Abstract
Here we describe the results of some experimental laboratory studies aimed at verifying the efficacy of high dilutions of substances and of homeopathic medicines in models of inflammation and immunity. Studies carried out on basophils, lymphocytes, granulocytes and fibroblasts are reviewed. This approach may help to test under controlled conditions the main principles of homeopathy such as ‘similarity’ of drug action at the cellular level and the effects of dilution/dynamization on the drug activity. The current situation is that few and rather small groups are working on laboratory models for homeopathy. Regarding the interpretation of data in view of the simile principle, we observe that there are different levels of similarity and that the laboratory data give support to this principle, but have not yet yielded the ultimate answer to the action mechanism of homeopathy. Evidence of the biological activity in vitro of highly diluted-dynamized solutions is slowly accumulating, with some conflicting reports. It is our hope that this review of literature unknown to most people will give an original and useful insight into the ‘state-of-the-art’ of homeopathy, without final conclusions ‘for’ or ‘against’ this modality. This kind of uncertainty may be difficult to accept, but is conceivably the most open-minded position now.
Keywords: in vitro test, laboratory models, homeopathy, high-dilution, immune system, basophils, granulocytes, lymphocytes, similia principle
Introduction
The reliability of homeopathic principles (similia, globality of cure and use of high dilutions of ‘natural’ medicines) and their possible scientific bases can be assessed using various theoretical and experimental approaches. In this contribution we analyze the experimental evidence on cells and laboratory systems. Our aim is not to justify the clinical use of homeopathic medicines but to present evidence showing that substances prepared according to the homeopathic method have some effects on the immune system and inflammation. This may be the first step for a re-evaluation of homeopathy as a worthwhile field for basic and clinical investigations.
We are perfectly aware that the classical experimental approach to immunology based on the laboratory, animal and clinical experiments, typical of academic medicine and the current international scientific literature, can help us to understand only some of the aspects of homeopathy. Even bearing in mind the limitations of this ‘reductionistic’ approach, we still believe that it can build some bridgeheads toward the construction of a united medicine. This is mainly because we are not so much seeking to ‘demonstrate’ a single mechanism of action, but to understand general ‘rules’ of physiology, pathology and pharmacology that are the same in different fields of biology and pharmacology, and that can also be applied to homeopathy.
Immunoallergology represents a bridge between homeopathy and modern medicine insofar as it is a field in which it is easier to apply concepts such as the effect of substances administered on the basis of the logic of the ‘similarity’ and the great sensitivity of living systems to modulations induced by ultra-low doses or high dilutions of natural or endogenous substances (1).
Below, we describe the principal results of experimental studies aimed at verifying the efficacy of homeopathic medicines or, more limitedly, at verifying the main principles of homeopathy (e.g. dilution, similarity) in models of inflammation and immunity. We begin with in vitro studies of inflammatory cells (basophils, neutrophils, lymphocytes, macrophages and fibroblasts), and, in a subsequent paper, we shall examine animal studies before describing clinical trials in humans. Many of these experiments and observations are normally ignored by the modern biomedical literature.
We have performed experiments in our laboratory and have monitored the literature on the subject of this paper for the past 15 years. Here the best of our knowledge of all experimental work published is reported, irrespective of results (e.g. positive or negative results, in favor or against to homeopathy). All literature available in Medline, conference proceedings and books was searched. Due to the relative scarcity of literature in this field and the heterogeneity of experiments, we have not performed pooling and meta-analysis of data. Where indicated, a few comments on reliability of findings and on problems of replication of specific studies have been provided.
Basophils/Mast Cells
One of the laboratory models in which the phenomena of similarity and of high-dilution effects have been most widely investigated is the regulation of basophils and mast cells, which are fundamental cells in acute inflammation. In fact, one of the first biological events in acute inflammation—and immediate hypersensitivity in the case of pathology—is activation of basophils/mast cells triggered by their binding to IgE antibodies bound to high-affinity receptors as a result of sensitization. Since this is the most investigated model of high-dilution effects, some technical details may help understanding the results.
Biology of Basophil Activation
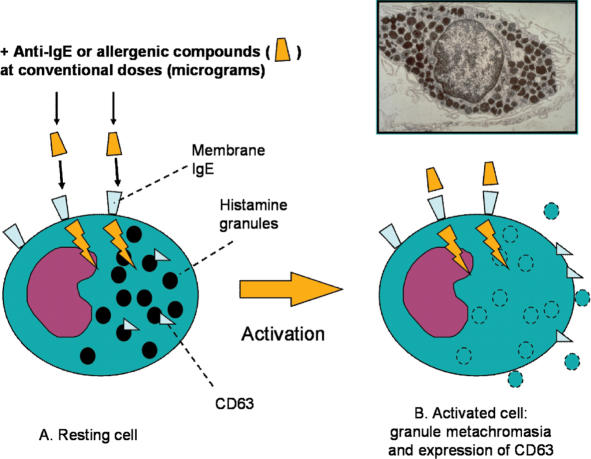
In these cells, internal activation is driven not only by specific foreign substances such as allergens but also by the binding of antibodies (anti-IgE) against heavy chains of IgE, which are the receptors of antigens in these cells. The cell activation involves changes in membrane ion fluxes (particularly calcium ions), changes in cell membrane electrical polarity, and other mechanisms that eventually lead to exocytosis and the release of mediators (Fig. 1). It is known that one of the main mediators is histamine, which is produced by the decarboxylation of histidine, stored in granules of basophils and mast cells, and released a few seconds after activation. Histamine in tissues exert vasodilating and permeabilizing actions (and therefore causes the formation of wheals and edema).
Figure 1.
Normal activation of basophil degranulation caused by anti IgE antibodies. This activation is not only driven by specific allergens, but also by the binding of antibodies against IgE heavy chains (anti-IgE) and involves changes in membrane ion fluxes (particularly calcium ions), changes in cell membrane electrical polarity, and other mechanisms that eventually lead to exocytosis and the release of mediators. Cell activation is evaluated by optical microscopy as a loss of staining properties. To be precise, loss of staining properties is not exactly the same biological phenomenon as cell degranulation, but indicates a change of the granule membrane permeability. Another typical response to activation is the increased expression of CD63 proteins, which are translocated from internal pools to the cell surface. Insert: electron microscopy of a mast cell.
At the end of the 1980s, when the first published studies aroused considerable international controversy (2,3), there were two ways of evaluating the reactivity of basophils: the histamine release test, which measures histamine released by activated basophils into the extracellular environment, and the basophil ‘degranulation’ test, which analyzes changes in coloring of granules in presence of stains such as toluidine blue or alcian blue (metachromasia). In practice, a microscope count is made of the unstained (‘degranulated’) cells in relation to the total number of basophils (4) (Fig. 1). This is erroneously called a ‘degranulation’ test because metachromasia may occur at activator doses that are much lower than the threshold required to trigger degranulation (5). Metachromasia probably reflects biochemical changes (the exchange of cations) that alter the interaction of basic dyes with proteoglycans of granule. Although it is easy to establish a correlation between the two methods using strong stimuli, this is not true when weak stimuli or very low agonist doses are used: the activation of basophils without true degranulation (i.e. without histamine release) has been observed under many circumstances (6).
Early Studies
Experimental studies of this cell type have been carried out by two groups of French researchers (Sainte-Laudy with Belon and Poitevin with Aubin) using homeopathic dilutions of Apis mellifica and Lung histaminum. The choice of these compounds was probably due on their frequent use in the treatment of allergic syndromes (7) and partially on the known ability of some of their molecular components (mellitin and histamine, respectively) to activate basophils or to have regulatory feed-back effects on them. The studies were based on the hypothesis that, even in homeopathic dilutions, these substances can regulate basophils activated by ponderal doses of an active agent. The first publications describing the effect of these homeopathic dilutions on basophils (8,9) reported that the in vitro degranulation induced by various allergens (domestic dust, house mites) was stimulated (+20%) by low dilutions (5c) (centesimal and decimal homeopathic dilutions, or ‘potencies’ in homeopathic terms, are designed as ‘c’ and ‘x’, respectively.) of bee venom (Apis mellifica), whereas higher dilutions (9c and 15c) had an opposite effect (>50% inhibition). These effects were obtained by including the medicines in the incubation mixture and were statistically significant. The inhibition by Histaminum and Apis is particularly interesting because when released at normal doses into a tissue both histamine and bee venom have pro-inflammatory powers and irritant properties. This experiment therefore illustrates application of the principle of similarity in an experimental model: a substance known to stimulate inflammation at conventional doses can, at different doses, inhibit cells responsible for many of the phenomena of the inflammatory process.
