Abstract
Indole compounds, related to the metabolism of tryptophan, constitute an extensive family, and are found in bacteria, plants and animals. Indolic compounds possess significant and complex physiological roles, and especially indole alkaloids have historically constituted a class of major importance in the development of new plant derived drugs. The indole alkaloid alstonine has been identified as the major component of a plant-based remedy, used in Nigeria to treat mental illnesses by traditional psychiatrists. Although it is certainly difficult to compare the very concept of mental disorders in different cultures, the traditional use of alstonine is remarkably compatible with its profile in experimental animals. Even though alstonine in mice models shows a psychopharmacological profile closer to the newer atypical antipsychotic agents, it also shows important differences and what seems to be an exclusive mechanism of action, not entirely clarified at this point. Considering the seemingly unique mode of action of alstonine and that its traditional use can be viewed as indicative of bioavailability and safety, this review focuses on the effects of alstonine in the central nervous system, particularly on its unique profile as an antipsychotic agent. We suggest that a thorough understanding of traditional medical concepts of health and disease in general and traditional medical practices in particular, can lead to true innovation in paradigms of drug action and development. Overall, the study of this unique indole alkaloid may be considered as another example of the richness of medicinal plants and traditional medical systems in the discovery of new prototypic drugs.
Keywords: alstonine, indole alkaloids, traditional psychiatry, antipsychotic, ethnopharmacology
Introduction
Indole compounds constitute an extensive family of compounds found in bacteria, plants and animals (1); in general, these compounds are related to the metabolism of tryptophan and present substitutions in different positions of the indole ring (2). Indolic compounds with significant and complex physiological roles include those related to melatonin and serotonin (5-hydroxytryptamine). Indole alkaloids possess an indole ring in its structure, constituting a versatile heterocyclic discovery in 1866 (1); this extensive group of alkaloids received more attention after the isolation of reserpine from Rauwolfia serpentina Benth., an alkaloid that changed the history of conditions as diverse as schizophrenia and hypertension. Indolic alkaloids include various plant-derived medicinal products, including the well-known anti-tumor vinblastine, vincristine, vincamin and camptothecin isolated from Catharanthus roseus L. Don. (3).
Among the chemical classes present in medicinal plant species, alkaloids stand as a class of major importance in the development of new drugs because alkaloids possess a great variety of chemical structures and have been identified as responsible for pharmacological properties of medicinal plants. Some plant families have the genetic capability of producing more than one alkaloid, reflected in the structural diversity of these compounds (3).
We have previously identified the indole alkaloid alstonine (3,4,5,6,16,17-hexadehydro-16-(methoxycarbonyl)-19 alpha-methyl-20 alpha-oxyhoimbanium, see Fig. 1) as the major component of a plant-based remedy traditionally used in Nigeria to treat mental illnesses (4). In Nigeria, alstonine can be found in various plants species: Alstonia boonei De Wild (Apocynaceae), C. roseus (L.)G. Don (Apocynaceae), Picralima nitida (Stapf) Th. & H. Dur (Apocynaceae), Rauwolfia caffra Sond. (Apocynaceae) and Rauwolfia vomitoria Afzel. (Apocynaceae) (5). A. boonei is used in African ethnomedicine for the treatment of malarial disease, and can be used as astringent, antifebrile, antihelmintic, antispasmodic among other indications (5). C. roseus has been indicated as a purgative, to treat indigestion and dyspepsia, and has been considered to be useful in the treatment of diabetes; nevertheless, the studies to verify antidiabetic properties of C. roseus extracts led instead to the discovery and isolation of the above mentioned complex indole alkaloids vinblastine and vincristine (6).
Figure 1.
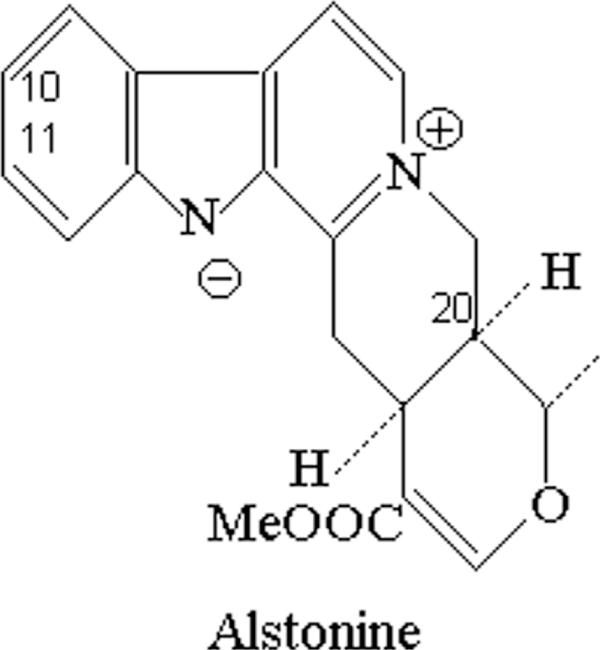
Chemical structure of indolic alkaloid alstonine.
R. caffra is traditionally used to treat fever, insomnia, rheumatism and pneumonia, whereas R. vomitoria can be associated with Xylopia aethiopica (Dun) A. Rich. (Annonaceae) to treat convulsions in children (5); these two species possess anti-inflammatory activity (7). The methanol extract of P. nitida fruit showed potent and dose-dependent anti-inflammatory, antipyretic and antimalarial activities (8); in addition, it has also been indicated in the treatment of trypanosomiasis (9).
This review focuses on the effects of alstonine in the central nervous system, particularly as an antipsychotic agent.
Ethnopharmacology of a Traditional Antipsychotic
The study of alstonine as antipsychotic arose in the course of an ethnopharmacological expedition (February 1993) in Nigeria, among the Igbo people. The field research methodology involved an ethnopharmacologist–physician team conducting research in collaboration with local scientists (botanists) and local experts such as healers of various kinds. The team was invited by a traditional leader to visit Dr C.O., a traditional psychiatrist who agreed to collaborate with the project. On his grounds Dr C.O. maintains a shrine for healing practices, part of a clinic (that includes patient sleeping quarters), so that ‘mad’ patients can stay under his care until restored to health. He presented a plant medicine named ‘uhuma obi-nwok’, in Igbo ‘the heart of man’. The plant is used to treat different types of ‘madness’, epilepsy, and is in general considered to be a sedative. To treat severe ‘madness’ the ground root is boiled in water (a piece of root about 10 cm long and 5 cm in diameter is prepared in 250 ml of water) until the liquid turns into a reddish-brown in color; the initial loading dose for an adult is one cup. Dr C.O. reported that 1 h after taking the medicine the person would fall asleep as long as 2–3 days; thereafter, the daily dose is half-a-cup three times a day for 2 weeks, and subsequently the dose is tapered down. As the dose is reduced the patient would gradually be awake for longer periods of the day. If the patient does not present symptoms of ‘madness’ during these alert periods the dose is further reduced. Dr C.O. reports considerable success of this treatment, with patients eventually being free of both medicine and ‘madness’ symptoms.
If the patient arrives in an exceedingly agitated state, one teaspoon of the powdered root is prepared in 20 ml Gin (a shot) to be taken by the patient as a single dose, inducing some 3 h of deep sleep. The roots and leaves may also be soaked in water, and bathing with this water has sedative effects; in fact, big pots with soaked root could be found at the clinic in each room. The leaf sap of this same species can also be taken orally (applied directly to the tongue) to treat convulsions.
