal sections (thickness, 5 µm) obtained from mice at 7 days after the intravitreal injection of N-methyl-d-aspartate (NMDA). Representative photographs showing non-treated normal retina (A), NMDA-treated, vehicle-treated retina (B) and NMDA-treated, propolis-treated retina (C). Propolis (PW; extract with water) at 100 mg kg−1 (0.1 ml per 10 g) was intraperitoneally administered at 48 h, 24 h and 60 min before and at 6 h after the NMDA injection (40 nmol per eye). Treatment with propolis limited the damage to retinal ganglion cells (arrows) and to the IPL (vertical bars) induced by the NMDA injection. Also see Table 2. Vertical bars show thickness of plexiform layer. Horizontal bar represents 25 µm.
Discussion
In the present study, we examined the in vitro effects of propolis (i) against H2O2- and staurosporine-induced cell damage in cultures of RGC-5 (an established transformed rat retinal ganglion cell line), and (ii) against lipid peroxidation in pig retina homogenates. In addition, we examined the in vivo effect of propolis on the retinal damage seen after the intravitreal injection of NMDA in mice. Propolis protected retinal ganglion cells (RGC-5) against both H2O2- and staurosporine-induced in vitro retinal damage (such as apoptosis and necrosis). Furthermore, intraperitoneally administered propolis partly prevented the in vivo retinal damage induced by NMDA in mice. Our results, therefore, indicate that propolis inhibits neuronal damage both in vitro and in vivo.
Clinical and experimental data suggest that in both the retina (1) and brain (26–28), ischemic neuronal damage is at least partly induced by the free radical production and/or lipid peroxidation that occurs either during the ischemia itself or following reperfusion. Recently, Ferreira et al. (29) observed that markers of oxidative stress (such as total reactive antioxidant potential and the activities of the antioxidant enzymes superoxide dismutase, catalase and glutathione peroxidase) were increased in the aqueous humor of primary open-angle glaucoma patients, as compared with their levels in cataract patients (controls). These findings indicate that oxidative stress may play a pivotal role in the pathogenesis of retinal damage.
Reactive oxygen species (ROS) such as H2O2, superoxide anion (O2−) and hydroxyl radical (•OH) have been implicated in the regulation of many important cellular events, including transcription-factor activation (30), gene expression (31) and cellular proliferation (32). However, excessive production of ROS gives rise to events that lead to the death of several types of cells (33). It is known that cells possess antioxidant systems that serve to control the redox state, a control that is important for their survival, and H2O2 is often used to investigate the mechanism underlying ROS-induced cell death (34,35). The concentration of H2O2 used in the present study (0.3 mM) was sufficient to induce cell damage, such as apoptosis and necrosis, as shown in Figs 1 and 2. In the present study, propolis inhibited both the H2O2- and staurosporine-induced cell death. Different mechanisms may be involved in cell death induce by H2O2 and staurosporine. However, common pathways such as ROS have been reported by Shimizu et al. (36). Briefly, staurosporine rapidly increased the intracellular level of ROS, and N-acetyl-cystein (NAC), an antioxidant inhibited the production of ROS, activation of caspase-3 and cell death. Taken together, the protective effects of propolis on H2O2- and staurosporine-induced cell death may be derived from its common mechanism.
In total, at least 200 compounds have been identified in different samples of propolis, with more than 100 being present in any given sample. Propolis has a variety of botanical origins, and its chemical composition can also be variable. The propolis used in the present study was Brazilian green propolis (from Minas Gerais state, Brazil), and the plant of origin was B. dracunculifolia (16). The main constituents are shown in Table 1. Using Brazilian green propolis, we found that extracts made by using ethanol (PE) or water (PW) inhibited H2O2-induced cell damage in RGC-5 cultures, the potency of the former being not greatly different from that of the latter. Since both propolis extracts had neuroprotective effects against in vitro cell damage, common constituents (such as 3,4-di-O-caffeoylquinic acid, 3,5-di-O-caffeoylquinic acid and/or p-coumaric acid; see Table 1) may be responsible for the effects. Further experiments will be needed to identify the main constituent(s) responsible for the neuroprotective effects of propolis.
