Abstract
Non-parenchymal cells might play an important role in the modulation of xenobiotic metabolism in liver and its pharmacological and toxicological consequences. Therefore, the role of cell-to-cell interactions in herbal induced liver toxicity was investigated in monocultures of cells from the human hepatocyte cell line (HepG2) and in co-cultures of cells from the HepG2 cell line and cells from the human monocyte cell line (THP1). Cells were treated with various concentrations (1–500 µg ml−1) of extracts of Pistacia palaestina, Juglans regia and Quercus ithaburensis for 24 h. Extracts from Cleome droserifolia, a known toxic plant, were taken as positive control. In the co-culture system, toxic effects were observed after exposure to extracts of Pistacia palestina and C. droserifolia. These two extracts significantly reduced by cell viability as measured the MTT test and the LDH assay. Whereas in hepatocyte cultures, only extracts of C. droserifolia were found to affect the cell viability. The production levels of albumin from hepatocytes were not affected by treatment with plant extracts in both culture systems. It seems that the observed reduction in cell viability after exposure to extracts of P. palestina in co-cultures but not in monocultures is a result of monocyte-derived factors. The use of liver cell co-cultures is therefore a useful approach to investigate the influence of intercellular communication on xenobiotic metabolism in liver.
Keywords: biosafety, co-cultures, hepatocyte, in vitro, medicinal plants
Introduction
The currently observed rapid increase in consumption of herbal remedies worldwide has been stimulated by several factors, including the notion that all herbal products are safe and effective (1–3). However, over the past decade, several news-catching episodes in developed communities indicated adverse effects, sometimes life-threatening, allegedly arising consequential to taking herbal products or traditional medicines from various ethnic groups (4,5). In some cases, adulteration, inappropriate formulation, or lack of understanding of plant and drug interactions or uses has led to adverse reactions that are sometimes life-threatening or lethal to patients (6–10).
Hepatotoxicity of Herbal Remedies
Most reports of toxic effects due to the use of herbal medicines and dietary supplements are associated with hepatotoxicity although reports of other toxic effects including kidney, nervous system, blood, cardiovascular and dermatologic effects, mutagenicity, and carcinogenicity have also been published in medical literature. On the basis of various case reports, liver injury from herbal remedies has ranged from mild elevations of liver enzymes to fulminated liver failure requiring liver transplantation. The reported toxicity of herbal formulations may be the result of several factors, including contamination with pesticides, microbes, heavy metals, toxins or adulteration with orthodox drugs (11). Therefore, for safety and quality assurances, chemical analytical techniques should be applied at different stages for good practices in quality assurances of natural or herbal products, including good agricultural practice by farmers, good sourcing and laboratory practices by pharmaceutical companies, good manufacturing practices and innovative clinical trial practices by researchers and physicians (5).
In Vitro Test Methods
In vitro culture models that employ human liver cells could be potent tools for predictive studies on drug toxicity and metabolism in the pharmaceutical industry. In general, in vitro test systems represent the first phase of the evaluation procedure. In the in vitro test systems, cells isolated from various tissues or cell lines are cultured (12,13). In vitro cell culture methods have the advantage of relatively well-controlled variables and are generally accepted as a very effective method for safety testing. Advantages of these systems over classical methods (such as long-term studies on experimental animals) include relatively well-controlled variables, decreased costs, a reduced time to completion and reduced numbers of animals necessary to complete the study. The fact that cells and tissues in vivo do not exist in isolation, but communicate with and are interdependent on neighboring tissue makes it essential to simulate the in vivo situation, where, for example, the microenvironment of the hepatocytes within the liver acinus involves gradients in oxygen tension, hormones, extracellular matrix components, non-parenchymal cells and effective exposure levels of xenobiotics from the periportal to the pericentral compartment (14,15). In addition to primary cultures, cell lines, such as HepG2 (express hepatocyte specific function, such as P450 and albumin production), provide an adequate in vitro liver model for many mechanistic studies of signal transduction, gene expression, metabolism and toxicology.
