Abstract
The lifelong spermatogonial stem cell divisions unique to male germ cell production are thought to contribute to a higher mutation frequency in males. The fact that certain de novo human genetic conditions (e.g., achondroplasia) increase in incidence with the age of the father is consistent with this idea. Although it is assumed that the paternal age effect is the result of an increasing frequency of mutant sperm as a man grows older, no direct molecular measurement of the germ-line mutation frequency has been made to confirm this hypothesis. Using sperm DNA from donors of different ages, we determined the frequency of the nucleotide substitution in the fibroblast growth factor receptor 3 (FGFR3) gene that causes achondroplasia. Surprisingly, the magnitude of the increase in mutation frequency with age appears insufficient to explain why older fathers have a greater chance of having a child with this condition. A number of alternatives may explain this discrepancy, including selection for sperm that carry the mutation or an age-dependent increase in premutagenic lesions that remain unrepaired in sperm and are inefficiently detected by the PCR assay.
Geneticists and evolutionary biologists debate the extent to which the lifelong spermatogonial stem cell divisions unique to male gametogenesis contribute to a higher mutation frequency in males (1–8). One source of support for the hypothesis that mutations increase with spermatogonial stem cell divisions comes from the observation of certain human genetic conditions where the incidence of new mutations increases with the age of the father. Epidemiological studies on a number of dominantly inherited conditions indicate that the average age of the fathers is older among unaffected couples having a child with the condition (a sporadic case caused by a new mutation), than the average paternal age in the population (1, 2, 4, 7). Achondroplasia, the most common form of dwarfism, is one of these conditions (9–11). Studies on sporadic achondroplasia cases have reported an exponential increase with paternal age (1, 2, 4, 7, 10).
In mice, a significant increase in the overall male germ cell mutation frequency as measured by the lacI assay was observed between 15 and 28 months of age (12). In humans, no direct molecular measurement of how germ-line nucleotide substitution frequencies change with age exists. Achondroplasia provides a unique opportunity to directly test the relationship between paternal age and sperm mutation frequency at the molecular level. First, 97–99% of the de novo mutations leading to this condition result from a G-to-A transition mutation at base pair 1138 (G1138A) in exon 10 of fibroblast growth factor receptor 3 (FGFR3) (13–15). The cytosine at base pair 1138 is part of a CpG dinucleotide and, if methylated, is highly susceptible to mutation caused by spontaneous deamination (16). Second, all sporadic achondroplasia cases have been found to inherit the G1138A mutation from their father (17). Third, recent data on the population incidence of sporadic achondroplasia (10, 18–20) predict the average frequency of sperm carrying the mutation in normal individuals will be in a range detectable by modern molecular methods (1/15,000 to 1/70,000). Our studies on sperm DNA from men of different ages suggest that the observed increase in G1138A mutation frequency cannot satisfactorily explain the exponential increase in sporadic achondroplasia cases with paternal age.
Methods
Sources of Semen Samples and DNA.
Semen samples were collected according to protocols approved by the institutional review boards of the University of Southern California (USC) (60 donors; mean age 40.65 years, range 18–72) and the University of California (Berkeley) and Lawrence Livermore National Laboratory (LNLL) (58 donors; mean age 41.66 years, range 22–80). At least 90% of the donors were of European ancestry. Pooled human blood DNA (catalog no. 6550-1) used in the standards was purchased from CLONTECH. The DNA cell line (NA08859; see below) homozygous for the G1138A mutation was obtained from Coriell Cell Repositories, Camden, NJ.
Sperm DNA Quantitation.
We quantitated the number of genomes containing the single-copy FGFR3 gene by using real-time or kinetic PCR (kt-PCR; ref. 21). An initial denaturation step at 94°C for 10 min was followed by cycling conditions of 94°C for 40 s and 66°C for 1 min. Reactions were carried out in 50 μl containing 1× Taq Gold Buffer (15 mM Tris⋅HCl/50 mM KCl at pH 8.0), 1.25 units of Taq Gold, 2 mM MgCl2, 80 μM of each dNTP, and 0.2 μM forward (5′-aggagctggtggagctga-3′) and reverse primer (5′-tgcgcaggcggcagagcgtc-3′), 0.2× SYBR Green I (Molecular Probes), and 2 μM 5,6 ROX dye (Sigma). kt-PCR was performed on a GeneAmp 5700 Sequence Detection System (Applied Biosystems).