When propolis (extracted with water; PW) was intraperitoneally administered at 100 mg kg−1 four times in the NMDA-induced retinal damage experiment, it reduced the overt signs of retinal damage (such as the decreases in RGC number and in the thickness of IPL) (Table 2). In our previous report, propolis extracted with ethanol (PE) or water (PW) protected against oxidative stress (lipid peroxidation in mouse forebrain homogenates) (22). In the present study, propolis (extracted with ethanol; PE) was effective against lipid peroxidation in pig retina homogenates. The effective concentrations of propolis in the present experiments were 2 µg ml−1 or more. Propolis has been reported to exhibit powerful scavenging activity in vitro towards both the superoxide anion radical and the NO radical (37). Collectively, these findings indicate that the antioxidant effects of propolis may make an important contribution to its neuroprotective potential.
In conclusion, in our in vitro experiments propolis exerted neuroprotective effects in RGC-5 culture, as well as antioxidant effects against lipid peroxidation. Propolis also exerted neuroprotective effects in vivo (against NMDA-induced retinal damage in mice). These findings are consistent with the observed neuroprotective effects of propolis being derived, at least in part, from its antioxidant properties.
Acknowledgments
This work was supported by a research grant from the Gifu Research and Development Foundation. The authors wish to express their gratitude to Dr Neeraj Agarwal, Department of Pathology and Anatomy, UNT Health Science Center, Fort Worth, TX, USA, for the kind gift of RGC-5, and to Miss Satomi Chikamatsu, Mr Yasuhisa Oida and Mr Nobutaka Morimoto for skillful technical assistance.
References
- 1.Bonne C, Muller A, Villain M. Free radicals in retinal ischemia. Gen Pharmacol. 1998;30:275–80. doi: 10.1016/s0306-3623(97)00357-1. [DOI] [PubMed] [Google Scholar]
- 2.Dreyer EB. A proposed role for excitotoxicity in glaucoma. J Glaucoma. 1998;7:62–7. [PubMed] [Google Scholar]
- 3.Neufeld AH. Nitric oxide: a potential mediator of retinal ganglion cell damage in glaucoma. Surv Ophthalmol. 1999;43:S129–35. doi: 10.1016/s0039-6257(99)00010-7. [DOI] [PubMed] [Google Scholar]
- 4.McKinnon SJ. Glaucoma, apoptosis, and neuroprotection. Curr Opin Ophthalmol. 1997;8:28–37. doi: 10.1097/00055735-199704000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Clark AF, Yorio T. Ophthalmic drug discovery. Nat Rev Drug Discov. 2003;2:448–59. doi: 10.1038/nrd1106. [DOI] [PubMed] [Google Scholar]
- 6.Aga H, Shibuya T, Sugimoto T, Kurimoto M, Nakajima SH. Isolation and identification of antimicrobial compounds in Brazilian propolis. Biosci Biotechnol Biochem. 1994;58:945–6. [Google Scholar]
- 7.Marcucci MC, De Camargo FA, Lopes CMA. Identification of amino acids in Brazilian propolis. Z Naturforsch [C] 1996;51:11–4. [Google Scholar]
- 8.Bankova V, Marcucci MC, Simova S, Nikolova N, Kujumgiev A. Anti-bacterial diterpenic acids from Brazilian propolis. Z Naturforsch [C] 1996;51:277–80. doi: 10.1515/znc-1996-5-602. [DOI] [PubMed] [Google Scholar]
- 9.Drago B, Mombelli B, Vecchi EDE, Fassina MC, Tocalli L, Gismondo MR. In vitro antimicrobial activity of propolis dry extract. J Chemother. 2000;12:390–5. doi: 10.1179/joc.2000.12.5.390. [DOI] [PubMed] [Google Scholar]