Another group has unsuccessfully tried to repeat the same experiments (10), but the authors of the first study report that the same protocol was used by a third and independent laboratory upon the request of the French Academy of Medicine and that, in this case, all three dilutions of Apis mellifica (5c, 9c, 15c) had a statistically significant inhibitory effect (11).
In order to explore the question further, and with the support of the team of Benveniste (which at that time was still working at INSERM U200 in Paris), Poitevin carried out another series of ‘blind’ experiments using 1c–15c dilutions of Apis mellifica and Lung histaminum on basophils activated by anti-IgE antibodies (12) (Fig. 2). Lung histaminum is an extract of lung from laboratory animals where anaphylactic reactions are experimentally induced, so it presumably contains, at least in the starting material (mother tincture), histamine as a mediator. The dilution-effect curves of these products showed an alternation of inhibition, inactivity and stimulation with a unusual and complex trend: Apis significantly inhibited basophil activation at the dilutions of 8c, 9c and 10c when the basophils were activated with high and low anti-IgE doses, and caused significant inhibition also at the dilutions of 5c, 7c, 13c and 20c when the basophils were activated with low anti-IgE doses. With Lung histaminum, significant inhibitions were observed at dilutions of about 5c and about 15c (from 12c to 18c). In the case of basophils activated using small anti-IgE doses, Apis 10c and Lung histaminum 18c caused ∼100% inhibition.
Figure 2.
Inhibition of basophil degranulation by homeopathic dilutions of Apis mellifica and Lung histamine (8,11,12).
The Benveniste Affair
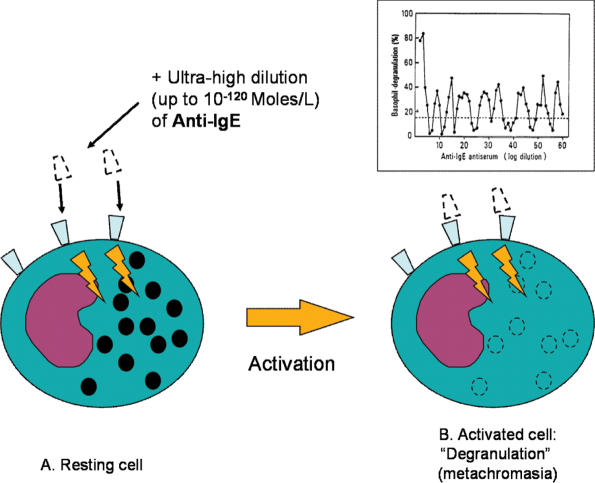
The famous multicenter study, led by J. Benveniste and involving four other laboratories, reported that human basophils undergo ‘degranulation’ (metachromasia) not only at usual anti-IgE antibody doses (10−3 mg/ml) but also at extremely high dilutions (1060 or 10120 times lower than the concentrations usually leading to a molecular interaction) (2) (Fig. 3). The dose–response curves at decreasing doses first showed the disappearance of activity and then its re-appearance followed by various peaks of alternating activity and inactivity up to extremely high dilutions corresponding to practically zero antibody concentrations. This type of a dose–response curve is also called ‘pseudo-sinusoidal’. In addition to anti-IgE antibodies, significant results were also obtained using high dilutions of substances such as calcium ionophores and phospholipase A2, known to have stimulatory effect at ponderal doses. The specificity of action was proved by the lack of effect of other highly diluted substances such as anti-IgG antibodies (basophils are only activated by anti-IgE antibodies) and phospholipase C, which have a different biochemical specificity on membranes (2). It is observed interesting to note that the high-dilution effect was observed only when the serial dilution was followed by strong succussion (‘dynamization’ in homeopathic terminology) of the solutions.
Figure 3.
The scheme of the study reporting that basophils undergo ‘degranulation’ (metachromasia) not only at usual anti-IgE antibody doses (10−3 mg/ml) but also at extremely high dilutions (1060 or 10120 times lower than the concentrations usually leading to a molecular interaction) (2). The dose–response curves of Anti-IgE (see insert) at increasing dilutions first showed the disappearance of activity and then its re-appearance followed by various peaks of alternating activity and inactivity up to extremely high dilutions.
The work of Benveniste's group was published in the authoritative scientific journal Nature and had considerable resonance as the presumed demonstration of the ‘memory of water’, but it was heavily criticized on theoretical grounds (the ‘incredibility’ of the data) and because of the difficulty in reproducing the results, as well as for methodological weaknesses (a sort of inspection of Benveniste's laboratory organized by Nature) (3,13,14).
The group subsequently repeated tests using more reliable methods and more complete evaluations made by expert statisticians, and confirmed many times the existence of an effect at high dilutions, although it was not so marked as that described in the first paper (15–18). These further experiments are not known by the general public, and possibly because they were published in less renowned journals or in conference proceedings they have not been welcomed by the scientific community.
A Dutch group reported its inability to reproduce the effect of high IgE dilutions (19). This study failed to demonstrate any action of high anti-IgE antibody dilutions on mast cells, and the authors (one of whom had learned the technique in Benveniste's laboratory) concluded that it was a difficult to reproduce model. Similar negative results have also been reported by another group (20). However, according to Benveniste, these studies apparently refuting his results were marred by a number of methodological and statistical errors (21).
As pointed out by Vecchi (22), in data of Table 2 from Hirst et al. (20), the probability that the successive high dilution behaves as the control solution is 0.0027 (Fischer P-value). This represents the probability of obtaining such experimental data by chance, under the assumption that there will be no difference between successive high dilutions and control treatments. In other words, the experimental data confirm within a 99.7% level of confidence that there is a difference between successive high dilutions and control treatments. The authors appear to recognize that their data are incompatible with the null hypothesis, i.e. with the assumption that there is no difference between potentized solutions and placebo (p. 527, right column): ‘According to conventional scientific theory, there should be no differences within a session between the control treatment and the eight high-dilution treatments. … This is not the case …’, but they attribute the effect to unknown causes. It is difficult to determine the difference between the ‘unknown variation source’ in Hirst et al. and the perplexing intermittency that appears in Benveniste's original paper and that was construed there in terms of ‘dilution waves’. What appears to be happening is largely consistent with the findings of Davenas et al. (2): successive anti-IgE strongly enhances the variation in basophil counts, while affecting the mean counts only moderately. The high-dilution story of basophils is therefore still open at the experimental level, as is demonstrated by other authors working on this type of research (23,24) and by other results reported below.
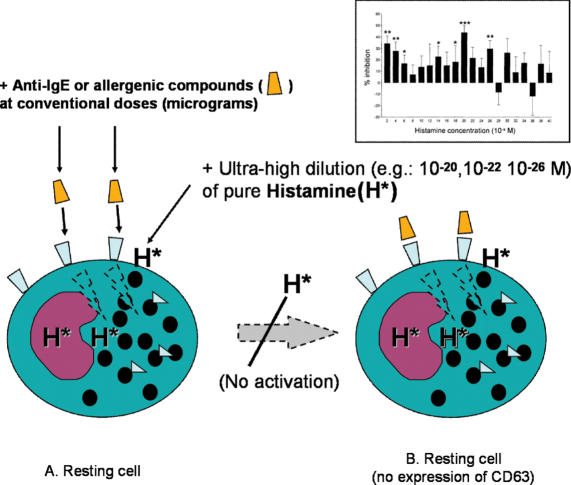
Inhibition by Pure Histamine
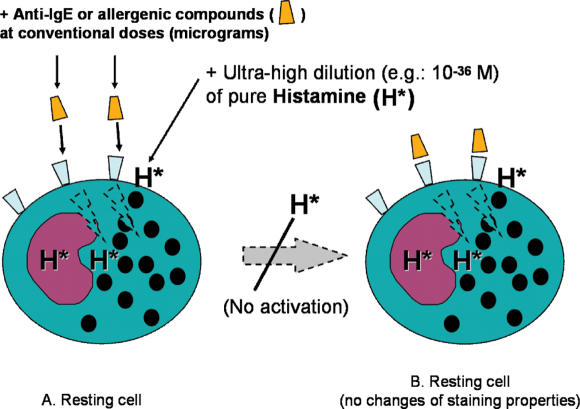
The group of Belon/Sainte-Laudy obtained the inhibition of basophil ‘degranulation’ (according to metachromasia) by using high dilutions of pure histamine (Fig. 4). If the initial concentration is known, the use of pure substances as the starting material makes it possible to determine the theoretical molar concentrations in the subsequent dilutions, which, in this case, are expressed as negative powers of 10. A first series of experiments led to two inhibitory peaks at dilutions with theoretical histamine concentrations of between 10−10 and 10−17 M and between 10−30 and 10−38 M (25). All of the experiments were carried out under blind conditions in the sense that the researcher did not know with which dilution s/he was working. A control experiment showed that dilutions of histidine (the carboxylated precursor of histamine) were inefficacious, thus confirming specificity of the molecular information and reducing the likelihood of laboratory artifacts.