Quantitation of G1138A Mutations in Sperm DNA.
Sperm DNA was extracted from all semen samples by using Puregene DNA Isolation Kits (Gentra Systems). The concentration and purity of the sperm DNA samples was determined spectrophotometrically after HaeIII or BseDI digestion to reduce the DNA viscosity. Neither enzyme has a recognition sequence in the amplification target. Sperm DNA from 1/3 of the samples was also quantitated by using kt-PCR (see above). This process confirmed the accuracy of the OD measurements.
In the first step of the assay, FGFR3 fragments containing base pair 1138 were amplified from 1 μg of sperm DNA (duplicate aliquots) with eight PCR cycles (94°C for 40 s and 66°C for 1 min) preceded by a 94°C 10-min Taq Gold activation step. Each 50-μl reaction contained 1× Taq Gold Buffer (15 mM Tris⋅HCl/50 mM KCl at pH 8.0), 1.25 units of Taq Gold (Applied Biosystems), 2 mM MgCl2, 80 μM of each dNTP, and 0.2 μM forward primer (5′-tgtgtatgcaggcatcctcagctcc-3′) and reverse primer (5′-tgcgcaggcggcagagcgtc-3′). In the second step, 20 units of MspI was added to the amplification reaction and incubated for 30 min at 37°C followed immediately by 16 additional amplification cycles. The amount of PCR product produced in these 24 cycles was confirmed by kt-PCR. In the third step, two 5-μl aliquots of a 40-fold dilution of each of the above duplicates were analyzed by using allele-specific kt-PCR (22). The reactions contained 0.1 μM of mutant-specific primer (5′-atgcaggcatcctcagctaca-3′) and reverse nested primer (5′-gccgccaccaccaggatgaac-3′), 0.8 units of Taq Gold, 1× Taq Gold buffer, 3.5 mM MgCl2, 50 μM of each dNTP, 0.2× SYBR Green I, and 2 μM 5,6 ROX dye in a total volume of 50 μl. An initial Taq Gold activation step was followed by 35 cycles of 40 s at 94°C for denaturation and 60 s at 68°C for annealing and extension. To confirm that the PCR product was the one desired and not an amplification artifact (e.g., primer-dimer), we used the GeneAmp 5700 denaturation profile function to examine every allele-specific reaction.
To avoid carryover contamination by PCR product, each step of the protocol was carried out in a different biological safety cabinet UV-irradiated for 60 min. The kt-PCR tubes were discarded unopened. The controls with no DNA were not positive in any experiment. Finally, the first 1/3 of the data from sperm samples throughout the age range was collected by using the UNG protocol for preventing PCR contamination (Amersham Pharmacia).
In each experiment the standards also consisted of four measurements each of three different amounts (15, 45, and 135 copies or 20, 60, and 180 copies) of G1138A genomes from the homozygous achondroplasia cell line diluted in blood cell DNA (1 μg). Blood DNA alone also served as a control in every experiment. Note that the number of G1138A mutants estimated for any sperm sample by using the standard curve can be taken as an estimate of the true value without any additional corrections because the blood DNA in the standards are subject to the same background-producing PCR processes as sperm DNA (see below). The number of G1138A mutants per μg of DNA defines the “counts” for any sample.
PCR Product Quantitation.
The number of PCR templates in a 5-μl aliquot of a 1:40 dilution of the PCR product made in the first two steps of the assay was quantitated by kt-PCR. Each 50-μl reaction contained 1× Taq Gold Buffer, 1 unit of Taq Gold, 2 mM MgCl2, 80 μM of each dNTP, and 0.2 μM forward primer (5′-tgtgtatgcaggcatcctcagctcc-3′) and reverse primer (5′-gccgccaccaccaggatgaac-3′), 0.2× SYBR Green I, and 2 μM 5,6 ROX dye. Amplification followed an initial Taq Gold activation step (10 min at 94°C) and cycling conditions of 40 s at 94°C and 60 s at 68°C.