Co-cultures of Parenchymal and Non-parenchymal Cells
Conventional homotypic hepatocyte cultures do not include the possible contribution of non-parenchymal liver cells, particularly Kupffer cells, to the pharmacological and toxicological consequences after exposure to xenobiotics. Therefore, in the present study we evaluated the hepatotoxicity of three plant extracts using co-cultures of cells from the human hepatocyte cell line (HepG2) and cells from the human monocyte cell line (THP1). In this co-culture system both cell types have direct cell-to-cell contacts and are maintained in more ‘in vivo-like’ culture conditions than in the monoculture system. Results obtained here indicate that one out of three tested plant extracts significantly reduced cell viability in the co-culture system but not in the monoculture system. It seems that the observed reduction in cell viability is a result of monocyte-derived factors.
Material and Methods
Preparation of Plant Extracts
Fifty grams of air-dried Pistacia palaestina (leaves), Juglans regia (leaves), Quercus ithaburensis (fruits) and Cleome droserifolia (taken as positive control) were added to 1 liter of distilled water and boiled for 10 min. The boiled water extracts were filtered through filter paper and freeze-dried in a lyophilizer. The freeze-dried extracts were stored at −70°C for further evaluation.
Cell Culture
The effect of P. palaestina (RDC 1089), J. regia (RDC 1060), Q. ithaburensis (RDC 1097) and C. droserifolia (1) on cell viability was assessed in a cell culture system using cells from the human hepatoplastoma cell line HepG2 and cells from the monocyte cell line THP1. HepG2 cell line retains differentiated parenchymal functions of normal hepatocytes, including the expression of P450 isoenzymes (16) thus permitting long-term studies to be performed. The cells from both cell lines were grown in Dulbecco's modified Eagle's medium (DMEM) with a high glucose content (4.5 g l−1) supplemented with 10% vol/vol inactivated fetal calf serum, 1% non-essential amino acids, 1% glutamine, 100 U ml−1 penicillin, and 10 µg ml−1 streptomycin. Cells were maintained in humidified atmosphere with 5% CO2 at 37°C. The medium of cells from both cell lines was changed twice a week. At 70–80% confluence, cells were trypsinized and seeded in 96-well plates in cell density of 1.5 × 104 HepG2 cells and 5 × 103 THP1 cells. Twenty-four hours after cell seeding, cells were exposed to various concentrations of the plant extracts in fresh serum-free medium.
MTT Assay
The tetrazolium dye, MTT, is widely used to assess the viability and/or the metabolic state of cells (17). This colorimetric assay is based on the conversion of the yellow tetrazolium bromide (MTT) to the red formazan derivative by mitochondrial succinate dehydrogenase in viable cells. Twenty-four hours after cell seeding, cells were incubated with varying concentrations of water extracts of the three plants for 24 h at 37°C. Following removal of the plant extracts from each well, cells were washed in phosphate-buffered saline. The cells were then incubated in serum-free DMEM to which MTT (0.5 mg ml−1) was added to each well (100 µl), and incubated for a further 4 h. Then the medium was removed and the cells were incubated for 15 min with 100 µl of acidic isopropanol (0.08 N HCl) to dissolve the formazan crystals. The absorbance of the MTT formazan was determined at 570 nm in an enzyme-linked immunosorbent assay (ELISA) reader. Viability was defined as the ratio (expressed as a percentage) of absorbance of treated cells to untreated cells.
Lactate Dehydrogenase
In the lactate dehydrogenase (LDH) assay, leakage of the cytoplasmic located enzyme LDH into the extracellular medium is measured. The presence of the exclusively cytosolic enzyme, LDH, in the cell culture medium is indicative of cell membrane damage (17).
For the LDH assay, 1.5 × 104 HepG2 cells and 5 × 103 THP1 were seeded per well of 96-microtiter plates. Twenty-four hour after cell seeding, cells were exposed to varying concentrations of the plant extracts (0.001–0.5 mg ml−1). After 24 h of treatment, the supernatants were collected from each well. Cell monolayers were then treated with a cell lysis solution for 30 min at room temperature to lyse. The cells and the lysate were collected. LDH activity was measured in both the supernatants and the cell lysate fractions using CytoTox 96, a non-radioactive cytotoxicity assay kit (Promega, WI, USA), in accordance with the manufacturer's instruction. The intensity color is proportional to LDH activity. The absorbance is determined at 490 nm with 96-well plate ELISA reader. The percent of LDH release from the cells was determined using the following formula: LDH release = (Absorbance of the supernatant)/(absorbance of the supernatant and cell lysate) × 100.