Figure 4.
The effects of ultra-high dilutions of pure histamine on basophil activation, assessed by optical evaluation of granule staining (25–27).
The same group has reported further data confirming that high dilutions of histamine (pure histamine chloride) significantly inhibit the degranulation of basophils (sensitized by IgE antibodies against dermatophagoid) induced in vitro by dermatophagoid extracts (26). Using a series of 16 progressive one-hundredth dilutions (from 5c to 20c), the authors observed inhibitory activity at dilutions of about 7c and 18c. The addition of pharmacological doses of cimetidine (an antagonist of histamine H2 receptors) abolished effects of all of active dilutions. Adding of the histaminase enzyme (which destroys histamine) inhibited the effect of ponderal doses (6c and 7c) but not that of high dilutions (18c), thus indicating that the latter was not due to the histamine molecule but to other mechanisms. The authors therefore tend to believe that the action of high dilutions involves an effect of the solvent (water) on H2 receptors, although they do admit that it is paradoxical to think in terms of molecular biology when there are theoretically no molecules of the effector in some of the active dilutions tested. A further paper confirmed that the IgE activation of human basophils is greatly and significantly (P < 0.001) inhibited by histamine dilutions (27). In these experiments, the ‘degranulation’ of basophils was inhibited at theoretical histamine concentrations of 10−16, 10−18, 10−20, 10−22 and 10−36 M.
New Techniques: Role of CD Markers
As the results obtained with the ‘degranulation’ test have never been replicated using the histamine release test in vitro (probably because of the different sensitivity of the two methods in relation to the different stages of basophil activation), it was important to replicate them by means of the different test of flow cytometry (28). Basophil membranes express many proteins: adhesion molecules and high-affinity receptors for IgE, aggregated IgG, CD26, CD33, CD40, CD45 and CD63. The last one is particularly interesting because it is expressed on cytoplasmic granules and on the external membrane after activation, and can thus be used as a marker of the functional status of the cell and also for ex vivo allergological diagnoses (29). Flow cytometry has the further advantage of being objective because it does not rely on judgment of the observer, whereas the degranulation test requires a visual evaluation as to whether the cells are light or dark after staining.
This technique has been used to demonstrate that basophil activation by anti-IgE antibodies (Fig. 5), observed as an increased expression of CD63, is blocked by ‘conventional’ histamine doses of between 10−2 and 10−4 M, as well as by (theoretical) ultra-low doses of 10−22 and 10−34 M (30). The same authors have also demonstrated that the inhibition induced by very high histamine dilutions (theoretical dose of 10−30 to 10−34 M) is abolished by cimetidine, thus confirming the receptor specificity already reported by their previous data (31).
Figure 5.
The effects of ultra-high dilutions of pure histamine on basophil activation, assessed by flow-cytometric measurement of CD63 expression (24,30–34). Data reported in the insert are from Brown and Ennis (32).
Is the Phenomenon Reproducible?
Furthermore, similar results have been obtained by three of the four laboratories involved in a multicenter collaboration (24) and independently reproduced (32–34). Only one group was not able to confirm the previously reported effects of high histamine dilutions on basophil function (35). In seven independent experiments, basophils of the same human donor were incubated with diluted histamine (up to 10−34 M) or water controls and activated with anti-IgE antibodies. Basophil activation was determined by using bi-color flow cytometry. Experiments were blinded and performed with a randomized arrangement of solutions on microtiter plates. Histamine at the dilutions 10−2 M and 10−22 M was associated with a significant inhibition of basophil degranulation (P = 0.018) of 23.1 and 5.7%, respectively, if compared with control (‘diluted’ water treated in an identical manner). However, if all controls were pooled, only histamine 10−2 M had a significant effect. The authors suggest that minor variables of the experimental set up, such as the position of samples in different rows of the same laboratory microplate, can lead to significant differences of results if not properly controlled.
In conclusion, this experimental model, using the highly sensitive basophil, has been very fruitful, and can be considered particularly consolidated and credible. These data are summarized in Table 1. However, the research in this field has been till now unable to give a clear-cut technical explanation of the observed discrepancies between different laboratories. Hopefully, in the future, use of rigorously controlled experimental conditions and multicenter cooperations will help to identify and to minimize confounding factors.
Table 1.
Summary of laboratory studies on basophils/mast cells
| ✓ High dilutions (up to 10−120) of Anti-IgE antibodies stimulate basophil degranulation (2). This result was not confirmed by two independent groups (19,20). |
| ✓ Homeopathic dilutions of Apis Mellifica and Lung Histamine inhibit basophil degranulation (8,11,12). |
| ✓ High dilutions of Histamine (10−20 to 10−38 M) influence the activation of human basophils measured by alcian blue staining (25–27). A multicenter study confirmed this finding in different laboratories, using several different techniques (24,30,32). |
| ✓ Inhibition of basophil activation by high dilutions of histamine is reversed by anti-H2 and is not observed with histidine, these results being in favor of the specificity of this effect (31). |
| ✓ An independent team confirmed the inhibitory effect of high dilutions of histamine (10−22 to 10−25M histamine) (33,34). |
| ✓ One report (35) failed to replicate these findings: histamine consistently inhibited basophil degranulation only when used in low dilutions. |
Lymphocytes
Studies dating back several years have tested the in vitro effects of medium dilutions of plants used in homeopathic medicines on lymphocytes. Two particularly interesting studies report the effect of Phytolacca on lymphocyte proliferation, measured by means of 3H-thymidine incorporation (36,37). Phytolacca contains a glycoprotein (the mitogenic Pokeweed) that is known to induce lymphoblastic transformation in cultured B-lymphocytes. Phytolacca has also been empirically used in homeopathy for a long time (since before its in vitro immunological action was discovered) in order to treat numerous diseases involving lymphoadenopathies, such as infectious mononucleosis and otorhinolaryngoiatric viral disease (38). Phytolacca dilutions of 5c, 7c and 15c have no mitogenic effect on resting lymphocytes but on lymphocytes stimulated with ponderal doses of phytohemagglutinin they inhibit mitosis by 28−73%: one study using rabbit lymphocytes found that the 15c dilution had the greatest effect (36); another study using human lymphocytes found that the maximum effect was achieved by the 7c dilution (37). Once again, these experiments underline the concepts of biological tropism (according to which an ultra-diluted solution acts on the same target system as the undiluted substance) and ‘similarity’ as effect inversion (according to which the diluted solution inhibits the effects of original or a similar substance). Unfortunately, this line of research has not been expanded by further experimental studies.
In vitro studies may also help to characterize homeopathic medicines for their active principles, which are undoubtedly present in crude extracts (mother tinctures) and low dilutions. Arbor vitae (Thuja occidentalis L.) is a native European tree widely used in homeopathy and phytotherapy. A recent review has presented the phytochemistry properties of its essential oil (Thujone), its antiviral action and immunopharmacological potential, such as stimulatory and co-stimulatory effects on cytokines, antibody production, and activation of immunocompetent cells and macrophages. The in vitro effects have been observed using ponderal doses (low dilutions) of the plant extract and of its active principles (39).
Hormesis and Sensitization
Wagner's group (40) has tackled experimentally the problem of the effect at cell level (i.e. on leukocytes) of low doses of vegetable extracts used in homeopathy and, in addition, of unusual changes in effect observed in dose–response curves. Among the various studies made by this group, of particular interest are those reporting that relatively high concentrations (100 µg−10 ng/ml) of the naphthoquinones (plumbagin, alkannin and others) and cytostatic agents (vincristine, methotrexate and fluorouracil) inhibit lymphoblastic transformation and granulocyte phagocytosis, whereas low concentrations (10 pg−10 fg/ml) have a stimulatory effect: intermediate doses are ineffective. The authors suggested that a number of the antitumoral effects of vegetable extracts might be explained by this dose-related double-effect mechanism. This is an immunological example of U-shaped dose–response curves that have been described earlier in toxicology as the ‘Arndt-Schultz law’ (41,42) and then theoretically developed as the ‘hormesis’ effect (43–46). This phenomenon could be a possible mechanism underlying the inversion of drug effects, or paradoxical effects of drugs, that recalls the traditional ‘similarity’ principle of homeopathy. However, hormesis can explain only a few aspects of homeopathy (47–49).