BfmI Digestion Experiments.
Duplicate aliquots, each containing 1 μg of sperm DNA or standards consisting of blood DNA with known amounts of added G1138A genomes (180, 60, and 20 copies), were incubated with 5 units of active or heat-inactivated BfmI enzyme (Fermentas, Hanover, MD) overnight at 37°C. Each sample was analyzed 6–8 times by our assay.
Accuracy of the Assay.
Coded samples with known numbers of genomes with the G1138A mutation were mixed with 1 μg of blood DNA. The counts in the mixtures were determined by using the same series of standards and number of replicates used to measure the number of G1138A mutations in the sperm samples.
Sensitivity of Allele-Specific PCR.
Reconstruction experiments were used to determine the sensitivity of the allele-specific kt-PCR step. PCR product obtained by amplifying the homozygous achondroplasia cell line DNA was mixed with blood DNA PCR product. Both PCRs used the same conditions as in the first two steps of the assay except there was no MspI digestion. We found that the ratio of one G1138A template per 600 WT templates can be distinguished from WT templates alone by a difference of two PCR cycles (data not shown). This is equivalent to detecting one G1138A mutant in 20,000 genomes because the MspI digestion in assay step two increases the ratio of mutant to WT by ≈35-fold.
Data Collection and Analysis.
In each experiment, two 1-μg aliquots of sperm DNA per individual from 6–10 different donors of varying ages were analyzed. The allele-specific step for each aliquot was done in duplicate. The mean of the four measurements was taken as the count for that sperm donor in that experiment. The final count for any donor was calculated by taking the median of the count values obtained in different experiments. In our first statistical analysis we assume that the assay produced an unbiased estimate of the true counts in any sperm sample.
Relationship Between Sporadic Achondroplasia Births and Mutation Frequency.
Comparison of the sperm G1138A mutation counts with the data on sporadic achondroplasia cases was made by using seven age groups (in 5-year intervals from ages 18 to 49 and a 10-year interval of 50–59 years). From the observed number of affected births in the various age groups, we computed the proportion of all affected births that occurred in each age group. The mean of the measured counts for the donors in a given age group i, when divided by 300,000, represents the proportion P(M|i) of all sperm in age group i that are mutant. For comparison to the birth data, we needed to compute the proportion P(i|M) of all mutants involved in births that belonged to fathers in age group i. To compute the quantity P(i|M), we used the quantities P(M|i) from our experimental data, along with the quantities P(i), which represent the proportion of all births that occur to fathers in age group i. We first computed P(M), the proportion of all births that involve a mutant sperm, by summing the products P(M|i) P(i) over all age groups i. Then P(i|M) was computed from the equation P(i|M) = P(M|i)P(i)/P(M). We calculated the predicted age distribution of the separate USC and LNLL cohorts in the same way except for compensating for their lower sample size.
To test the relationship between the birth data and our sperm data, we performed a generalized likelihood ratio test by using age categories. We used a Student's t distribution for each age-specific mean mutant frequency, after normalizing by its sample standard error. The observed numbers of births in the age categories were treated as multinomial. Because there are seven age groups, there are 13 parameters: seven mutant frequency means and six free parameters for the multinomial distribution. Under the null hypothesis, each of the multinomial probabilities can be expressed as a function of the mutant count means, leaving only seven parameters. The log likelihood ratio has a nominal χ2 distribution with 6 degrees of freedom. To account for the possibility that the distribution of age-specific mean mutant frequencies may not be well approximated with a Student's t distribution, we used the bootstrap to approximate the P value of the log likelihood ratio. We performed 10,000 bootstrap iterations. For each iteration, we drew a sample with replacement from each of the seven age groups to represent a bootstrap sample of mutant counts. We then created a bootstrap sample of observed births by generating a multinomial vector with probabilities proportional to the original age-specific mutant count means. We then computed the log likelihood ratio for the bootstrap data set consisting of these two bootstrap samples. The 10,000 bootstrap iterations provided us with 10,000 values of the log likelihood ratio. The proportion of these 10,000 values that exceeded the value obtained from the real data were used to estimate the P value.