- 10.Mizoeva OK, Calder PC. The effect of propolis and its components on eicosanoid production during the inflammatory response. Prostaglandins Leukot Essent Fatty Acids. 1996;55:441–9. doi: 10.1016/s0952-3278(96)90129-5. [DOI] [PubMed] [Google Scholar]
- 11.Krol W, Czuba Z, Scheller S, Gabrys J, Grabiec S, Shani J. Anti-oxidant property of ethanolic extract of propolis (EEP) as evaluated by inhibiting the chemiluminescence oxidation of luminol. Biochem Int. 1990;21:593–7. [PubMed] [Google Scholar]
- 12.Scheller S, Wilczok T, Imielski S, Krol W, Gabrys J, Shani J. Free radical scavenging by ethanol extract of propolis. Int J Radiat Biol. 1990;57:461–5. doi: 10.1080/09553009014552601. [DOI] [PubMed] [Google Scholar]
- 13.Chen CN, Weng MS, Wu CL, Lin JK. Comparison of radical scavenging activity, cytotoxic effects and apoptosis induction in human melanoma cells by Taiwanese propolis from different sources. Evid Based Complement Alternat Med. 2004;1:175–85. doi: 10.1093/ecam/neh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuno T. Isolation and characterization of the tumoricidal substance from Brazilian propolis. Honeybee Sci. 1992;13:49–54. [Google Scholar]
- 15.Kumazawa S, Yoneda M, Shibata I, Kanaeda J, Hamasaka T, Nakayama T. Direct evidence for the plant origin of Brazilian propolis by the observation of honeybee behavior and phytochemical analysis. Chem Pharm Bull. 2003;51:740–2. doi: 10.1248/cpb.51.740. [DOI] [PubMed] [Google Scholar]
- 16.Kumazawa S, Yoneda M, Nakayama T. Constituents in Brazilian propolis and its plant of origin. FFI J. 2004;209:132–9. [Google Scholar]
- 17.Midorikawa K, Banskota AH, Tezuka Y, Nagaoka T, Matsushige K, Message D, et al. Liquid chromatography-mass spectrometry analysis of propolis. Phytochem Anal. 2001;12:366–73. doi: 10.1002/pca.605. [DOI] [PubMed] [Google Scholar]
- 18.Park YK, Alencar SM, Aguiar CL. Botanical origin and chemical composition of Brazilian propolis. J Agric Food Chem. 2002;50:2502–6. doi: 10.1021/jf011432b. [DOI] [PubMed] [Google Scholar]
- 19.Teixeira EW, Negri G, Meira RM, Message D, Slatino A. Plant origin of green propolis: bee behavior, plant anatomy and chemistry. Evid Based Complement Alternat Med. 2005;2:85–92. doi: 10.1093/ecam/neh055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simoes LM, Gregorio LE, Da Silva Filho AA, de Souza ML, Azzolini AE, Bastos JK, et al. Effect of Brazilian green propolis on the production of reactive oxygen species by stimulated neutrophils. J Ethnopharmacol. 2004;94:59–65. doi: 10.1016/j.jep.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 21.Marcucci MC, Ferreres F, Garcia-Viguera C, Bankova VS, De Castro SL, Dantas AP, et al. Phenolic compounds from Brazilian propolis with pharmacological activities. J Ethnopharmacol. 2001;74:105–12. doi: 10.1016/s0378-8741(00)00326-3. [DOI] [PubMed] [Google Scholar]
- 22.Shimazawa M, Chikamatsu S, Morimoto N, Mishima S, Nagai H, Hara H. Neuroprotection by Brazilian green propolis against in vitro and in vivo ischemic neuronal damage. Evid Based Complement Alternat Med. 2005;2:201–7. doi: 10.1093/ecam/neh078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimazawa M, Yamashima T, Agarwal N, Hara H. Neuroprotective effects of minocycline against in vitro and in vivo retinal cell damage. Brain Res. 2005;1053:185–94. doi: 10.1016/j.brainres.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 24.Tam BB, Siu AW, Lam AF, Lee EY. Effects of vitamin E and pinoline on retinal lipid peroxidation. Clin Exp Optom. 2004;87:171–4. doi: 10.1111/j.1444-0938.2004.tb03170.x. [DOI] [PubMed] [Google Scholar]