A study of the action of successive substances on human lymphocytes stimulated with phytohemagglutinin (PHA) was conducted by a team in Bucharest (50). Human peripheral blood lymphocytes from healthy controls, immunodepressed patients presenting with chronic bacterial infections or neoplasias and allergic patients were stimulated in vitro with PHA in a culture medium supplemented or not with high dilutions (10−7, 10−15 or 10−30) of bee venom or phosphorus in tridistilled water. The most significant inhibition due to DNA incorporation was noted in lymphocytes from allergic patients cultivated in media supplemented with 10−30 dilutions in the presence of PHA. The cells from immunodepressed patients showed no significant inhibition at 10−30 dilution. According to the authors, these data suggest a possible effect of high dilutions on structures of cell membranes from sensitized subjects. In this case, the sensitization due to pathology would make the individual susceptible to the homeopathic dilution.
In vitro Testing of Complex Formulations
A complex homeopathic medicine (Engystol-N, composed of Vincetoxicum at dilutions 6× to 30× and of Sulfur at dilutions 4× to 10×) stimulates cytokine(s) production by T lymphocytes in whole blood (51). Culture media of T lymphocytes treated with 10−4 and 10−8 dilutions of Engystol-N show an inhibiting effect on superoxide anion generation by neutrophils. From these data it was concluded that the drug stimulates secretion of lymphokine(s) with an inhibiting action on superoxide anion generation by neutrophils that prevail over the direct stimulating effect, confirming and extending the suggested immunomodulatory ability of the drug.
A homeopathic complex medicine (‘Canova’) is used as an immune modulator (52). The formula is composed of 19× Thuya occidentalis, 18× Bryonia alba, 11× Aconitum napellus, 19× Arsenicum album and 18× Lachesis muta (Viperidae) venom, all extracted and diluted in 70% alcohol, in equal parts. Studies of its mechanism of action have shown that it stimulates the immune system by activating macrophages, but has no genotoxic properties in vitro on lymphocytes (53).
Traumeel S, a homeopathic formulation containing low potencies (4× to 12×) of Arnica montana and other plant extracts and minerals (Calendula officinalis, Hamamelis virginiana, Achillea millefolium, Atropa belladonna, Aconitum napellum, Hepar sulfuris, Symphytum, Mercurius solubilis, Bellis perennis, Chamomilla, Echinacea angustifolia, Echinacea purpurea, Hypericum) is widely used in humans to relieve trauma, inflammation and degenerative processes. However, little is known about its possible effects on the behavior of immune cells. The effects of Traumeel were examined in vitro on human T cells, monocytes and gut epithelial cells (54). Traumeel inhibited the secretion of IL-1beta, TNF-alpha and IL-8 secretion by 50−70% in both resting and activated cells (P < 0.01 for all cells). Interestingly, the effect appeared to be inversely dose-related: maximal inhibition was seen with dilutions of 10−3 to 10−6 of the medicine stock material. This finding suggests that Traumeel does not inhibit immune cell functions by exerting a toxic effect. Although additional studies are needed to clarify the mode of action of this homeopathic formulation (see also the studies on neutrophil granulocytes reported below), the in vitro results may offer a mechanism for its anti-inflammatory effects.
The main results on lymphocytes are summarized in Table 2.
Table 2.
Summary of laboratory studies on lymphocytes
| ✓ Phytolacca (5c, 7c and 15c) have no mitogenic effect on resting lymphocytes but on lymphocytes stimulated with ponderal doses of phytohemagglutinin they inhibit mitosis (36,37). |
| ✓ Naphthoquinones (plumbagin, alkannin and others) and cytostatic agents (vincristine, methotrexate and fluorouracil) inhibit lymphoblastic transformation at relatively low dilutions, whereas higher dilutions have a stimulatory effect (38). |
| ✓ High dilutions of Bee venom (Apis) or Phosphorus inhibit blood lymphocytes (stimulated in vitro with PHA) from healthy subjects while the cells from immunodepressed patients do not show any significant inhibition (40). |
| ✓ A homeopatic complex formulation containing Vincetoxicum 6× to 30× and Sulfur 4× to 10× stimulate cytokine production by human lymphocytes (51). |
| ✓ The homeopathic immunostimulant complex Canova has no genetoxic properties on human lymphocytes (53). |
| ✓ Traumeel S inhibits IL-beta, TNF-alpha, and IL-8 production by human T cells, monocytes and gut epithelial cells (54). |
Polymorphonuclear Granulocytes (Neutrophils)
Among studies of possible regulatory effects of homeopathic dilutions on inflammation, mention must be made of those involving phagocytic cells and in particular polymorphonuclear leukocytes (neutrophil granulocytes). In this case, the tested substances were those used by homeopaths in situations of acute inflammation with a major polymorphonucleat component.
It has been reported (55) that 5c and 9c dilutions of Belladonna and Ferrum phosphoricum inhibit the production of free oxygen radicals (measured as chemiluminescence, i.e. release of light generated during metabolic reactions) by granulocytes stimulated by opsonized zymosan. The inhibition was highly significant and as much as 30–40%, approximately the same as that obtained using 10 µM of dexamethasone and 0.1 mM of indomethacin. The authors pointed out that there was a considerable difference in individual sensitivity to these drugs, and this problem of the different sensitivity of cells isolated from different subjects has also been highlighted by others (56) who have investigated the effect of Belladonna, Hepar sulfur, Pyrogenium, Silicea and Staphylococcinum on chemotaxis and obtained conflicting results.
It has also been reported (in a preliminary communication) that Bryonia 4c and 9c (family: Cucurbitaceae) had a stimulatory effect on oxidative metabolism of polymorphonuclear leukocytes, which may be both direct and indirect (increasing the response to chemotactic peptides) (57).
Our Investigations of Homeopathic Medicines In Vitro
Our own group has also investigated the effects of homeopathic medicines on inflammatory cells and has obtained significant results particularly in relation to low dilutions (58–63). We first explored the possible direct effect of homeopathic dilutions on cell systems by evaluating their in vitro effects on oxidative metabolism of cultured neutrophils activated by formylated peptides (60). Results of the first series of studies, based on analysis of multiple dilutions of a large series of compounds, can be summarized as follows: (i) Manganum phosphoricum 6× and 8×, Magnesium phosphoricum 6× and 8×, Sulphur 6×, Acidum citricum 3× and Acidum succinicum 3× and 4× have highly reproducible inhibitory effects on our in vitro assay system; ii) Acidum fumaricum and Acidum malicum (both at a dilution of 4×) have a slightly potentiating effect on oxidative metabolism; (iii) during the course of various experiments, Phosphorus and Magnesium phosphoricum often showed inhibitory effects even at very high dilutions (>15×), but these did not always appear at the same dilutions, thus making it difficult to analyze them statistically; however, by pooling all of the data concerning the effects of high phosphorus dilutions, it has been possible to identify a small (10–15%) but statistically significant inhibition of cell activation.
These results can be biochemically interpreted in many ways. First of all, they demonstrate that, at medium–high doses, the solutions have certain effects on blood cells. Furthermore, they seem to suggest that most of the tested remedies interfere with subtle cell most regulatory mechanisms known to be based on ion exchanges, phosphorylation processes and reduced oxidation. In normal cell most physiology, phosphorus, sulphur, magnesium, manganese, calcium and other elements play a major role in such mechanisms, and so it is particularly interesting that some homeopathic medicines can act at these levels of control.
We then carried out studies aimed at improving knowledge of the anti-inflammatory action of Traumeel S (61,62). Results showed a decrease in paw edema associated with the process of healing, which was more rapid in the treated rats (P < 0.05 after three and P < 0.01 after 5 h) than in controls treated with saline (0.9% NaCl) solvent. The effects of Traumeel S on two important cellular functions, namely superoxide anion production and human platelet adhesion, were tested. This medicine did not affect either of these cellular functions, suggesting that its anti-inflammatory effects are not due to granulocyte and platelet inhibition. These findings also suggest that the antimicrobial functions of immune cells such as granulocytes are not disrupted by Traumeel S.