Quantification of Albumin Secretion
For the determination of albumin levels in culture supernatants, 1.5 × 104 HepG2 cells and 5 × 103 THP1 were seeded in 96-microtiter plates. Twenty-four hours after cell seeding, cells were exposed to varying concentrations of the plant extracts (0.001–0.5 mg ml−1). After 24 h of treatment, the supernatants were collected from each well. The amount of albumin in the culture supernatant was measured using ELISA. In brief, supernatants were incubated in 96-well microtiter plates for 1 h at 37°C or overnight at 4°C. After washing in PBS, non-specific binding sites were blocked in PBS containing 0.5% bovine serum albumin (BSA) for 1 h at room temperature. After another washing step in PBS, peroxidase-conjugated goat anti-rat albumin antibody was added in PBS containing 1% BSA and incubated for 2 h at room temperature (this antibody cross-reacts with human albumin). The microtiter plates were then washed, the substrate (0.5 mg 2.2-azino-di-3-ethylbenzothiazoline-6-sulfonic acid per ml of 100 mM sodium acetate, 50 mM sodium phosphate and 9 × 10−3 % H2O2) added and the absorption was measured at 405 nm in an ELISA reader. All washing steps were carried out with PBS at room temperature. Background values were measured in the absence of culture supernatant and subtracted from the experimental values. All ELISA determinations were carried out in duplicates.
Statistical Analysis
Error limits cited and error bars plotted represent simple standard deviations of the mean. Usually, numerical results are only accurate enough to specify the least significant digit. When comparing different samples, results were considered to be statistically different when P < 0.05 (Student's t-test for unpaired samples).
Results
The effect of extracts of P. palaestina, J. regia, Q. ithaburensis and C. droserifolia was evaluated here in co-cultures of cells from the hepatocyte cell line HepG2 and cells from the monocyte cell line THP1. MTT, LDH assay and albumin determination (ELISA) were carried out 24 h after treatment with increasing concentrations (1–500 µg ml−1 of culture medium) of extracts from the four plants.
Evaluation of Effects on Cell Viability Using MTT Test
Metabolic activity can be evaluated by measuring the activity of a mitochondrial enzyme succinate dehydrogenase using MTT test. MTT is designed to be used for the quantification of both cell proliferation and cell viability in cell population using 96-well plate format. This test is widely used in the in vitro evaluation of the biosafety of plant extracts. In the present study we applied the MTT test to evaluate the biosafety of three medicinal plants in hepatocyte monolayers and in co-cultures of hepatocytes and monocytes. Therefore, HepG2 cells and co-cultures of HepG2 and THP1 were exposed to increasing concentrations (1–500 µg ml−1 of culture medium) of the three plant extracts for 24 h. Following removal of the plant extracts from each well, cells were washed in phosphate-buffered saline, and the MTT assay was carried out as described. Unattached HepG2 cells and THP1 cells (grow in suspension) are removed from the co-cultures during the washing process.
HepG2 Cells
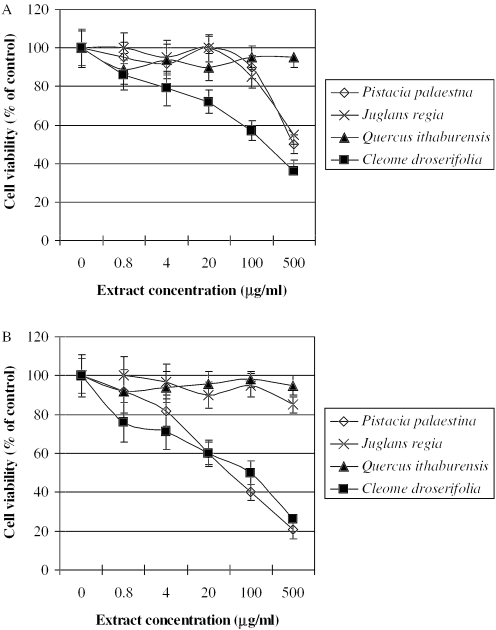
Extracts from Q. ithaburensis exhibited no sign of any negative effects at all concentrations tested (Fig. 1A). Extracts from J. regia and P. palaestina reduced MTT formation by about 60% at concentration of 500 µg ml−1 (Fig. 1A). Cell viability was decreased with increasing extract concentrations of C. droserifolia, which was taken as a positive control. The cell viability was decreased by about 60% at 500 µg of C. droserifolia per ml.