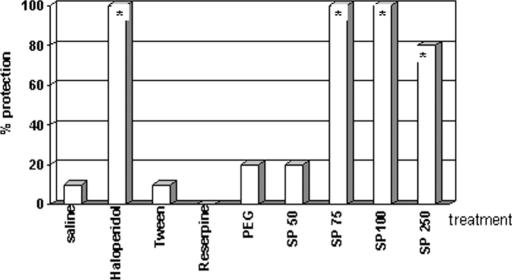
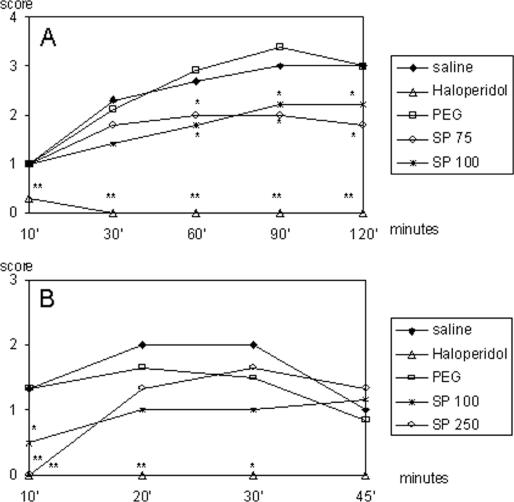
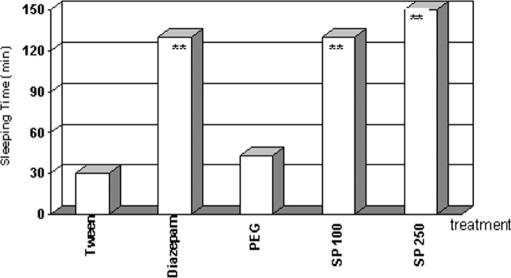
When asked to assist in collecting the species, Dr C.O. told us that we would have to travel 2–3 days to get to the proper location, and that he did not have time to take us there. Nevertheless, he gave us circa 45 g of dried root powder from his office stash and told us to analyze it in the laboratory. A crude ethanol extract (SP49000) was obtained from this powdered root material and analyzed for psychopharmacological activity, particularly antipsychotic properties. SP49000 clearly showed a neuroleptic-like activity in various mice models, such as protection of lethality induced by amphetamine in grouped mice (Fig. 2) and reversal of amphetamine-induced (Fig. 3A) and apomorphine-induced (Fig. 3B) stereotypy; additionally, the extract potentiated barbiturate-induced sleeping time (Fig. 4) (4).
Figure 2.
Effects of haloperidol (HAL), reserpine (RES), SP49000 (SP) and respective vehicles (SAL, TWE, PEG) on amphetamine-induced lethality in grouped mice. Drugs were administered intraperitoneally *P < 0.05, Fisher.
Figure 3.
Effects of haloperidol (HAL), SP49000 (SP) and respective vehicles (SAL, PEG) on amphetamine- (A) and apomorphine-induced (B) stereotypies. Drugs were administered intraperitoneally *P < 0.05, **P < 0.01, Mann–Whitney.
Figure 4.
Potentiation of barbiturate sleeping time by diazepam (DPZ), SP49000 (SP) and vehicles (TWE, PEG). Drugs were given intraperitoneally **P < 0.01, ANOVA.
The chemical analysis of SP49000 revealed reserpine-like alkaloids, alstonine being the dominant component. Possible local sources of these alkaloids have been mentioned earlier; R. caffra, commonly used in Igboland for medicinal purposes (10) is a good candidate as Dr C.O. source. Dr C.O. was visited on a couple of other occasions and demonstrated interest in the research, but did not reveal his plant source. Dr C.O and one of the authors (E.E.) actually exchanged amicable correspondence, discussing the data and other matters.
Antipsychotic Properties of Alstonine
Schizophrenia is a neuropsychiatric disorder characterized by diverse symptoms including hallucinations, delusional thinking, thought disorder, attention deficit and cognitive dysfunction; typically symptoms start relatively early in life and once the illness begins there is rarely a full return to normal levels of functioning. Studies of the pathophysiology of this disorder have focused on the heritability of schizophrenia, the affected neurotransmitter systems and neuroanatomical abnormalities (11).
Schizophrenia is associated with abnormalities of multiple neurotransmitter systems, including dopamine, serotonin, γ-aminobutyric acid (GABA) and glutamate. Typical and atypical antipsychotic agents differ in their receptor-binding affinities, which are related to their differing side-effect profiles. Novel therapeutic strategies include normalization of synaptic dopamine or serotonin levels, serotonin receptor antagonism and modulation of cerebral protein synthesis (12).
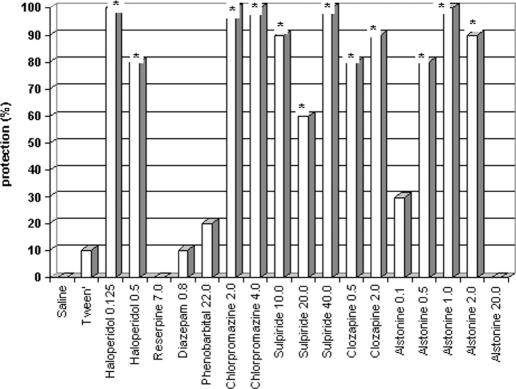
In mice models alstonine shows a clear, dose-dependent (inverted U shape), potent antipsychotic profile (13). The increased lethality induced by amphetamine in grouped mice is considered to be a specific model, since the lethality is prevented by antipsychotics, but not other tranquilizers (e.g. barbiturates and benzodiazepines). This effect is related to the ability of antipsychotics to block D2 receptors (14). Alstonine (i.p.) prevented amphetamine-induced lethality, with active doses within the range of 0.5–2.0 mg kg−1 (Fig. 5).
Figure 5.
Effects of classical (haloperidol and chlorpromazine) and atypical antipsychotics (clozapine and sulpiride), reserpine, barbiturate (pentobarbital) and benzodiazepine (diazepam), alstonine and vehicles (saline, tween) on amphetamine-induced lethality in grouped mice (doses in mg kg−1). Drugs were administered intraperitoneally *P < 0.05, Fisher.
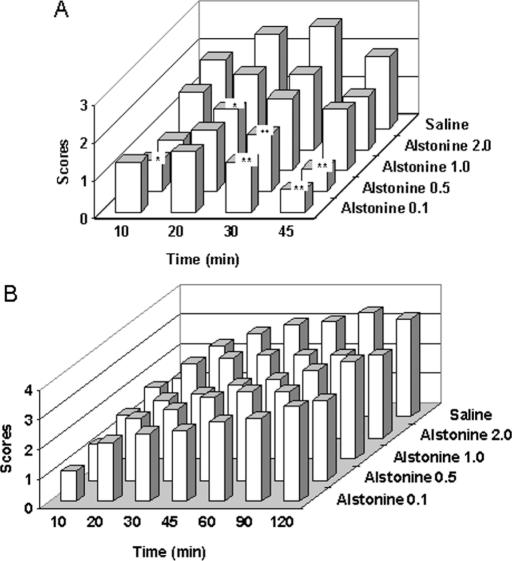
Ellenbroek (15) discussed the correlation of antipsychotic agents in preventing amphetamine- and apomorphine-induced stereotypies and their capacity to induce extrapyramidal symptoms in patients. Classical and atypical antipsychotics have different effects on these models. Alstonine reduced apomorphine-induced (6A) but not amphetamine-induced (Fig. 6B) stereotypy. Interestingly enough, in this regard, alstonine differs from the original plant extract effective against amphetamine but not apomorphine-induced stereotypy.
Figure 6.
Effects of alstonine and vehicle (saline) on apomorphine- (A) and amphetamine-induced (B) stereotypies (doses in mg kg−1). Drugs were administered intraperitoneally *P < 0.05, **P < 0.01, Mann–Whitney.