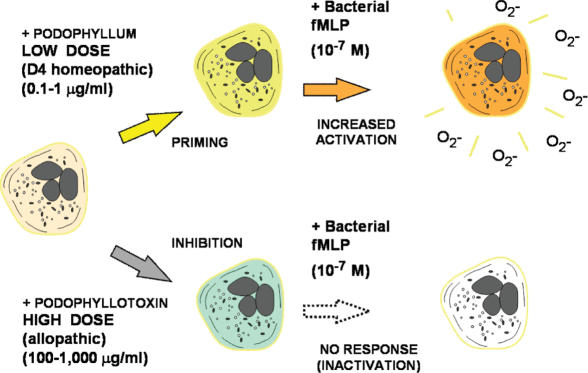
Human blood neutrophilic granulocytes (neutrophils) treated with low dilutions (4×) of a homeopathic drug extract (Podophyllum peltatum) had specific stimulating effects on their metabolic responses: an enhanced oxidative response to a subsequent challenge with bacterial formyl-peptides (63) (Fig. 6). This priming effect was related to superoxide anion (O2 −) release (respiratory burst) and was quantitatively similar to the priming of the effect of TNF-α. The phenomenon was observed with a homoeopathic preparation containing, among other things, podophyllum extract (Podophyllum compositum) and a 4× homeopathic dilution of Podophyllum (the final concentration of the active principle was about 0.025 µg/ml), whereas no enhancement of O2 − release was caused by homoeopathic Podophyllum 12× or the other components contained in the complex homoeopathic preparation. Purified podophyllotoxin caused the same effect at doses of 0.1–10 µg/ml, whereas doses of more than 100 µg/ml inhibited the respiratory burst so that pure toxin showed a typical bi-phasic dose–response curve.
Figure 6.
Dual effects of Podophyllum and podophyllotoxin on the human neutrophil metabolism according to the dose (63). For explanation see text.
Similar effects were obtained with purified colchicine (1−1000 µg/ml), a microtubule-disrupting agent. No priming by any Podophyllum-derived compound was observed on neutrophils stimulated with 50 ng/ml phorbol ester (PMA). Furthermore, both homeopathic podophyllum-derived compounds and pure podophyllotoxin inhibited cell adhesion to the serum-coated surface of culture microplates. These results show that low dilutions of a homeopathic drug extract have stimulant-specific effects on the activation of neutrophil metabolism. Interest in this drug also comes from the fact that much higher doses of podophyllotoxin are used by conventional pharmacology to inhibit cell proliferation and these doses appear to be efficacious against condilomata of the skin. Priming doses are those contained in the homeopathic preparation; toxic doses are those contained in the allopathic preparation.
Electromagnetic Regulation
A paper dealing with regulation of neutrophil metabolism, published by the group of Benveniste (64), contains apparently ‘incredible’ data that, if confirmed, may have relevance for the interpretation of homeopathic phenomena. The authors claimed to have electronically transferred the information of a potent leukocyte activator (PMA: 4-phorbol-12-beta-myristate-13-acetate, the active ingredient of croton oil) to a cell suspension contained in a test tube placed in contact with a solenoid crossed by an electrical current. In brief, a suspension of neutrophils was positioned at a temperature of 37°C inside a solenoid connected to an oscillator that was also connected to another solenoid containing a solution of PMA. The oscillator was operated for 15 min, after which neutrophil activation was recorded by measuring the release of radical species for a further 45 min. Cell activation occurred only when the oscillator was operating and not when it was switched off. As a control, no cell activation occurred when, instead of PMA, the other solenoid contained its inactive analogue 4-alpha-phorbol 12,13-didecanoate. The authors interpreted this by hypothesizing that PMA emits a specific signal that can be electronically transmitted to neutrophils without any chemical contact. It is clear that if these results are confirmed they would strongly support the existence of ‘meta-molecular’ modulations in living systems and open up an extremely fascinating field of research.
In studies not related to homeopathy, it has been shown that the adenosine receptors of neutrophils (65), and a number of biochemical functions (66) are sensitive to externally applied periodically pulsed weak magnetic fields, in the case of neutrophil metabolism when the pulses are matched in frequency to the metabolic oscillations. These observations and the subtle entanglement among different systems such as homeopathy and acupuncture (67) suggest that high dilutions of biologically active substances may affect, also through biophysical pathways, the signaling and transcriptional levels of cellular homeostasis.
The main results on granulocytes are summarized in Table 3.
Table 3.
Summary of laboratory studies on granulocytes
| ✓ Belladonna, Hepar Sulphur, Pyrogenium, Silicea stimulate or inhibit the leukocyte chemotaxis, with variable effects in different individuals (55). |
| ✓ Belladonna and Ferrum Phosphoricum 5c and 9c (not Apis 9c) inhibit zymosan-stimulated metabolism, with individual sensitivity (55). |
| ✓ Bryonia 4c and 9c stimulate the oxidative metabolism of granulocytes (57). |
| ✓ Manganum phosphoricum (6× to 8×), Magnesium phosphoricum, (6× to 8×) and Phosphorus (30× to 200×) inhibit the oxidative metabolism of granulocytes (60). The high-dilution effects of Phosphorus are not evident in all experiments. |
| ✓ No effects of Traumeel S on the oxidative metabolism of human granulocytes (61). |
| ✓ Podophyllum (4×) and low dilutions of podophyllotoxin prime the oxidative metabolism of granulocytes; higher doses of the same toxin exert an inhibitory effect (63). |
| ✓ Phorbol myristate acetate, a known activator of granulocytes, seems to act on these cells also through ‘electronic transmission’ of the signal (64). |
Fibroblasts, Osteoblasts and Other Enzyme Studies
Some in vitro tests have used a model of cytotoxicity in order to investigate whether homeopathic dilutions of toxic substances (mainly those having cytotoxic properties when used at high doses) may have protective effects on cell cultures of connective tissue cells involved in reparation processes. Boiron's group (68) reported that minimal doses (5c) of mercuric chloride (HgCl2) protect fibroblast cultures from intoxication by high doses of mercury. The parameter studied was the mitotic index. This model is obviously based on the hypothesis of the inverse or paradoxical effects of dose variations. Others (69) have observed a cytotoxic effect of HgCl2 on cultured mouse lymphocytes at doses ranging from 10−5 to 10−7 M, whereas a growth inhibiting effect without cytotoxicity was observed at doses ranging from 10−16 to 10−17 M. However, this effect was not found by another group studying the action of dilutions ranging from 10−10 to 10−18 M using the same model (70).
More recently, it has been reported that osteogenesis in vitro in rat tibia-derived osteoblasts is promoted by a homeopathic preparation (FMS-Calciumfluor) containing ultra-low doses of calcium fluoride associated with calcium monophosphate (71,72). Alkaline phosphatase, an indicator of osteoblast maturation, and the incorporation of radiolabelled Ca into the matrix were increased as compared with untreated control cultures. The effects of the homeopathic drug were concentration dependent and specific for its modalities of preparation and were observed at a concentration about three orders of magnitude lower than similar effects reported in the literature by treatment of osteoblast cultures in vitro with NaF.
In order to elucidate potential action mechanisms of an anti-inflammatory homeopathic complex (Zeel comp. N) and of its constituents, inhibition of synthesis of leukotriene B4 and prostaglandin by 5-lipoxygenase (5-LOX) and cyclo-oxygenase 1 and 2 (COX 1 and 2), respectively, were examined in vitro (73). A reconstituted Zeel comp. N combination as well as its constituent mother tinctures of Arnica montana, Sanguinaria canadensis and Rhus toxicodendron (Toxicodendron quercifolium) showed distinct inhibitory effects on the 5-LOX and on the COX 1 and COX 2 enzymes. The effects were observed using low doses of these compounds, in the range of micrograms of original mother tincture.
Discussion
Laboratory studies of cells and especially of leukocytes represent a fertile field in which homeopathic and conventional researchers have worked together. The well-established methods used in modern immunology and cell biology have been adopted for testing the in vitro effects of commercially available homeopathic drugs or of active principles diluted according to the traditional homeopathic methods. Moreover, the similar principle has been exploited also in reductionistic models based on different responses of inflammatory cells on changing the experimental conditions.
The data are still scanty and consider several different experimental models. So, we could not apply quantitative methods of evaluation that have been developed by modern epidemiology for conventional drugs. Since the whole field has been undergoing difficulties related to its lack of acceptance by the academy, as shown in the case of Benveniste (18,74), the quality of data is still too preliminary, rendering statistical analysis of papers impossible.
As in clinical research, it is highly conceivable that a publication bias in favor of papers reporting positive findings do exist also in basic research and the existence of this bias should be taken into account to formulate a global judgment about the field. Due to lack of consensus or criteria of quality in this type of (admittedly quite preliminary) research, we found it practically impossible to assign a score of quality to each referenced paper. As a matter of fact, the studies here described mirror the relative paucity of scientific homeopathic production concerning action on cells of the immune system.
The Uncertain ‘State-of-the-Art’
The most consistent model in the field of high dilutions is that of basophil regulation by anti-IgE, histamine and homeopathic dilutions (75). However, also in this case there is no international agreement on the validity of the model as a demonstration of the ‘high-dilution effect’ or ‘dynamization phenomenon’. The experiments have not been published in high-impact journals and have found difficulties in replication even in the same multicenter trials (24), and, these findings are not known to the majority of medical scientists. Lack of reproducibility in different laboratories, small differences with the placebo and, possibly, prejudices have limited acceptance of these apparently paradoxical results, which would otherwise make a breakthrough in the field.