The sample sizes in the individual LLNL and USC cohorts were too small to bootstrap. We therefore used the χ2 distribution with 6 degrees of freedom. Note that the bootstrap results from the complete data set suggested that the χ2 approximation for the pooled data are actually fairly good. For each of the seven age groups we calculated the P value to measure the significance of the difference between the observed and expected numbers of births by using a two-sample t test.
Results
Characterization of the Specificity and Sensitivity of the Assay.
We determined the frequency of the G1138A mutation in sperm samples by using PCR. Sperm DNA was extracted and the frequency of genomes carrying the G1138A mutation was estimated by using a three-step protocol. First, an FGFR3 fragment containing base pair 1138 was amplified by using 1 μg (300,000 genomes) of sperm DNA. The initial proportion of mutant to WT genomes is unchanged. As a result of PCR primer design, a MspI site was created in WT PCR products, but not in products containing the G1138A mutation. In the second step, MspI was added to the PCR to digest WT products. This process increased the mutant-to-WT ratio ≈35-fold (data not shown). The digestion was followed by additional PCR cycles. In the final step, real-time or kt-PCR (21) is used to quantitate the number of mutant templates with a primer whose 3′ base matches the mutant base at 1138, but not the WT base. To measure the number of G1138A mutations, sperm DNA was compared with standards consisting of a constant amount of blood cell DNA (a pool from >500 individuals) mixed with varying numbers of genomes from a human cell line homozygous for the G1138A mutation.
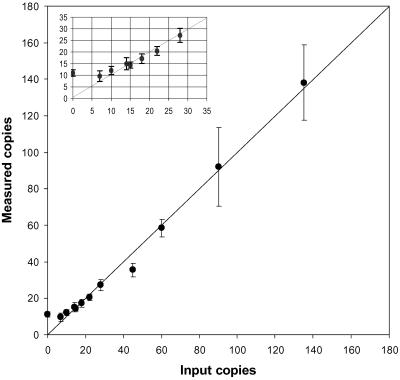
The accuracy of the method was determined by assaying coded samples containing 1 μg of blood DNA with known numbers of added G1138A mutants by using the standards discussed above. Fig. 1 compares the measured number of G1138A mutants per 300,000 blood cell genomes or counts to the actual input number of mutant genomes. The assay is quite accurate down to about 15 counts. Below 15 counts, the measured values tend to overestimate the true copy number because blood DNA with no added mutants gave an average value of 11 counts [95% confidence interval (C.I.) 10,12]. This background could result from the presence of G1138A mutants present in blood DNA or PCR artifact. At the high temperatures of PCR, deamination of the cytosine opposite the guanine at base pair 1138 could produce either a T or a U depending on whether the C was or was not methylated at the 5′ position, respectively. Both deamination products would be a template for the incorporation of dATP and create mutant PCR product. Second, PCR misincorporation by Taq polymerase could also result in G1138A product. Finally, during kt-PCR, the G1138A mutant-specific primer might get extended rarely on nonmutant templates.
Fig 1.
The accuracy of the assay is shown by the relationship between the median value of the measured counts (• with 95% C.I. indicated; in some cases the C.I. is too small to be seen) and the actual number of G1138A genomes added to blood DNA. Points falling along the diagonal would indicate a perfect assay. (Inset) An expansion of the data for the lower count values.
We asked whether PCR artifact rather than the presence of real G1138A mutants gave the background signal in blood DNA alone (data not shown). A fragment from a WT FGFR3 gene was cloned into a plasmid in Escherichia coli. The mutation assay was carried out by using 300,000 copies of the plasmid (in 1 μg of salmon sperm DNA) and compared with two sets of standards. One set was the same as described for G1138A mutation analysis in sperm DNA. The second set contained 300,000 copies of the plasmid (plus 1 μg salmon sperm DNA) and varying amounts of human achondroplasia genomes. Using either standard set, WT plasmid had approximately the same mutation frequency (≈1/27,000) as blood DNA alone. The frequency of a GC to AT mutation at a single nucleotide site measured in an E. coli reversion assay is ≈10−8 (23), and thus any sample of 300,000 plasmids is highly unlikely to contain any G1138A mutants. This finding suggests that the PCR assay is responsible for the production of the background counts in blood DNA. The same background value was obtained when the plasmids were enzymatically methylated with SssI (New England Biolabs) at the cytosines of CpG sites. Even boiling the methylated DNA for 10 min did not increase the background even though deamination of 5-methyl cytosine increases substantially with temperature. These data suggest that deamination during PCR does not increase the assay signal. We also found that cytosine methylation levels at base pair 1138 were the same in young and old sperm donor DNA (and blood DNA). Thus, differences in G1138A mutation frequency between young and old donors are not the result of different susceptibilities to PCR-induced deamination events at the cytosine of base pair 1138. We do not know to what extent the background is caused by the accumulation of G1138A PCR products arising during the first two assay steps or to misextensions of the mutant-specific primer on WT templates during kt-PCR (step 3). Artifact production during the latter step may be more likely because the background level did not change when a mixture of proofreading and nonproofreading polymerases was used in the first PCR step.