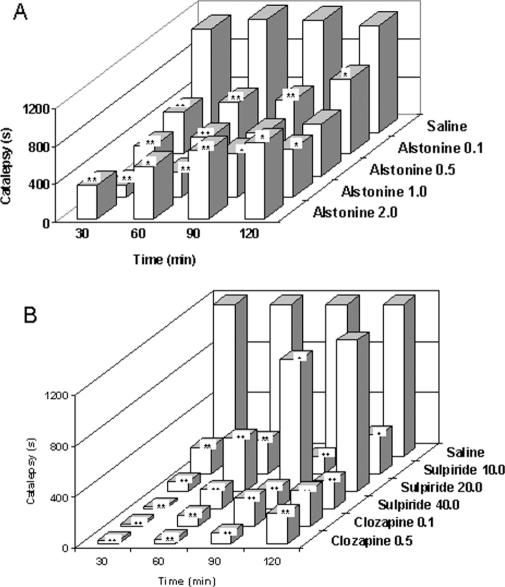
As can be seen at Fig. 7, similar to clozapine and sulpiride (atypical antipsychotics) (Fig. 7B) alstonine prevents haloperidol-induced catatonia (Fig. 7A). Several classes of drugs can prevent haloperidol-induced catalepsy, such as anticholinergics, D1 and D2 agonists, 5HT modulators and glutamate NMDA antagonists (16–18).
Figure 7.
Effects of alstonine (A), sulpiride, clozapine (B) and vehicle (saline) on haloperidol-induced catatonia (doses in mg kg−1). Drugs were administered intraperitoneally *P < 0.05, **P < 0.01, Mann–Whitney.
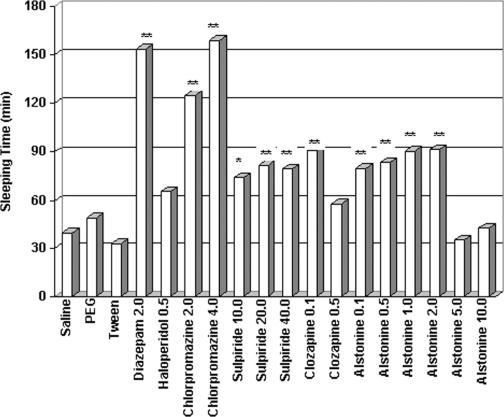
Regarding potentiation of barbiturate-induced sleeping time, alstonine exhibited hypnotic properties, compliant with most antipsychotics (Fig. 8). This result is also consistent with the emphasis on the hypnotic effects of alstonine containing plant preparation given Dr C.O.
Figure 8.
Effects of classical antipsychotics (haloperidol and chlorpromazine), atypical antipsychotics (clozapine and sulpiride), reserpine, benzodiazepine (diazepam), alstonine and respective vehicle (saline, tween and PEG) on barbiturate-induced sleeping time (doses in mg kg−1). Drugs were administered intraperitoneally *P < 0.05, **P < 0.01, Mann–Whitney.
As the clinical effectiveness of antipsychotic drugs has been associated with the blockade of mesolimbic D2 receptors, the studies of antipsychotics have been centered on drugs that modulate dopaminergic activity. However, side effects induced by antipsychotic drugs also results from D2 receptors blockade at the nigroestriatal system (19). A detailed understanding of specific roles of dopamine nuclei, projections, receptor subtypes and the cross talk among neurotransmitter systems have disclosed new ways of looking at the effects of antipsychotics (20), as well as the contribution of neurotransmitters other than dopamine to both schizophrenia and its treatments. As mentioned above, alstonine was active in the range 0.5 and 2.0 mg kg−1 against amphetamine-induced lethality, following an inverted U shape dose–effect relationship. This pattern has not been reported for any other antipsychotic drug; this unique pattern argues in favor of a multiple mechanism of action, a complex interaction with more than one neurotransmitter system.
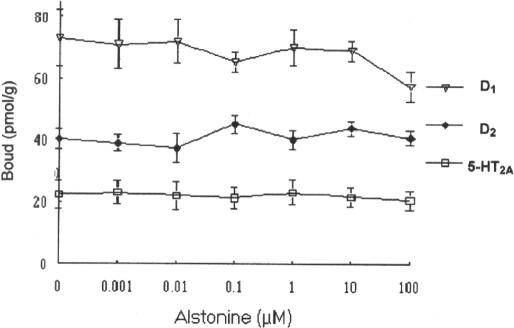
Adding to the hypothesis of alstonine singular mechanism of action, a neurochemical investigation revealed that alstonine does not seem to bind to dopamine D2 receptor subtype; displacement curves of [3H]Spiperone and [3H]SCH23390 also revealed lack of significant interaction of alstonine with dopamine D1 and serotonin 5-HT2A receptors obtained from striatum and cortex membranes, respectively (Fig. 9) (13). Hence, although close to atypical antipsychotics in mouse behavioral models, alstonine differs from these in the neurochemical basis of its mechanism of action.
Figure 9.
Influence of alstonine on [3H]SCH23390 specific binding to striatum (for D2 receptors), [3H]Spiperone specific binding to striatum (for D1 receptors) and [3H]Spiperone specific binding to frontal cortex (for 5-HT2A receptors).
Anxiolytic Properties of Alstonine
Symptoms of anxiety are very common, and thought to accompany many disorders, including schizophrenia. Antipsychotic therapy reduces anxiety concomitant with alleviation of the psychosis (21); in fact, a significant amount of attention has been given to the links between anxiety and schizophrenia (22). It has been argued that the anxiolytic property of certain antipsychotics is a crucial feature for ameliorating the so-called negative symptoms that affect the quality of life of some schizophrenic patients (23–25). It has been suggested that drug differences in relevant behavioral models presenting may be indicative of a likewise diverse clinical profile (26).
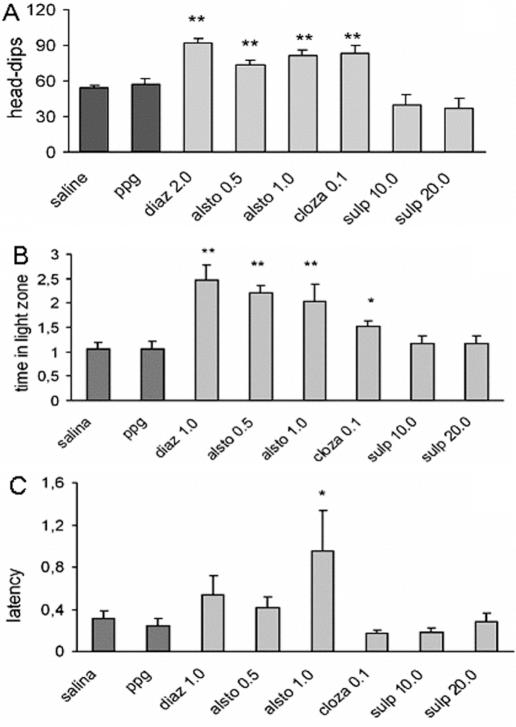
Experimental animals demonstrate anxiety, fear and curiosity when placed in a new environment, and an overall assessment of behavior can be determined by observing freezing, grooming (fear) or rearing, head-dips (curiosity), and the number of fecal boluses (stress) (27–29). The ‘hole-board’ and ‘light/dark’ models are frequently used to detect and evaluate anxiolytic/anxiogenic properties of drugs (30–34). Alstonine clearly behaves as anxiolytic in both of these animal models (35). In the hole-board model, alstonine significantly increases the number of head-dips (Fig. 10A); in the light/dark test, it significantly increases the time spent in light area exploration (Fig. 10B) as well as the latency for first crossing from light to dark compartment (Fig. 10C). Alstonine per se (0.5 and 1.0 mg kg−1) neither interfered with spontaneous locomotion (see Fig. 13A), nor induced deficits in the rota-rod test (data not shown), indicating that at doses presenting antipsychotic and anxiolytic properties, alstonine does not cause motor deficits (35). An increase in the latency for the first crossing is considered a reliable parameter to measure the disinhibitory behavior and decreased anxiety (36); in this case, false positive due to sedation is ruled out by the absence of effects of alstonine in ambulation.