Notwithstanding these experimental advancements, the data in favor of the high-dilution effect in immunological models are not so consistent and reproducible as it should be for a general acceptance by the scientific community. The present state of physico-chemical knowledge does not allow definite conclusions in favor or against the existence of specific physical states of highly diluted homeopathic remedy. Skeptics are not convinced by the available evidence. On the other hand, assuming that the phenomena described in many ‘high-dilution’ experiments are not laboratory artifacts and that they do really exist, their difficult reproducibility could be conceivably attributed to the sensitive methods used, which are unavoidably affected by minimal technical differences and conditions, including skill of the operator, type of blood donors, season and day of the experiment, perhaps atmospheric pressure, the electromagnetic ‘pollution’ of the laboratory, trace contaminants of the water solutions used to make dilutions, stirring or succussing procedure, time left between a dilution and subsequent one, the material of which test tubes are made and similar factors. More extensive and systematic research would be necessary in order to discriminate between these possible sources of laboratory artifacts or of discrepancy between laboratories.
Methodological Issues
The mast cell/basophil degranulation studies should be focused on more actively by other laboratories, because this appears to be the most sensitive model, and if many other replication studies are carried out we could more easily discover possible artifacts and/or mechanisms of action. At this stage of research, pooling and meta-analysis could be performed only if the database in scarcely investigated areas (e.g. research in lymphocyte biology, neutrophil metabolism) is increased and implemented with more consistent results.
A specific methodological issue that should be better investigated considers methods of dilution and of succussion (dynamization). These methods are well known and are described in the homeopathic pharmacopoeias, but in the scientific literature high-dilution effects of non-homeopathic substances often are not reported with suitable detail to allow easy replication.
The discussion regarding reproducibility in homeopathic research is a hot topic also in clinical studies, where elusive phenomena, such as a ‘weak quantum entanglement’, possibly occurring in the triad composed by doctor–drug–patient, have been suggested (76–78): the remedy would act in the context of a tripartite relationship with the patient and the practitioner. What may be the physical basis of such an entanglement (correlation by quantum entanglement is known and accepted only in submicroscopic world) is still a matter of speculation. In any case, assuming that a type of correlation between the tester intentionality and the drug effect could also take place in the laboratory system, this would affect the experimental reproducibility by introducing a further and difficult to control variable. We may expect a contribution of scientific complexity that can give conceptual tools and methods of analysis of these kind of interactions (79). Because till now efforts seem to have concentrated more on homeopathic medicine as an entity in itself, others also considered the effect of the medicine on the body or on its subcomponents.
The Sense of Subtle Regulations
The fact that the laboratory-revealed actions are often small should not be viewed as reducing the significance of findings because what counts in homeopathy is that a remedy is capable of ‘regulating’ or ‘triggering’ the body's response when it is on a far-from equilibrium state, and not that it acts as directly or dramatically as a chemical enzyme inhibitor. When the body is particularly sensitive, characterized by a high degree of instability and in the proximity of ‘bifurcation points’ of its evolution, even a small influence could orient the entire homeodynamic system involved in the disease and thus become a determinant factor in the final result of the reaction. Complex systems typically include subsystems that amplify the small perturbations and chaotic dynamics known to require fine and repeated impulses rather than drastic changes. Such considerations are particularly true in the case of inflammation and the immune system, in which the same mechanisms can be used for defensive or offensive purposes (i.e. cure or self-destruction) depending on their site and the timing and entity of the reaction. This ‘double-faced’ nature of the phenomena makes them susceptible to fine regulation.
Limitations and Prospects
It is worth admitting that one considerable limitation of in vitro studies is their highly sectorial nature insofar as they investigate a local phenomenon in standardized conditions, rather than a complex systemic manifestation such as disease. A number of in vitro effects of homeopathic medicines provided examples of U-shaped dose–response curves, which may be seen as special applications of the principle of similia at the biological and pathophysiological level, but it is important to say that this kind of inverse effect is not ‘the’ explanation of homeopathic effects, which may have further and more complex implications on the level of the organism as a whole. The demonstration that a given medicine stimulates or inhibits activity of a blood cell is interesting in itself, but it does not allow extrapolation (in homeopathy or allopathy) of an action of the same medicine in humans.
In conclusion, the importance of laboratory studies lies in the fact that they have made it possible to obtain some preliminary evidence of the effects of high dilutions/dynamisations under conditions that exclude any possible effect of suggestion. Furthermore, the data reviewed may give indications of some biochemical and molecular targets of homeopathic agents. There are many other reasons that make this type of research worth pursuing. In the future, if reliable and reproducible models for analyzing the effects of homeopathic dilutions are established, it will be possible to deepen our knowledge of the biological and physical bases of the phenomenon, evaluate the drug stability over time, identify any causes of decay, and standardize preparations by comparing activity of different sources and dilutions of raw materials.
Acknowledgments
This study was carried out using funds provided by the Italian Ministry of University Scientific and Technological Research (MURST 60%).
References
- 1.Bellavite P, Conforti A, Piasere V, Ortolani R. Immunology and homeopathy. 1. Historical background. Evid Based Complement Alternat Med. 2005;2:441–52. doi: 10.1093/ecam/neh141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davenas E, Beauvais F, Amara J, Robinson M, Miadonna A, Tedeschi A, et al. Human basophil degranulation triggered by very dilute antiserum against IgE. Nature. 1988;333:816–8. doi: 10.1038/333816a0. [DOI] [PubMed] [Google Scholar]
- 3.Maddox J, Randi J, Stewart WW. ‘High-dilution’ experiments a delusion. Nature. 1988;334:287–90. doi: 10.1038/334287a0. [DOI] [PubMed] [Google Scholar]
- 4.Benveniste J. The human basophil degranulation test as an in vitro method for the diagnosis of allergies. Clin Allergy. 1981;11:1–11. doi: 10.1111/j.1365-2222.1981.tb01559.x. [DOI] [PubMed] [Google Scholar]
- 5.Beauvais F, Bidet B, Descours B, Hieblot C, Burtin C, Benveniste J. Regulation of human basophil activation. I. Dissociation of cationic dye binding from histamine release in activated human basophils. J Allergy Clin Immunol. 1991;87:1020–8. doi: 10.1016/0091-6749(91)90426-o. [DOI] [PubMed] [Google Scholar]
- 6.Knol E, Mulf F, Kujipers T, Verhoeven A, Roos D. Intracellular events in anti-IgE activated non releasing human basophil. J Allergy Clin Immunol. 1992;90:92–103. doi: 10.1016/s0091-6749(06)80015-1. [DOI] [PubMed] [Google Scholar]
- 7.Boucinhas JC, DeMadeiros, Boucinhas ID. Prophylaxie des crises d'asthme bronchique chez l'enfant par l'usage de Poumon histamine 5 CH. Homéopathie Franc. 1990;78:35–39. [Google Scholar]