Figure 1.
MTT assay in HepG2 cells (A) and in the co-culture of HepG2 and THP1 cells (B) after an overnight incubation with various concentrations of extracts from P. palaestina, J. regia, Q. ithaburensis and C. droserifolia (positive control). The absorbance of the MTT formazan was determined at 570 nm in an ELISA reader. Cell viability was defined as the ratio (expressed as a percentage) of absorbance of treated cells to untreated cells. Values given represent the mean ± standard deviations of three independent experiments carried out in triplicates.
Co-cultures of HepG2 with THP1
No sign of any negative effect at all concentrations tested in the co-culture system was seen after exposure to extracts from J. regia and Q. ithaburensis (Fig. 1B). Cell viability with P. palestina was enhanced in the co-cultures. Exposure to Pistacia palestina extracts for 24 h dose-dependently decreased cell viability, reaching about 20% of untreated cells (Fig. 1B).
Evaluation of Effects on Cell Viability Using LDH-Release Test
Membrane integrity can be evaluated by measuring LDH activity. LDH, an enzyme located in the cytoplasm, catalyses the conversion of lactate and pyruvate. When LDH is found within the media on the cells, there are two possible causes: the first is cellular death and the second may be a ‘leak’ in a cell membrane. When cells are disrupted, LDH activity is elevated.
HepG2 Cells
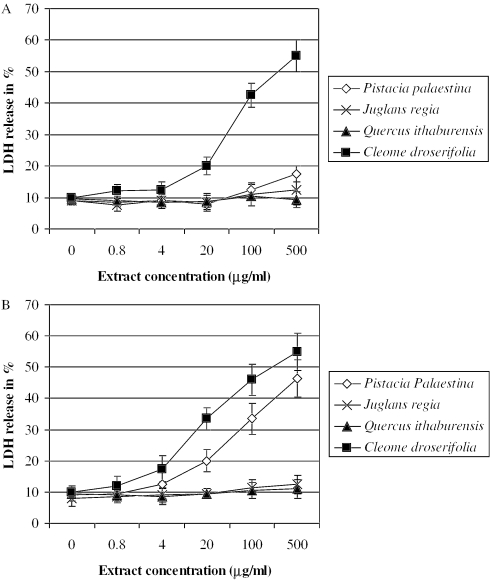
Results obtained in the present in vitro study indicate no significant changes of LDH levels in the culture medium were seen after 24 h of exposure to extracts from J. regia and Q. ithaburensis (Fig. 2A). Pistacia palaestina extract significantly increased the extracellular LDH levels at concentrations higher than 100 µg ml−1 (Fig. 2A). Cell viability was significantly decreased with increasing concentrations of C. droserifolia extract, the positive control.
Figure 2.
LDH leakage from HepG2 cells (A) and from co-cultures of HepG2 and THP1 cells (B) after an overnight incubation with various concentrations of extracts from P. palaestina, J. regia, Q. ithaburensis and C. droserifolia (positive control). The leakage of the cytoplasm located LDH into the extracellular medium is measured. LDH activity was measured in both the supernatants and the cell lysate fractions. Values given represent the mean ± standard deviations of three independent experiments carried out in triplicates.
Co-cultures of HepG2 with THP1
As seen with HepG2, LDH levels were not significantly increased after treatment with extracts from J. regia and Q. ithaburensis. The effect of P. palaestina extract was similar to that found in the MTT assay. Concentrations higher than 20 µg ml−1 significantly increased the extracellular LDH levels reaching about 100% at the highest concentration tested (Fig. 2B).