Figure 10.
Effects of alstonine, diazepam, clozapine and sulpiride in mice models of anxiety. Alstonine (alsto, 0.5 and 1.0 mg kg−1 i.p.), diazepam (diaz, 2.0 mg kg−1 i.p.), clozapine (cloza, 0.1 mg kg−1 s.c.) and sulpiride (sulp, 10.0 and 20.0 mg kg−1 i.p.), or vehicles (saline or PPG) were injected 30 min prior to the behavior measurements: (A) head-dips; (B) time in the light zone; (C) latency for the first crossing. Each column represents the mean ± SEM. (N = 10–14). **P < 0.01, *P < 0.05 versus vehicle-treated group, ANOVA followed by Student–Newmann–Keuls.
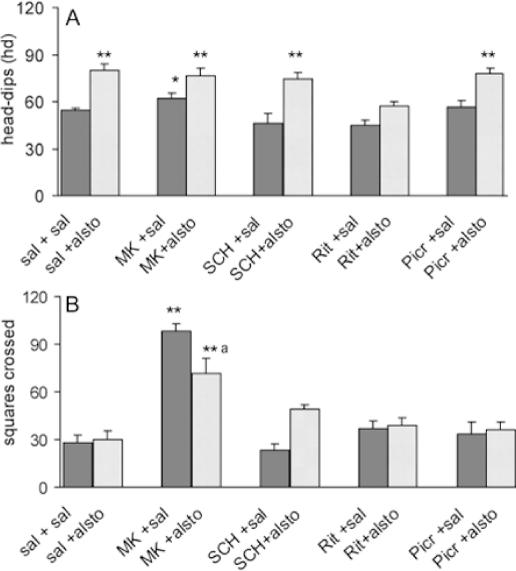
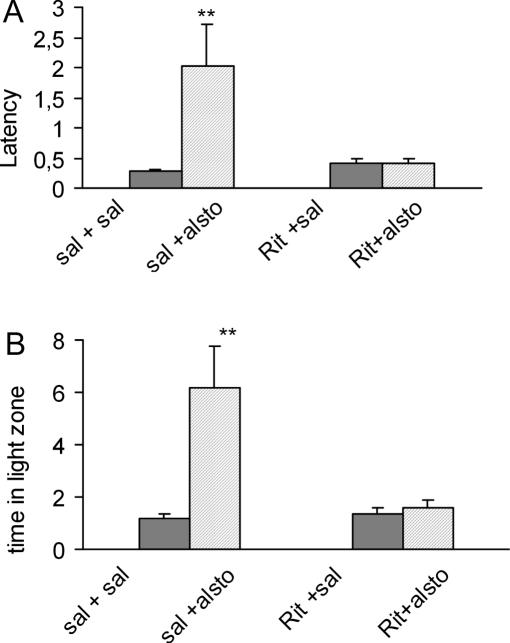
The participation of D1, 5-HT2, NMDA and GABA receptors in the effects of alstonine was examined in these models, by pretreatments with appropriate antagonists. In the hole-board model, the increase in head-dips induced by alstonine was not modified by previous administration of SCH23390 (D1 antagonist), MK-801 (NMDA antagonist) or picrotoxine (GABAA antagonist), but prevented by ritanserin (5-HT2A/C antagonist) (Fig. 11A). Alstonine also attenuated the number of squares crossed, increased in this model by MK-801 (Fig. 11B). In the black and white model, the alstonine-induced increase in the latency for first crossing (Fig. 12A) and the time spent in the light zone (Fig. 12B) was also prevented by previous administration of ritanserin (2.0 mg kg−1).
Figure 11.
Effect of the interaction of alstonine and antagonists in the mice hole-board model. Animals were treated with saline or the antagonists SCH23390 (SCH, i.p., 0.1 mg kg−1), ritanserin (Rit, 2.0 mg kg−1), MK-801 (MK, 0.1 mg kg−1) and picrotoxine (Picr, 1.0 mg kg−1) 30 min prior to vehicle (solution saline 0.9%) or alstonine (alsto, 1.0 mg kg−1). The test was performed 30 min after the last drug administration. (A) Head-dips; (B) squares crossed. Each column represents the mean ± SEM. (N = 10–14). **P < 0.01, *P < 0.05 versus vehicle-treated group, a = P < 0.01, versus MK-801-saline, ANOVA followed by Student–Newmann–Keuls.
Figure 12.
Effect of the interaction of alstonine and ritanserin in the light/dark model. Animals were treated with saline (sal) or the antagonist ritanserin (Rit, 2.0 mg kg−1) prior to saline or alstonine (alsto, 1.0 mg kg−1). The test was performed 30 min after the last drug administration. (A) Latency; (B) time spent in the light zone. Each column represents the mean ± SEM. (N = 10–12) **P < 0.01 vehicle-treated group. ANOVA followed by Student–Newmann–Keuls.
Although data from binding assays (13) do not suggest direct interaction of alstonine with 5HT2 receptors, the results with the hole-board and light/dark models are indicative of the involvement of 5-HT2A/2C receptors in the mode of action of alstonine. The findings that 5-HT2 or 5-HT3 receptors blockade by ritanserin, ondansetron and other agents has a disinhibitory profile in animal models of anxiety (37) provided further support for the serotonergic modulation of anxiety (38). A serotonergic involvement with the anxiolytic profile of alstonine would be, therefore, not entirely surprising, and correlates well with the previously reported disinhibitory effects of clozapine and thioridazine (39). In fact, it has been proposed that new agents that modulate the 5-HT2 family of serotonin receptors could have a significant impact on mental disease management (40,41), including anxiety, depression, schizophrenia and other disorders (42).
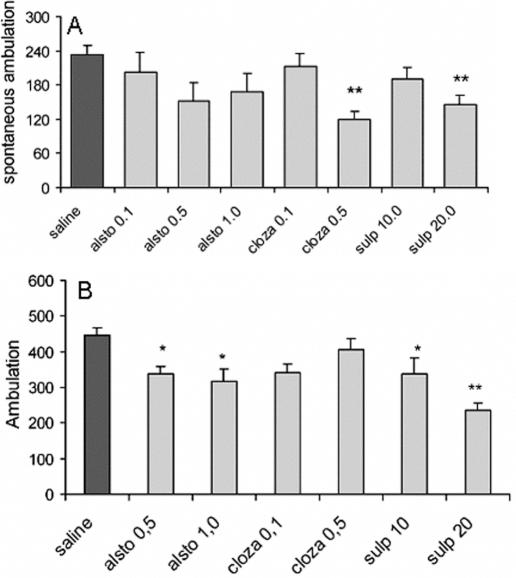
Pharmacological studies have revealed that the blockade of NMDA receptors in vivo causes behavioral abnormalities accompanied by functional alteration of monoaminergic neuronal systems. For example, noncompetitive NMDA receptor antagonist MK-801 or phencyclidine (PCP) induces characteristic rodent behavioral syndromes, including hyperlocomotion and stereotypy, which are accompanied by an increase in dopaminergic and serotonergic neuronal activities in various brain regions (43). MK-801-induced hyperlocomotion is therefore used as a simple test associated with dopaminergic alterations and considered by some as an experimental model useful to study antipsychotics (44). Alstonine (0.1, 0.5 and 1.0 mg kg−1) prevented MK-801 induced hyperlocomotion (Fig. 13B). Because alstonine partially reversed MK-801-induced increase in locomotion both at the hole-board and locomotor activity cages, a possible role of NMDA glutamate receptors in alstonine mechanism(s) of action should also be considered.