- 8.Poitevin B, Aubin M, Benveniste J. Effect d'Apis Mellifica sur la degranulation des basophiles humains in vitro. Homéopathie Franc. 1985;73:193–8. [Google Scholar]
- 9.Poitevin B, Aubin M, Benveniste J. Approche d'une analyse quantitative de l'effet d'apis mellifica sur la degranulation des basophiles humains in vitro. Innov Tech Biol Med. 1986;7:64–8. [Google Scholar]
- 10.Murietta M, Leynadier F, Dry J. Dégranulation des basophiles et substances ‘dites homéopathiques’. Bull Acad Natl Med. 1985;169:611–22. [PubMed] [Google Scholar]
- 11.Poitevin B. Experimental study of homoeopathy in allergology. Biological studies. Br Homeopath J. 1998;87:154–64. [Google Scholar]
- 12.Poitevin B, Davenas E, Benveniste J. In vitro immunological degranulation of human basophils is modulated by lung histamine and Apis mellifica. Br J Clin Pharmacol. 1988;25:439–44. doi: 10.1111/j.1365-2125.1988.tb03327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Picart CJ. Scientific controversy as farce: the Benveniste-Maddox counter trials. Soc Stud Sci. 1994;24:7–37. doi: 10.1177/030631279402400102. [DOI] [PubMed] [Google Scholar]
- 14.Pool R. Unbelievable results spark a controversy. Science. 1988;241:407. doi: 10.1126/science.3393908. [DOI] [PubMed] [Google Scholar]
- 15.Benveniste J, Davenas E, Ducot B, Spira A. Basophil achromasia by dilute ligand: a reappraisal. FASEB J. 1991;5:A3706. [Google Scholar]
- 16.Benveniste J, Davenas E, Ducot B, Cornillet B, Poitevin B, Spira A. L'agitation de solutions hautement diluées n'induit pas d'activitébiologique spécifique. C R Acad Sci Paris. 1991;312:461–6. [Google Scholar]
- 17.Benveniste J. Further biological effects induced by ultra high dilutions. Inhibition by a magnetic field. In: Endler PC, Schulte J, editors. Ultra High Dilution. Dordrecht: Kluwer Acad. Publ.; 1994. pp. 35–8. [Google Scholar]
- 18.Schiff M. The Memory of Water. Homeopathy and the Battle of Ideas in the New Science. London: Thorsons; 1995. [Google Scholar]
- 19.Ovelgonne JH, Bol AWJM, Hop WCJ, Van Wijk R. Mechanical agitation of very dilute antiserum against IgE has no effect on basophil staining properties. Experientia. 1992;48:504–8. doi: 10.1007/BF01928175. [DOI] [PubMed] [Google Scholar]
- 20.Hirst SJ, Hayes NA, Burridge J, Pearce FL, Foreman JC. Human basophil degranulation is not triggered by very dilute antiserum against human IgE. Nature. 1993;366:525–7. doi: 10.1038/366525a0. [DOI] [PubMed] [Google Scholar]
- 21.Benveniste J. Memory of water revisited (letter) Nature. 1994;370:322. doi: 10.1038/370322a0. [DOI] [PubMed] [Google Scholar]
- 22.Vecchi I. On high dilution experiments. Frontier Perspect. 1999;8:58–61. [Google Scholar]
- 23.Wiegant FAC. Memory of water revisited (letter) Nature. 1994;370:322. doi: 10.1038/370322b0. [DOI] [PubMed] [Google Scholar]
- 24.Belon P, Cumps J, Ennis M, Mannaioni PF, Sainte-Laudy J, Roberfroid M, et al. Inhibition of human basophil degranulation by successive histamine dilutions: results of a European multi-centre trial. Inflamm Res. 1999;48:S17–8. doi: 10.1007/s000110050376. [DOI] [PubMed] [Google Scholar]
- 25.Cherruault Y, Guillez A, Sainte-Laudy J, Belon P. E'tude mathematique et statistique des effets de dilutions successives de chlorhydrate d'histamine sur la réactivitédes basophiles humains. Bio-Sciences. 1989;7:63–72. [Google Scholar]
- 26.Sainte-Laudy J, Sambucy JL, Belon P. Biological activity of ultra low doses I. Effect of ultra low doses of histamine on human basophil degranulation triggered by D. pteronissinus extract. In: Doutremepuich C, editor. Ultra Low Doses. London: Taylor and Francis; 1991. pp. 127–38. [Google Scholar]
- 27.Sainte-Laudy J, Belon P. Inhibition of human basophil activation by high dilutions of histamine. Agent Actions (Inflamm Res) 1993;38:C245–7. [Google Scholar]
- 28.Gane P, Pecquet C, Lambin P, Abuaf N, Leynadier F, Rouger P. Flow cytometric evaluation of human basophils. Cytometry. 1993;14:344–8. doi: 10.1002/cyto.990140316. [DOI] [PubMed] [Google Scholar]
- 29.Sainte-Laudy J, Vallon C, Gutrin JC. Analyse de l'expression membranaire du marqueur CD63 par activation du basophil humain. Application au diagnostique allergologique. Allergie et Immunologie. 1994;26:211–4. [PubMed] [Google Scholar]
- 30.Sainte-Laudy J, Belon P. Analysis of immunosuppressive activity of serial dilutions of histamine on human basophil activation by flow cytometry. Inflamm Res. 1996;45:S33–4. doi: 10.1007/BF03354076. [DOI] [PubMed] [Google Scholar]
- 31.Sainte-Laudy J, Belon P. Application of flow cytometry to the analysis of the immunosuppressive effect of histamine dilutions on human basophil activation: effect of cimetidine. Inflamm Res. 1997;46:S27–8. [PubMed] [Google Scholar]
- 32.Brown V, Ennis M. Flow-cytometric analysis of basophil activation: inhibition by histamine at conventional and homeopathic concentrations. Inflamm Res. 2001;50:S47–8. doi: 10.1007/PL00022402. [DOI] [PubMed] [Google Scholar]
- 33.Lorenz I, Schneider EM, Stolz P, Brack A, Strube J. Influence of the diluent on the effect of highly diluted histamine on basophil activation. Homeopathy. 2003;92:11–18. doi: 10.1054/homp.2002.0082. [DOI] [PubMed] [Google Scholar]
- 34.Lorenz I, Schneider EM, Stolz P, Brack A, Strube J. Sensitive flow cytometric method to test basophil activation influenced by homeopathic histamine dilutions. Forsch Komplementarmed Klass Naturheilkd. 2003;10:316–24. doi: 10.1159/000075885. [DOI] [PubMed] [Google Scholar]
- 35.Guggisberg AG, Baumgartner SM, Tschopp CM, Heusser P. Replication study concerning the effects of homeopathic dilutions of histamine on human basophil degranu-lation in vitro. Complement Ther Med. 2005;13:91–100. doi: 10.1016/j.ctim.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Colas H, Aubin M, Picard P, Lebecq JC. Inhibition du test de transformation lymphoblastique (TTL) αla phytohemagglutinine (PHA) par phytolacca americana en diluition homeopathiques. Ann Homéopath Fr. 1975;6:1–11. [Google Scholar]
- 37.Bildet J, Dupont H, Aubin M, Baronnet S, Berjon JJ, Gomez H, Manlhiot JL. Action in vitro de dilutions infinitesimales de Phytolacca Americana sur la transformation lymphoblastique αla phytohemagglutinine. Ann Homéopath Fr. 1981;23:102–11. [Google Scholar]
- 38.Poitevin B. Relation generale entre Homéopathie et immunoallergologie. Encycl Med Chir/Homeopathie. 1988:38255A. [Google Scholar]
- 39.Naser B, Bodinet C, Tegtmeier M, Lindequist U. Thuja occidentalis (Arbor vitae): a review of its pharmaceutical, pharmacological and clinical properties. Evid Based Complement Alternat Med. 2005;2:69–78. doi: 10.1093/ecam/neh065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner H, Kreher B, Jurcic K. In vitro stimulation of human granulocytes and lymphocytes by pico-and femtogram quantities of cytostatic agents. Arzneimittelforschung. 1988;38:273–5. [PubMed] [Google Scholar]
- 41.Schulz H. Uber die Theorie der Arzneimittelwirkung. Virchow's Arch. 1877;108:423–34. [Google Scholar]
- 42.Martius F. Das Arndt-Schulz Grundgesetz. Muench Med Wschr. 1923;70:1005–6. [Google Scholar]
- 43.Calabrese EJ, McCarthy ME, Kenyon E. The occurrence of chemically induced hormesis. Health Phys. 1987;52:531–41. doi: 10.1097/00004032-198705000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Calabrese EJ. Hormesis: from marginalization to mainstream: a case for hormesis as the default dose-response model in risk assessment. Toxicol Appl Pharmacol. 2004;197:125–36. doi: 10.1016/j.taap.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 45.Rozman KK, Doull J. Scientific foundations of hormesis. Part 2. Maturation, strengths, limitations, and possible applications in toxicology, pharmacology, and epidemiology. Crit Rev Toxicol. 2003;33:451–62. doi: 10.1080/713611037. [DOI] [PubMed] [Google Scholar]
- 46.Pickrell JA, Oehme FW. Examining the risks and benefits of replacing traditional dose-response with hormesis. Hum Exp Toxicol. 2005;24:259–64. doi: 10.1191/0960327105ht521oa. [DOI] [PubMed] [Google Scholar]