Effects on the Biofunctionality of Cultured Cells
The effects of the three plant extracts on expression of liver specific functions were measured by assessing albumin secretion by HepG2. Levels of albumin in the cell culture medium were unchanged by treatment with any of the three test extracts (data not shown).
Discussions and Conclusions
Recently, the number of people using medicinal plants has been rapidly increasing and therefore national health authorities are beginning to express concern over the safety and efficacy of these products since almost all of them are sold over the counter and are not registered (18,19). The purpose of the present study is to develop a new way to evaluate both biosafety and biofunctionality of herbal extracts.
In vitro Test Methods
A very sensitive method for testing the toxicity of plant extracts and their degradation products is the assessment of in vitro toxicity. In vitro cell culture methods have the advantage of relatively well-controlled variables and are generally accepted as very effective for biosafety testing. Their sensitivity is equal to or greater than that of in vivo tests. In vitro test methods are based on the extraction of the bioactive compounds. After exposure of cells to extracts, cytotoxicity is assessed by various methods, such as microscopical evaluation of cell morphology, the methyltetrazolium assay (MTT test), measurement of DNA and protein synthesis, LDH activity, and neutral red uptake. However, it has long been realized that while growth in two dimensions is a convenient way of preparing and observing a culture and allows a high rate of cell proliferation, it lacks the cell–cell and cell–substrate interactions characteristic of the whole tissue in vivo. The interactions of a cell with a substratum play an important role not only in the development, differentiation and regeneration of multicellular organisms but also in preserving the specific phenotype of cultured cells in vitro.
Co-cultures of Hepatocytes and Monocytes
The development of a new in vitro test system that enables cultured cells to grow at higher cell density and to maintain more in vivo like cell-to-cell interactions is very important in order to obtain more reliable results in vitro. Non-parenchymal cells, in particular Kupffer cells, might play an important role in the modulation of xenobiotic metabolism in liver and its pharmacological and toxicological consequences (20). Therefore, we used co-cultures of human hepatoplastoma cell line HepG2 cells and monocyte cell line THP1. HepG2 cell line retains differentiated parenchymal functions of normal hepatocytes and can be grown indefinitely, thus permitting long-term studies to be performed. After treatment of HepG2 cells and co-cultures of HepG2 cells and THP1 cells with various concentrations of extracts of P. palestina, J. regia and Q. ithaburensis for 24 h, the biosafety (MTT and LDH release) and biofunctionality (albumin production) were evaluated.
The Role of Monocyte-derived Mediators
The observed difference in cytotoxicity of P. palaestina extract in hepatocytes and in co-culture of hepatocytes and monocytes may be the result of cell-to-cell interactions and/or of cytokines produced by monocytes. In LPS-induced liver injury, activated hepatic Kupffer cells play an essential role (21,22). Following contact with the cluster of differentiation (CD) 14 protein, the complex triggers a signal cascade involving the nuclear factor kappa B. This factor enhances the expression of inflammation-related genes. The acute-phase response is regulated by cytokines released by activated Kupffer cells, notably interleukin-1 (IL-1), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) (23). Among these cytokines, IL-6, also known as hepatocyte-stimulating factor, is a major inducer of the acute phase response. IL-6 is produced not only by Kupffer cells but also other cell types including monocytes/macrophages, fibroblasts, keratinocytes, endothelial cells, mesanglial cells, chondrocytes, T cells, B cells, human hepatoma cells (HepG2) and primary human hepatocytes. Hepatocytes also produce nitric oxide (NO) during chronic hepatic inflammation (24) and in vitro in response to conditioned medium from activated Kupffer cells (25) or to a mixture of LPS and TNF-α, IL-1 and IFN-γ. In the liver, TNF-α production is not restricted to Kupffer cells.