Figure 13.
Effects of alstonine (alsto, 0.1, 0.5 and 1.0 mg kg−1), clozapine (cloza, 0.1 and 0.5 mg kg−1), sulpiride (sulp, 10.0 and 20.0 mg kg−1) or saline on spontaneous locomotion (A) and MK-801-induced hyperlocomotion (B). Each column represents the mean ± SEM. (N = 10). **P < 0.01 versus vehicle-treated group, ANOVA followed by Student–Newmann–Keuls.
The regulation of fear and anxiety is strongly associated with the central GABA and serotonergic (5-HT) systems (28). Recently however, other neurotransmitter systems (such as cholinergic, dopaminergic and glutamatergic) in modulating emotional behavior have received attention (45). It has been suggested that NMDA antagonists are potential nonclassical anxiolytics (46).
Alstonine Lacks Epileptogenic Property
A serious risk of antipsychotic drugs to induce seizures is commonly acknowledged, and the administration of these drugs in epileptic patients requires specific measures for a safe use (47). The pro-convulsant property of antipsychotic has been related to their dopamine D2-receptor blocking activity, and partially, to their activity on histaminergic receptors (48). However, the new antipsychotic drugs with anti-serotonergic mechanisms changed the concept of this somehow simplistic anti-dopaminergic hypothesis. As a matter of fact, the occurrence of seizures with atypical agents is highest among antipsychotics. Clozapine (a phenotiazine) is the most widely studied antipsychotic in this regard; it carries the highest seizure risk, with an incidence of 3.5%, compared to 0.9% for olanzapine, 0.9% for quetiapine and 0.3% for risperidone (49).
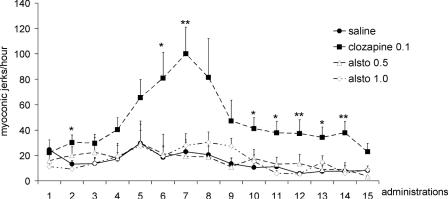
The experimental profile of alstonine showed similarities with clozapine; it was thus of interest to verify if alstonine possessed pro-convulsant properties. The evaluation was done by analyzing the effects of repetitive administrations of antipsychotic doses of alstonine. The repeated brief low-intensity sub-convulsive focal electrical stimulation of certain brain areas, at intervals of hours or days, resulting in a progressive development of generalized seizures is known as ‘the Kindling phenomenon’, originally described by Goddard et al. (49). It is understood that the kindling-induced enhanced seizure activity reflects a long-lasting alteration of the neuronal excitability, suggestive of neuronal plasticity (50). Alstonine (0.5 and 1.0 mg kg−1) did not induce kindling throughout a 30-day period, suggesting that alstonine, unlike clozapine, lacks pro-convulsant properties (51) (Fig. 14).
Figure 14.
Number of myoclonic jerks (MJs) induced by repeated administration of alstonine (0.5 and 1.0 mg kg−1), clozapine (1.0 mg kg−1) and saline. Data expressed as media and standard error (SE). Observations were done in the hour following alternate days of drug administrations. N = 6 mice per group. *P < 0.5 and **P < 0.01, ANOVA/SNK.
Alstonine does not affect dopamine binding in striatal membranes, indicating lack of direct interaction with dopamine D1 or D2 receptors (13). The anxiolytic property of alstonine is not antagonized by the GABAA antagonist picrotoxine (35); this lack of GABAA modulation by alstonine may be one important factor in its apparent lack of pro-convulsant activity. Despite the fact that drugs acting as 5-HT2 antagonists enhanced NMDA-mediated transmission (52), the lack of pro-convulsant activity found with repeated alstonine administration suggests that the enhancement of NMDA transmission produced by a possible serotonergic antagonism of alstonine is not a significant factor for inducing seizures.
A differential effect of alstonine in 5HT2A receptors at cortex and 5HT2A/C receptors in areas of relevance to anxiety (such as amygdala and septo-hypocampal system) (53) could be the basis for these apparent conflicting results. It can be suggested that alstonine may act restoring the appropriate levels of excitability in areas relevant to the symptoms of schizophrenia, without inducing concomitant excess excitability that results in seizures.
Alstonine and Glutamate
Despite playing important roles in diverse physiological functions, the NMDA mediated glutamatergic neurotransmission is related to various pathologies, including neurodegenerative diseases, ischemia, epilepsy and schizophrenia (54–56). There are several arguments in favor of a glutamate involvement in schizophrenia. Pharmacological evidences indicate that a glutamate hypoactivity may be associated with the disease, more specifically in reference to NMDA-mediated glutamate activity (57). It is believed that schizophrenia has a neurodevelopmental component and NMDA receptors are crucial for axonal signaling during development (58). Schizophrenic patients present a marked cognitive deficit and cognition is associated with neuronal plasticity mediated by NMDA receptors (59). Another relevant observation is that in schizophrenia a reduction in grey matter is verified, arguably the result of NMDA-mediated neurotoxicity (60). Additionally, ketamine (an NMDA antagonist) increases the sensibility to audio hallucinations in schizophrenics, perhaps by accentuating a subjacent glutamatergic deficit in regions linked to audition (61). Therefore, a variety of symptoms found in schizophrenia could be partially explained through an alteration of NMDA-mediated glutamatergic neurotransmission (57).
As seen earlier, in mouse models alstonine (0.5–1.0 mg kg−1) (i.p.) reverses MK-801-induced effects (35). A preliminary neurochemical analysis indicates that alstonine does not interfere directly with [3H] glutamate release in cortical synaptosomes (data not shown). If alstonine does not affect directly NMDA receptors or glutamate release and uptake, a possible interpretation of alstonine reversal of MK-801-induced effects could be an indirect modulation of glutamate transmission exerted by alstonine through the serotonin system. It has been observed that some antipsychotics inhibit 5-HT2A serotonin receptors reducing the response to NMDA antagonists (62,63). Such diminished response is, in turn, compatible with the fact that 5-HT2 antagonists increase NMDA glutamatergic transmission (52,64).
Attuned with this interpretation is the demonstration that the anxiolytic effects of alstonine are blocked by previous administration of ritanserine, suggesting that the anxiolityc properties of this alkaloid depend on 5-HT2A/2C serotonin receptors (35). Interaction with 5-HT2A/2C serotonin receptors is common to other atypical antipsychotics, such as clozapine and olanzapine (65), again illustrating the relevance of the complex cross talk between glutamate and serotonin for antipsychotic effects. Since there is no indication that a direct stimulation of 5-HT2A/2C result in anxiolytic effects, and given the blocking effect of previous administration of ritanserine, it is tempting to speculate that alstonine may act as an inverse agonist of 5-HT2A/2C serotonin receptors; nevertheless binding studies are necessary to clarify the exact mode of the anxiolytic action of alstonine.
Other Properties of Alstonine
Alstonine has the capacity to distinguish cancer DNA from healthy tissue DNA; it inhibits DNA in vitro synthesis when DNA from different cancerous tissues or cells is used as template. Practically without effects on DNA from healthy tissues, the inhibitory effect of alstonine is due to its capacity to form an ‘alkaloid-cancer DNA’ complex (66).