- 47.Oberbaum M, Cambar J. Hormesis: dose-dependent reverse effects of low and very low doses. In: Endler PC, Schulte J, editors. Ultra High Dilution. Dordrecht: Kluwer Acad. Publ.; 1994. pp. 5–18. [Google Scholar]
- 48.Bellavite P, Lussignoli S, Semizzi M, Ortolani R, Signorini A. The similia principle. From cellular models to regulation of homeostasis. Br Homeopath J. 1997;86:73–85. [Google Scholar]
- 49.Calabrese EJ. Toxicological awakenings: the rebirth of hormesis as a central pillar of toxicology. Toxicol Appl Pharmacol. 2005;204:1–8. doi: 10.1016/j.taap.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 50.Chirila M, Hristescu S, Manda G, Neagu M, Olinescu A. The in vitro action of a succussed substance on the proliferative response of human lymphocytes stimulated with phytohemagglutinin. Rom J Intern Med. 1992;30:63–7. [PubMed] [Google Scholar]
- 51.Fimiani V, Cavallaro A, Ainis O, Bottari C. Immunomodulatory effect of the homoeopathic drug Engystol-N on some activities of isolated human leukocytes and in whole blood. Immunopharmacol Immunotoxicol. 2000;22:103–15. doi: 10.3109/08923970009016409. [DOI] [PubMed] [Google Scholar]
- 52.Sato DY, Wal R, de Oliveira CC, Cattaneo RI, Malvezzi M, Gabardo J, et al. Histopathological and immunophenotyping studies on normal and sarcoma 180-bearing mice treated with a complex homeopathic medication. Homeopathy. 2005;94:26–32. doi: 10.1016/j.homp.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Seligmann IC, Lima PD, Cardoso PC, Khayat AS, Bahia MO, Buchi Dde F, et al. The anticancer homeopathic composite ‘Canova Method’ is not genotoxic for human lymphocytes in vitro. Genet Mol Res. 2003;2:223–8. [PubMed] [Google Scholar]
- 54.Porozov S, Cahalon L, Weiser M, Branski D, Lider O, Oberbaum M. Inhibition of IL-1beta and TNF-alpha secretion from resting and activated human immunocytes by the homeopathic medication Traumeel S. Clin Dev Immunol. 2004;11:143–9. doi: 10.1080/10446670410001722203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poitevin B, Aubin M, Royer JF. Effet de belladonna et ferrum phosphoricum sur la chemiluminescence des polynucleaires neutrophiles humains. Ann Homéopath Fr. 1983;3:5–12. [Google Scholar]
- 56.Moss VA, Roberts JA, Simpson HKL. The action of ‘low potency’ homeopathic remedies on the movement of guinea pig macrophages human leukocytes. Br Homeopath J. 1982;71:48–61. [Google Scholar]
- 57.Fletcher MP, Halpern GM. Effects of dilutions of Bryonia (4-9CH) and lung-histamine (4-9CH) on human neutrophil (PMN) activation responses as assessed by flow cytometry. Proceedings of the 2nd GIRI Meeting; Monte Carlo. 1988. p. A20. [Google Scholar]
- 58.Bellavite P, Chirumbolo S, Signorini A, Bianchi I, Dri P. Simultaneous measurement of oxidative metabolism and adhesion of human neutrophils and evaluation of multiple doses of agonists and inhibitors. In: Doutremepuich C, editor. Ultra Low Doses. London: Taylor and Francis Ltd.; 1991. pp. 93–117. [Google Scholar]
- 59.Bellavite P, Chirumbolo S, Santonastaso C, Biasi D, Lussignoli S, Andrioli G. Dose-dependence of the various functional responses of neutrophils to formylpeptides. Activation, regulation, and inverse effects according to the agonist dose and cell condition. In: Bastide M, editor. Signals and Images. Dordrecht: Kluwer Acad. Publ.; 1997. pp. 111–9. [Google Scholar]
- 60.Chirumbolo S, Signorini A, Bianchi I, Lippi G, Bellavite P. Effects of homeopathic preparations of organic acids and of minerals on the oxidative metabolism of human neutrophils. A controlled trial. Br Homeopath J. 1993;82:227–44. [Google Scholar]
- 61.Lussignoli S, Bertani S, Metelmann H, Bellavite P, Conforti A. Effect of Traumeel S, a homeopathic formulation, on blood-induced inflammation in rats. Complement Ther Med. 1999;7:225–30. doi: 10.1016/s0965-2299(99)80006-5. [DOI] [PubMed] [Google Scholar]
- 62.Conforti A, Bertani S, Metelmann H, Chirumbolo S, Lussignoli S, Bellavite P. Experimental studies on the anti-inflammatory activity of a homeopathic preparation. Biol Ther. 1997;15:28–31. [Google Scholar]
- 63.Chirumbolo S, Conforti A, Lussignoli S, Metelmann H, Bellavite P. Effects of Podophyllum peltatum compounds in various preparations dilutions on human neutrophil functions in vitro. Br Homeopath J. 1997;86:16–26. [Google Scholar]
- 64.Thomas Y, Schiff M, Belkadi L, Jurgens P, Kahhak L, Benveniste J. Activation of human neutrophils by electronically transmitted phorbol-myristate acetate. Med Hypotheses. 2000;54:33–39. doi: 10.1054/mehy.1999.0891. [DOI] [PubMed] [Google Scholar]
- 65.Varani K, Gessi S, Merighi S, Iannotta V, Cattabriga E, Spisani S, et al. Effect of low frequency electromagnetic fields on A2A adenosine receptors in human neutrophils. Br J Pharmacol. 2002;136:57–66. doi: 10.1038/sj.bjp.0704695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosenspire AJ, Kindzelskii AL, Simon BJ, Petty HR. Real-time control of neutrophil metabolism by very weak ultra-low frequency pulsed magnetic fields. Biophys J. 2005;88:3334–47. doi: 10.1529/biophysj.104.056663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ventura C. CAM and cell fate targeting: molecular and energetic insights into cell growth and differentiation. Evid Based Complement Alternat Med. 2005;2:277–83. doi: 10.1093/ecam/neh100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boiron J, Abecassis J, Cotte J, Bernard AM. L'étude de l'action de diluitions hannemanniennes de chlorure mercurique sur l'index mitotique de cultures de cellules animales. Ann Homéopath Fr. 1981;23:43–9. [Google Scholar]
- 69.van Mansvelt JD, Amons E. Inquiry into the limits of biological effects of chemical compounds in tissue culture. I. Low dose effects of mercure chloride. Z Naturforsch. 1975;30:643–9. [PubMed] [Google Scholar]
- 70.Kollerstrom J. Basic scientific research into the ‘low dose effect’. Br Homeopath J. 1982;71:41–7. [Google Scholar]
- 71.Palermo C, Filanti C, Poggi S, Manduca P. Osteogenesis in vitro in rat tibia-derived osteoblasts is promoted by the homeopathic preparation, FMS-Calciumfluor. Cell Biol Int. 1999;23:31–40. doi: 10.1006/cbir.1998.0312. [DOI] [PubMed] [Google Scholar]
- 72.Manduca P, Marchisio S, Astigiano S, Zanotti S, Galmozzi F, Palermo C, et al. FMS-Calciumfluor specifically increases mRNA levels and induces signaling via MAPK 42,44 and not FAK in differentiating rat osteoblasts. Cell Biol Int. 2005;29:629–37. doi: 10.1016/j.cellbi.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 73.Jaggi R, Wurgler U, Grandjean F, Weiser M. Dual inhibition of 5-ipoxygenase/cyclooxygenase by a reconstituted homeopathic remedy;possible explanation for clinical efficacy and favourable gastrointestinal tolerability. Inflamm Res. 2004;53:150–7. doi: 10.1007/s00011-003-1236-y. [DOI] [PubMed] [Google Scholar]
- 74.Vallance AK. Can biological activity be maintained at ultra-high dilution? An overview of homeopathy, evidence, and Bayesian philosophy. J Altern Complement Med. 1998;4:49–76. doi: 10.1089/acm.1998.4.1-49. [DOI] [PubMed] [Google Scholar]
- 75.Belon P, Cumps J, Ennis M, Mannaioni PF, Roberfroid M, Sainte-Laudy J, et al. Histamine dilutions modulate basophil activation. Inflamm Res. 2004;53:181–8. doi: 10.1007/s00011-003-1242-0. [DOI] [PubMed] [Google Scholar]
- 76.Walach H. Entanglement model of homeopathy as an example of generalized entanglement predicted by weak quantum theory. Forsch Komplementarmed Klass Naturheilkd. 2003;10:192–200. doi: 10.1159/000073475. [DOI] [PubMed] [Google Scholar]
- 77.Milgrom LR. Patient-practitioner-remedy (PPR) entanglement. Part 5. Can homeopathic remedy reactions be outcomes of PPR entanglement? Homeopathy. 2004;93:94–8. doi: 10.1016/j.homp.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 78.Hyland ME. Entanglement and some heretical thoughts about homeopathy. Homeopathy. 2005;94:105–6. doi: 10.1016/j.homp.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 79.Bellavite P. Complexity science and homeopathy. A synthetic overview. Homeopathy. 2003;92:203–12. doi: 10.1016/j.homp.2003.08.002. [DOI] [PubMed] [Google Scholar]