Saad et al. (26) showed that LPS affects the acute phase response via hepatocyte-derived IL-6 and TNF-α in an autocrine loop and the NO production of parenchymal liver cells. TNF-α is also involved in inducing cell damage by promoting oxidative stress in mitochondria that occurs as a result of an imbalance between oxidants and antioxidants in favor of oxidants (27). TNF-α stimulates the production of reactive oxygen species [ROS and reactive nitrogen species (RNS)]. ROS have been implicated in the pathogenesis of many forms of liver disease. When liver cells are exposed to excess ROS, oxidative stress occurs and affects many cellular functions. However, cells are equipped with antioxidant systems. The essential function of the cellular antioxidant systems is to lower the steady state of intracellular concentrations of free radicals. They are interrelated and fall into two broad categories as follows: enzymatic (e.g. glutathione peroxidase, catalase and superoxide dismutase) and non-enzymatic antioxidant [e.g. vitamin E, vitamin C and reduced glutathione (GSH)] defenses. It seems that the observed reduction in cell viability in co-cultures is the result of monocyte-derived factors produced after activation of these cells by extract from P. palestina. Therefore, cell–cell interactions must be taken into consideration by the in vitro evaluation of medicinal plant biosafety.
Conclusions
In conclusion, conventional homotypic hepatocyte cultures do not include the possible contribution of non-parenchymal liver cells, particularly Kupffer cells, to the pharmacological and toxicological consequences after exposure to xenobiotics. Results obtained here indicate that co-cultures improve the reliability of data obtained from organ-specific cell cultures and that they more closely stimulate the situation in the intact liver.
References
- 1.Said O, Khalil K, Fulder S, Azaizeh H. Ethnobotanical survey of medicinal herbs of the Middle Eastern region. J Ethnopharmacol. 2002;83:251–65. doi: 10.1016/s0378-8741(02)00253-2. [DOI] [PubMed] [Google Scholar]
- 2.Farnsworth NR, Soejarto DD. Potential consequence of plant extinction in the United States on the current and future availability of prescription drugs. Econ Bot. 1985;39:231–40. [Google Scholar]
- 3.Soejarto DD. Biodiversity prospecting and benefit-sharing: perspectives from the field. J Ethnopharmacol. 1989;51:1–15. doi: 10.1016/0378-8741(95)01345-8. [DOI] [PubMed] [Google Scholar]
- 4.Elvin-Lewis M. Should we be concerned about herbal remedies? J Ethnopharmacol. 2001;75:141–64. doi: 10.1016/s0378-8741(00)00394-9. [DOI] [PubMed] [Google Scholar]
- 5.Chan K. Some aspects of toxic contaminants in herbal remedies. A review. Chemosphere. 2003;52:1361–71. doi: 10.1016/S0045-6535(03)00471-5. [DOI] [PubMed] [Google Scholar]
- 6.Ernst E. Harmless herbs? A review of the recent literature. Am J Med. 1998;104:170–8. doi: 10.1016/s0002-9343(97)00397-5. [DOI] [PubMed] [Google Scholar]
- 7.Ernst E. Herbal medications for common ailments in the elderly. Drugs Aging. 1999;15:423–8. doi: 10.2165/00002512-199915060-00002. [DOI] [PubMed] [Google Scholar]
- 8.Abu-Irmaileh BE, Afifi FU. Herbal medicine in Jordan with special emphasis on commonly used herbs. J Ethnopharmacol. 2003;89:193–7. doi: 10.1016/s0378-8741(03)00283-6. [DOI] [PubMed] [Google Scholar]
- 9.Pak E, Esrason KT, Wu VH. Hepatotoxicity of herbal remedies: an emerging dilemma. Prog Transplant. 2004;14:91–6. doi: 10.1177/152692480401400203. [DOI] [PubMed] [Google Scholar]
- 10.Saad B, Azaizeh H, Said O. Tradition and perspectives of Arab herbal medicine: a review. Evid Based Complement Alternat Med. 2005;2:475–9. doi: 10.1093/ecam/neh133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Nahhal Y. Contamination and safety status of plant and food in Arab countries. J Appl Sci. 2004;4:411–7. [Google Scholar]