Alstonine, as well as serpentine and sempervirine, successfully treat a relatively important proportion of BALB/C mice inoculated with transplantable YC8 lymphoma ascites cells as well as Swiss mice bearing Ehrlich ascites carcinoma cells. However, the development of some solid tumors is only partially prevented by alstonine. These results illustrate the anticancer activity of alstonine (67).
Wright et al. (68) studied alkaloids present in various plant species as antimalarials. In a preliminary evaluation, extracts of A. constricta and alstonine demonstrated in vivo antimalarial activity (69).
The interaction of tetrahydroalstonine, an alkaloid closely related to alstonine, with the α-adrenergic receptors have been reported by Demichel and Roquebert (70). Raubasine, tetrahydroalstonine and akuammigine were shown to have inhibitory effects on sympathetic stimulation or adrenaline-induced hypertension, demonstrating specificity for presynaptic α2-adrenergic receptors (71).
Conclusion
Alstonine is an alkaloid present in a variety of plant species used in traditional medical systems. Biomedical examination revealed that alstonine is bioactive, distinct in its antipsychotic and anticancer properties.
The antipsychotic profile of alstonine is remarkably coherent with its use by Nigerian traditional psychiatrists. In many aspects the antipsychotic profile of alstonine is relevant to the development of new antipsychotic medications:
The traditional use of several species containing alstonine is indicative of bioavailability;
Although a thorough assessment would certainly be needed, traditional use is also indicative of safety (a relative concept in regard to all medicines);
Although it is certainly open to debate how to compare the very concept of mental disorders in different cultures, the traditional use of alstonine by Nigerian traditional psychiatrists is remarkably compatible with its profile in experimental animals, altogether indicative of effectiveness as antipsychotic;
Alstonine possesses clear anxiolytic activity, mediated by 5-HT2A/2C serotonin receptors, suggesting effectiveness against negative symptoms of schizophrenia;
Alstonine seems to interfere with the glutamate system in a manner consistent with resulting beneficial effects for schizophrenia;
Alstonine lacks the pro-convulsant property common to many antipsychotics, a considerable advantage for chronic use in general and epileptic schizophrenic patients in particular;
The lack of direct effects on the dopaminergic system suggests lack of significant extra pyramidal effects, the major drawback of many antipsychotic agents.
Rauwolfia alkaloids producing species usually produce several alkaloids, including reserpine—the first antipsychotic medication used in modern medicine. It is worth remembering that reserpine is the major component of R. serpentina used to treat mental illness in India for centuries before its re-discovery in the 20th century by the contemporary biomedical paradigm. The various pharmacological effects of reserpine are so prominent that it is not surprising that minor components likely to be present in Rauwolfia spp. crude extracts/formulations (including alkaloids like serpentine, ajmalicine and alstonine) would have been neglected as potential key active components in earlier psychopharmacology evaluations.
Progress in psychopharmacology and neurochemistry are constantly unveiling new cellular and molecular targets relevant to drug's mode of action; it is therefore arguable and expected that traditional treatments (including plant-based remedies) may act as disease modifiers by eliciting physiological responses through mechanisms yet to be elucidated. We suggest that a thorough understanding of traditional medical concepts of health and disease in general and traditional medical practices in particular, can lead to true innovation in paradigms of drug action and development. In sum, the study of this unique indole alkaloid may be considered as another example of the wealth of medicinal plants and traditional medical systems in the discovery of new prototypic drugs (72,73).
Acknowledgments
Alstonine was kindly provided by M. Iwu and Christopher Okunji. This work was supported by CNPq and the International Cooperative Biodiversity Group (ICBG) of the U.S. National Institutes of Health (ICBG coordinated by Dr M. Iwu). We are especially grateful for the pivotal assistance of Mr Cosmas Obialor Nkemjika, Mr Alfred Ozioko and Dr Charles O.C. Agwu (University of Nigeria, Nsukka) while in Igboland. We sincerely thank Dr C.O. for sharing his knowledge with us.
References
- 1.Williams JE. Review of antiviral and immunomodulating properties of plants of the Peruvian rainforest with a particular emphasis on uña de gato and sangre de grado. Altern. Med. Rev. 2001;6:567–79. [PubMed] [Google Scholar]
- 2.Cano A, Alcaraz O, Arnao MB. Free radical-scavenging activity of indolic compounds in aqueous and ethanolic media. Anal Bioanal Chem. 2003;376:33–7. doi: 10.1007/s00216-003-1848-7. [DOI] [PubMed] [Google Scholar]
- 3.Cordell GA, Quinn-Beattie ML, Farnsworth NR. The potencial of alkaloids in drug discovery. Phytother Res. 2001;15:183–205. doi: 10.1002/ptr.890. [DOI] [PubMed] [Google Scholar]
- 4.Costa-Campos L, Elisabetsky E, Lara DR, Carlson TJ, King SR, Ubilas R, et al. Antipsychotic profile of alstonine: ethnopharmacology of a traditional Nigerian botanical remedy. An Acad Bras Cienc. 1999;71:189–201. [PubMed] [Google Scholar]
- 5.Iwu MM, Klayman DL. Evaluation of the in vitro antimalarial activity of Picralima nitida extracts. J Ethnopharmacol. 1992;36:133–5. doi: 10.1016/0378-8741(92)90012-g. [DOI] [PubMed] [Google Scholar]
- 6.Noble RL. The discovery of the vinca alkaloids—chemotherapeutic agents against cancer. Biochem Cell Biol. 1990;68:1344–51. [PubMed] [Google Scholar]
- 7.Kweifio-Okai G. Antiinflammatory activity of a Ghanaian antiarthritic herbal preparation: I. J Ethnopharmacol. 1991;33:263–7. doi: 10.1016/0378-8741(91)90087-t. [DOI] [PubMed] [Google Scholar]
- 8.Ezeamuzie IC, Ojinnaka MC, Uzogara EO, Oji SE. Anti-inflammatory, antipyretic and anti-malarial activities of a West African medicinal plant—Picralima nitida. Afr J Med Med Sci. 1994;23:85–90. [PubMed] [Google Scholar]
- 9.Wosu LO, Ibe CC. Use of extracts of Picralima nitida bark in the treatment of experimental trypanosomiasis: a preliminary study. J Ethnopharmacol. 1989;25:263–8. doi: 10.1016/0378-8741(89)90032-9. [DOI] [PubMed] [Google Scholar]
- 10.Nasser AM, Court WE. Stem bark alkaloids of Rauvolfia caffra. J Ethnopharmacol. 1984;11:99–117. doi: 10.1016/0378-8741(84)90099-0. [DOI] [PubMed] [Google Scholar]