- 25.Yoneda S, Tanihara H, Kido N, Honda Y, Goto W, Hara H, et al. Interleukin-1β mediates ischemic injury in the rat retina. Exp Eye Res. 2001;73:661–7. doi: 10.1006/exer.2001.1072. [DOI] [PubMed] [Google Scholar]
- 26.Chan PH, Schmidley JM, Fishman RA, Longar SM. Brain injury, edema, and vascular permeability changes induced by oxygen-derived free radicals. Neurology. 1984;34:315–20. doi: 10.1212/wnl.34.3.315. [DOI] [PubMed] [Google Scholar]
- 27.Hara H, Sukamoto T, Kogure K. Mechanism and pathogenesis of ischemia-induced neuronal damage. Prog Neurobiol. 1993;40:645–70. doi: 10.1016/0301-0082(93)90009-h. [DOI] [PubMed] [Google Scholar]
- 28.Siesjo BK. Cell damage in the brain: a speculative synthesis. J Cereb Blood Flow Metabol. 1981;1:155–84. doi: 10.1038/jcbfm.1981.18. [DOI] [PubMed] [Google Scholar]
- 29.Ferreira SM, Lerner SF, Brunzini R, Evelson PA, Llesuy SF. Oxidative stress markers in aqueous humor of glaucoma. Am J Ophthalmol. 2004;137:62–9. doi: 10.1016/s0002-9394(03)00788-8. [DOI] [PubMed] [Google Scholar]
- 30.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–58. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo YY, Cruz TF. Involvement of reactive oxygen species in cytokine and growth factor induction of c-fos expression in chondrocytes. J Biol Chem. 1995;270:11727–30. doi: 10.1074/jbc.270.20.11727. [DOI] [PubMed] [Google Scholar]
- 32.Murrell GA, Francis MJ, Bromley L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem J. 1990;265:659–65. doi: 10.1042/bj2650659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolfe JT, Ross D, Cohen GM. A role for metals and free radicals in the induction of apoptosis in thymocytes. FEBS Lett. 1994;352:58–62. doi: 10.1016/0014-5793(94)00920-1. [DOI] [PubMed] [Google Scholar]
- 34.Goldshmit Y, Erlich S, Pinkas-Kramarski R. Neuregulin rescues PC12-ErbB4 cells from cell death induced by H2O2. Regulation of reactive oxygen species levels by phosphatidylinositol 3-kinase. J Biol Chem. 2001;276:46379–85. doi: 10.1074/jbc.M105637200. [DOI] [PubMed] [Google Scholar]
- 35.Kitamura Y, Ota T, Matsuoka Y, Tooyama I, Kimura H, Shimohama S, et al. Hydrogen peroxide-induced apoptosis mediated by p53 protein in glial cells. Glia. 1999;25:154–64. [PubMed] [Google Scholar]
- 36.Shimizu T, Numata T, Okada Y. A role of reactive oxygen species in apoptotic activation of volume-sensitive Cl− channel. Proc Natl Acad Sci USA. 2004;101:6770–3. doi: 10.1073/pnas.0401604101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ichikawa H, Satoh K, Tobe T, Yasuda I, Ushio F, Matsumoto K, et al. Free radical scavenging activity of propolis. Redox Rep. 2002;7:347–50. doi: 10.1179/135100002125000965. [DOI] [PubMed] [Google Scholar]
- 38.Mishima S, Narita Y, Chikamatsu S, Inoh Y, Ohta S, Yoshida C, et al. Effects of propolis on cell growth and gene expression in HL-60 cells. J Ethnopharmacol. 2005;99:5–11. doi: 10.1016/j.jep.2005.02.005. [DOI] [PubMed] [Google Scholar]