- 12.Ventura C. CAM and cell fate targeting: molecular and energetic insights into cell growth and differentiation. Evid Based Complement Alternat Med. 2005;2:277–83. doi: 10.1093/ecam/neh100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimazawa M, Chikamatsu S, Morimoto N, Mishima S, Nagai H, Hara H. Neuroprotection by Brazilian green propolis against in vitro and in vivo ischemic neuronal damage. Evid Based Complement Alternat Med. 2005;2:201–7. doi: 10.1093/ecam/neh078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saad B, Schawalder HP, Maier P. Crude liver membrane fractions maintain liver specific functions in long term, serum free rat hepatocyte cultures. In Vitro Cell Dev Biol. 1993;29:32–40. doi: 10.1007/BF02634369. [DOI] [PubMed] [Google Scholar]
- 15.Saad B, Scholl FA, Thomas H, Schawalder HP, Streit V, Waechter F, et al. Crude liver membrane fractions and extracellular matrix components as substrata regulate differentially the preservation and inducibility of P-450 isoenzymes in cultured rat hepatocytes. Eur J Biochem. 1993;213:805–14. doi: 10.1111/j.1432-1033.1993.tb17823.x. [DOI] [PubMed] [Google Scholar]
- 16.Medina-Diaz IM, Elizondo G. Transcriptional induction of CYP3A4 by o,p'-DDT in HepG2 cells. Toxicol Lett. 2005;16:41–7. doi: 10.1016/j.toxlet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Saad B, Abu-Hijleh G, Suter UW. Polymer biocompatibility assessment by cell culture techniques. In: Arshady R, editor. The PMB Series Volume 1: Introduction Polymeric Biomaterials. The Citus Books; 2003. pp. 263–99. [Google Scholar]
- 18.Cooper EL. Drug discovery, CAM and natural products. Evid Based Complement Alternat Med. 2004;1:215–7. doi: 10.1093/ecam/neh032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper EL. Complementary and alternative medicine, when rigorous, can be science. Evid Based Complement Alternat Med. 2004;1:1–4. doi: 10.1093/ecam/neh002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milosevic N, Schawalder H, Maier P. Kupffer cell-mediated differential down-regulation of cytochrome P450 metabolism in rat hepatocytes. Eur J Pharmacol. 1999;26:75–87. doi: 10.1016/s0014-2999(98)00988-1. [DOI] [PubMed] [Google Scholar]
- 21.Knolle P, Lohr H, Treichel U, Dienes HP, Lohse A, Schlaack J, et al. Parenchymal and nonparenchymal liver cells and their interaction in the local immune response. Gastroenterol. 1995;33:613–20. [PubMed] [Google Scholar]
- 22.Toth CA, Thomas P. Liver endocytosis and Kupffer cells. Hepatology. 1992;16:255–66. doi: 10.1002/hep.1840160137. [DOI] [PubMed] [Google Scholar]
- 23.Ramadori G, Armbrust T. Cytokines in the liver. Eur J Gastroenterol Hepatol. 2001;13:777–84. doi: 10.1097/00042737-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Billiar TR, Curran RD, Harbrecht BG, Stuehr DJ, Demetris AJ, Simmons RL. Modulation of nitrogen oxide synthesis in vivo: NG-monomethyl-l-arginine inhibits endotoxin-induced nitrite/nitrate biosynthesis while promoting hepatic damage. J Leukoc Biol. 1990;48:565–9. doi: 10.1002/jlb.48.6.565. [DOI] [PubMed] [Google Scholar]
- 25.Curran RD, Billiar TR, Stuehr DJ, Hofmann K, Simmons RL. Hepatocytes produce nitrogen oxides from l-arginine in response to inflammatory products of Kupffer cells. J Exp Med. 1989;170:1769–74. doi: 10.1084/jem.170.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saad B, Frei K, Scholl F, Fontana A, Maier P. Hepatocyte-derived IL-6 and TNF-a mediate the LPS-induced acute phase response and NO-release by cultured rat hepatocytes. Eur J Biochem. 1995;229:349–55. doi: 10.1111/j.1432-1033.1995.0349k.x. [DOI] [PubMed] [Google Scholar]
- 27.Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291–5. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]