- 11.Perlman WR, Weickert CS, Akil M, Kleinman JE. Postmortem investigations of the pathophysiology of schizophrenia: the role of susceptibility genes. J Psychiatry Neurosci. 2004;29:287–93. [PMC free article] [PubMed] [Google Scholar]
- 12.Lyne J, Kelly BD, O'Connor WT. Schizophrenia: a review of neuropharmacology. Ir J Med Sci. 2004;173:155–9. doi: 10.1007/BF03167931. [DOI] [PubMed] [Google Scholar]
- 13.Costa-Campos L, Lara DR, Nunes DS, Elisabetsky E. Antipsychotic-like profile of alstonine. Pharmacol Biochem Behav. 1998;60:133–41. doi: 10.1016/s0091-3057(97)00594-7. [DOI] [PubMed] [Google Scholar]
- 14.Bourin M, Poisson L, Larousse C. Piracetam interaction with neuroleptics in psychopharmacological tests. Neuropsychobiology. 1986;19:93–96. doi: 10.1159/000118305. [DOI] [PubMed] [Google Scholar]
- 15.Ellenbroek BA. Treatment of schizophrenia: a clinical and preclinical evaluation of neuroleptic drugs. Pharmacol Ther. 1993;57:1–78. doi: 10.1016/0163-7258(93)90036-d. [DOI] [PubMed] [Google Scholar]
- 16.Ezrin-Waters C, Muller P, Seeman P. Catalepsy induced by morphine and haloperidol: effects of apomorphine and anti-cholinergic drugs. Can J Physiol Pharmac. 1976;54:516–9. doi: 10.1139/y76-071. [DOI] [PubMed] [Google Scholar]
- 17.Moore NA, Blackman A, Awere S, Leander JD. NMDA receptor antagonists inhibit catalepsy induced by either dopamine D1 or D2 receptor antagonists. Eur J Pharmacol. 1993;237:1–7. doi: 10.1016/0014-2999(93)90085-v. [DOI] [PubMed] [Google Scholar]
- 18.Jackson DM, Westlind-Danielsson A. Dopamine receptors: molecular biology, biochemistry and behavioral aspects. Pharmac Ther. 1994;64:291–369. doi: 10.1016/0163-7258(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 19.Horacek J. Novel antipsychotics and extrapyramidal side effects: Theory and reality. Pharmacopsychiatry. 2000;33(Suppl 1):34–42. doi: 10.1055/s-2000-7660. [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- 21.Bourin M, Nic Dhonnchadha BA, Colombel MC, Dib M, Hascoët M. Cyamemazine as an anxiolytic drug on the elevated plus maze and light/dark paradigm in mice. Behav Brain Res. 2001;124:87–95. doi: 10.1016/s0166-4328(01)00238-8. [DOI] [PubMed] [Google Scholar]
- 22.Turnbull G, Bebbington P. Anxiety and the schizophrenic process: clinical and epidemiological evidence. Soc Psychiatr Epidemiol. 2001;36:235–43. doi: 10.1007/s001270170054. [DOI] [PubMed] [Google Scholar]
- 23.Cao BJ, Rodgers RJ. Dopamine D4 receptors and anxiety: behavioural profiles of clozapine, L-745,870 and L-741,742 in the mouse plus-maze. Eur J Pharmacol. 1997;335:117–25. doi: 10.1016/s0014-2999(97)01234-x. [DOI] [PubMed] [Google Scholar]
- 24.Sakamoto H, Matsumoto K, Yukihiro O, Nakumura M. Anxiolytic-like effects of perospirone, a novel serotonin-2 and dopamine-2 antagonist (SDA)-type antipsychotic agent. Pharmacol Biochem Behav. 1998;60:873–8. doi: 10.1016/s0091-3057(98)00068-9. [DOI] [PubMed] [Google Scholar]
- 25.Huppert JD, Weiss KA, Lim R, Pratt S, Smith TE. Quality of life in schizophrenia: contributions of anxiety and depression. Schizophr Res. 2001;51:171–80. doi: 10.1016/s0920-9964(99)00151-6. [DOI] [PubMed] [Google Scholar]
- 26.Moore NA. Behavioural pharmacology of the new generation of antipsychotics agents. Br J Psychiatry Suppl. 1999;38:5–11. [PubMed] [Google Scholar]
- 27.Kennedy B. Experimental approaches to the detection of anxiolytic activity in the rat. Ir J Med Sci. 1978;147:38–42. doi: 10.1007/BF02947902. [DOI] [PubMed] [Google Scholar]
- 28.File SE. The contribution of behavioral studies to the neuropharmacology of anxiety. Neuropharmacology. 1987;26:877–86. doi: 10.1016/0028-3908(87)90065-7. [DOI] [PubMed] [Google Scholar]
- 29.Dalvi A, Rodgers RJ. Behavioral effects of diazepam in the murine plus-maze: flumazenil antagonism of enhanced head-dipping but not the disinhibition of open-arm avoidance. Pharmacol Biochem Behav. 1999;62:727–34. doi: 10.1016/s0091-3057(98)00220-2. [DOI] [PubMed] [Google Scholar]
- 30.File SE, Wardill GA. Validity of head-dipping as a measure of exploration in a modified hole-board. Psychopharmacology (Berl.) 1975;44:53–9. doi: 10.1007/BF00421184. [DOI] [PubMed] [Google Scholar]
- 31.Takeda H, Tsuji M, Matsumiya T. Changes in head-dipping behavior in the hole-board test reflect the anxiogenic and/or anxiolytic state in mice. Eur J Pharmacol. 1998;350:21–9. doi: 10.1016/s0014-2999(98)00223-4. [DOI] [PubMed] [Google Scholar]
- 32.Ohl F, Roedel A, Storch C, Holsboer F, Landgraf R. Cognitive performance in rats differing in their inborn anxiety. Behav Neurosci. 2002;116:464–71. doi: 10.1037//0735-7044.116.3.464. [DOI] [PubMed] [Google Scholar]
- 33.Nic Dhonnchadha BA, Bourin M, Hascoet M. Anxiolytic-like effects of 5-HT2 ligands on three mouse models of anxiety. Behav Brain Res. 2003;140:203–14. doi: 10.1016/s0166-4328(02)00311-x. [DOI] [PubMed] [Google Scholar]
- 34.Isogawa K, Fujiki M, Akiyoshi J, Tsutsumi T, Horinouchi Y, Kodama K, Nagayama H. Anxiety induced by repetitive transcranial magnetic stimulation is suppressed by chronic treatment of paroxetine in rats. Pharmacopsychiatry. 2003;36:7–11. doi: 10.1055/s-2003-38085. [DOI] [PubMed] [Google Scholar]
- 35.Costa-Campos L, Dassoler SC, Rigo AP, Iwu M, Elisabetsky E. Anxiolytic properties of the antipsychotic alkaloid alstonine. Pharmacol Biochem Behav. 2004a;77:481–9. doi: 10.1016/j.pbb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Costall B, Naylor RJ. Anxiolytic effects of 5-HT3 antagonists in animals. In: Rodgers RJ, Cooper SJ, editors. 5-HT1A Agonists, 5-HT3 Antagonists and Benzodiazepines: Their Comparative Behavioural Pharmacology. Chichester: John Wiley and Sons; 1991. pp. 133–58. [Google Scholar]
- 37.Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- 38.Costall B, Naylor RJ. Behavioural interactions between 5-hydroxytryptophan, neuroleptic agents and 5-HT receptor antagonists in modifying rodent responding to aversive situations. Br J Pharmacol. 1995;116:2989–99. doi: 10.1111/j.1476-5381.1995.tb15954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones BJ, Blackburn TP. The medical benefit of 5-HT research. Pharmacol Biochem Behav. 2002;71:555–68. doi: 10.1016/s0091-3057(01)00745-6. [DOI] [PubMed] [Google Scholar]
- 40.Roth BL, Shapiro DA. Insights into the structure and function of 5-HT(2) family serotonin receptors reveal novel strategies for therapeutic target development. Expert Opin Ther Targets. 2001;5:685–95. doi: 10.1517/14728222.5.6.685. [DOI] [PubMed] [Google Scholar]
- 41.Van Oekelen D, Luyten WHML, Leysen JE. 5-HT2A and 5-HT2C receptors and their atypical regulation properties. Life Sci. 2003;72:2429–49. doi: 10.1016/s0024-3205(03)00141-3. [DOI] [PubMed] [Google Scholar]
- 42.Miyamoto Y, Yamada K, Noda Y, Mori H, Mishina M, Nabeshima T. Hyperfunction of dopaminergic and serotonergic neuronal systems in mice lacking the NMDA receptor epsilon1 subunit. J Neurosci. 2001;21:750–7. doi: 10.1523/JNEUROSCI.21-02-00750.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lourenço Da Silva A, Hoffmann A, Dietrich MO, Dall'Igna OP, Souza DO, Lara DR. Effect of riluzole on MK-801 and amphetamine-induced hyperlocomotion. Neuropsychobiology. 2003;48:27–30. doi: 10.1159/000071825. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura K, Kurasawa M. Anxiolytic effects of aniracetam in three different mouse models of anxiety and the underlying mechanism. Eur J Pharmacol. 2001;420:33–43. doi: 10.1016/s0014-2999(01)01005-6. [DOI] [PubMed] [Google Scholar]
- 45.Dunn RW, Corbett R, Fielding S. Effects of 5-HT1A receptor agonists and NMDA receptor antagonists in the social interaction test and elevated plus maze. Eur J Pharmacol. 1989;169:1–10. doi: 10.1016/0014-2999(89)90811-x. [DOI] [PubMed] [Google Scholar]
- 46.Koch-Stoecker S. Antipsychotic drugs and epilepsy: indications and treatment guidelines. Epilepsia. 2002;43(Suppl 2):19–24. doi: 10.1046/j.1528-1157.2002.043s2019.x. [DOI] [PubMed] [Google Scholar]
- 47.Torta R, Monaco F. Atypical antipsychotics and serotonergic antidepressants in patients with epilepsy: pharmacodynamic considerations. Epilepsia. 2002;43(Suppl 2):8–13. doi: 10.1046/j.1528-1157.2002.043s2008.x. [DOI] [PubMed] [Google Scholar]
- 48.Alldredge BK. Seizures risk associated with psychotropic rugs: clinical and pharmacokinetic consideration. Neurology. 1999;53(Suppl 2):S68–75. [PubMed] [Google Scholar]
- 49.Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969;95:294–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- 50.Cain DP. Long-term potentiation and kindling: how similar are the mechanisms. Trends Neurosci. 1989;57:37–43. doi: 10.1016/0166-2236(89)90146-x. [DOI] [PubMed] [Google Scholar]
- 51.Costa-Campos L, Iwu M, Elisabetsky E. Lack of pro-convulsant activity of the antipsychotic alkaloid alstonine. J Ethnopharmacol. 2004b;93:307–10. doi: 10.1016/j.jep.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 52.Arvanov VL, Wang RY. A selective 5-HT2A receptor antagonist and a potential antipsychotic drug, facilitates N-methyl-d-aspartate-receptor mediated neurotransmission in the medial prefrontal cortical neurons in vitro. Neuropsychopharmacology. 1998;18:197–209. doi: 10.1016/S0893-133X(97)00126-7. [DOI] [PubMed] [Google Scholar]
- 53.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 54.Meldrum BS. The role of glutamate in epilepsy and other CNS disorders. Neurology. 1994;44(Suppl. 8):S14–23. [PubMed] [Google Scholar]
- 55.Meldrum BS. Neurotransmission in epilepsy. Epilepsia. 1995;36:S30–35. doi: 10.1111/j.1528-1157.1995.tb01649.x. [DOI] [PubMed] [Google Scholar]
- 56.Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Prog Neurobiol. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- 57.Meador-Woodruff JH, Healy DJ. Glutamate receptor expression in schizophrenic brain. Brain Res Rev. 2000;31:288–394. doi: 10.1016/s0165-0173(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 58.Rakic P, Bourgeois JP, Goldman-Rakic PS. Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Prog Brain Res. 1994;102:227–43. doi: 10.1016/S0079-6123(08)60543-9. [DOI] [PubMed] [Google Scholar]
- 59.Daw NW, Stein PSG, Fox K. The role of NMDA receptors in information processing. Ann Rev Neurosci. 1993;16:207–22. doi: 10.1146/annurev.ne.16.030193.001231. [DOI] [PubMed] [Google Scholar]
- 60.Zipursky RB, Lim KO, Sullivan EV, Brown BW, Pfefferbaum A. Widespread cerebral grey matter volume deficits in schizophrenia. Arch Gen Psychiatry. 1992;49:195–205. doi: 10.1001/archpsyc.1992.01820030027004. [DOI] [PubMed] [Google Scholar]
- 61.Krystal JH, D'Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl) 2003;169:215–33. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- 62.Maurel-Remy S, Bervoets K, Millan MJ. Blockade of phencyclidine-induced hyperlocomotion by clozapine and MDL 100,907 in rats reflects antagonism of 5HT2A receptors. Eur J Pharmacol. 1995;280:R9–11. doi: 10.1016/0014-2999(95)00333-g. [DOI] [PubMed] [Google Scholar]
- 63.Zhang W, Bymaster FP. The in vivo effects of olanzapine and other antipsychotic agents on receptor occupancy and antagonism of dopamine D1, D2, D3, 5HT2A and muscarinic receptors. Psychopharmacology. 1999;141:267–78. doi: 10.1007/s002130050834. [DOI] [PubMed] [Google Scholar]
- 64.Spurney CF, Baca SM, Murray AM, Jaskiw GE, Kleinman JE, Hyde TM. Differential effects of haloperidol and clozapine on ionotropic glutamate receptors in rats. Synapse. 1999;34:266–76. doi: 10.1002/(SICI)1098-2396(19991215)34:4<266::AID-SYN3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 65.Herrick-Davis K, Grinde E, Teitler M. Inverse agonist activity of atypical antipsychotic drugs at human 5-hydroxytryptamine2C receptors. J Pharmacol Exp Ther. 2000;295:226–32. [PubMed] [Google Scholar]
- 66.Beljanski M, Beljanski MS. Selective inhibition of in vitro synthesis of cancer DNA by alkaloids of beta-carboline class. Exp Cell Biol. 1982;50:79–87. doi: 10.1159/000163131. [DOI] [PubMed] [Google Scholar]
- 67.Beljanski M, Beljanski MS. Three alkaloids as selective destroyers of cancer cells in mice. Synergy with classic anticancer drugs. Oncology. 1986;43:198–203. doi: 10.1159/000226363. [DOI] [PubMed] [Google Scholar]
- 68.Wright CW, Phillipson JD, Awe SO, Kirby GC, Warhurst DC, Quetinleclercq J, Angenot L. Antimalarial activity of criptolepine and some other anhydronium bases. Phytother Res. 1996;10:361–63. [Google Scholar]
- 69.Gandhi M, Vinayak VK. Preliminary evaluation of extracts of Alstonia scholaris bark for in vivo antimalarial activity in mice. J Ethnopharmacol. 1990;29:51–7. doi: 10.1016/0378-8741(90)90097-d. [DOI] [PubMed] [Google Scholar]
- 70.Demichel P, Roquebert J. Effects of raubasine stereoisomers on pre- and postsynaptic α-adrenoceptors in the rat vas deferens. Br J Pharmacol. 1984;83:505–10. doi: 10.1111/j.1476-5381.1984.tb16514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roquebert J, Demichel P. Inhibition of the α1- and α2-adrenoceptor-mediated pressor response in the pithed rats by raubasine, tetrahydroalstonine and akuammigine. Eur J Pharmacol. 1985;106:203–5. doi: 10.1016/0014-2999(84)90698-8. [DOI] [PubMed] [Google Scholar]
- 72.Shimazawa M, Chikamatsu S, Morimoto N, Mishima S, Nagai H, Hara H. Neuroprotection by Brazilian Green Propolis against In vitro and In vivo Ischemic Neuronal Damage. Evid Based Complement Alternat Med. 2005;2:201–7. doi: 10.1093/ecam/neh078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lindequist U, Niedermeyer THJ, Jülich WD. The pharmacological potential of mushroom. Evid Based Complement Alternat Med. 2005;2:285–99. doi: 10.1093/ecam/neh107. [DOI] [PMC free article] [PubMed] [Google Scholar]