Control experiments to confirm that sperm samples with counts above background reflected real G1138A mutations were carried out by digesting sperm DNA with BfmI before beginning the assay (data not shown). This enzyme only cuts the recognition site that includes base pair 1138 if the GC bp has been mutated to AT and thus renders templates carrying the G1138A mutation unamplifiable. Sperm samples from two 20-year-old individuals (23 and 27 counts) and two from donors over 50 years (42 and 58 counts) were analyzed. A highly significant reduction (P = 1.28 × 10−11) in counts was seen when samples incubated with BfmI were compared with samples incubated with inactivated enzyme. We showed by kt-PCR that the difference between samples with active and inactive enzyme was not caused by either promiscuous BfmI “star” activity in the active enzyme (BfmI did not digest our WT FGFR3 template) or a difference in PCR efficiency. BfmI digestion reduced the G1138A counts in the sperm samples with the highest counts by 85% but only by 65% in the young donors. This result is expected because the closer the count value is to the background of the assay the more difficult it is to observe a reduction in mutation frequency. These results confirm that our assay detects GC to AT mutations at base pair 1138 in sperm DNA.
Analysis of Sperm DNA Samples.
We analyzed the G1138A levels in 118 individual unaffected donors ranging in age from 18 to 80 years. The frequency of G1138A mutant counts, averaged over all ages, was 28 or 1/11,000 haploid genomes. Each sperm DNA sample was subjected to an average of 7.2 independent measurements (range 5–14) distributed among 3–6 experiments (average 3.7) in which each sample and standard series was analyzed in duplicate. See Table 2, which is published as supporting information on the PNAS web site, www.pnas.org, for the complete data set.
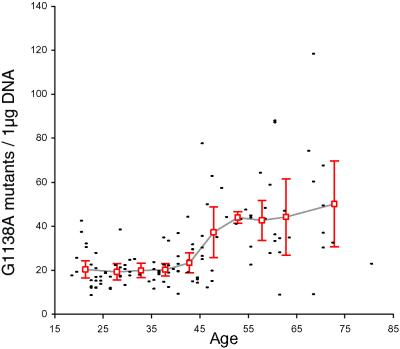
The sperm G1138A mutation counts for all donors is presented as a function of age in Fig. 2. There is little evidence for a change in the frequency of mutant sperm between the ages of 18 and 40 or between the ages of 55 and 80 years. However, an ≈2-fold increase between these two age groups occurs between 40 and 55 years. Because the change in mutation frequency is concentrated in one age range, neither a simple linear nor exponential model of mutation increase was found to fit the data satisfactorily (see Supporting Text, which is published as supporting information on the PNAS web site). Variation between individuals appears greater for those donors >45 years. This finding could be a consequence of population variation in a trait that influences mutation rate in an age-dependent manner because of a genetic or environmental factor. Preliminary assays based on a slightly different detection methodology [using allele-specific ligation (24, 25) in the last assay step] showed similar age trends as in Fig. 2 (data not shown).
Fig 2.
Relationship between donor age and the frequency of the G1138A mutation in sperm DNA. Each small dash represents one donor. The count value is taken as the median of the mean values obtained in 3–6 independent experiments on each donor. The open red squares show the average G1138A frequency (along with the 95% C.I.) for all individuals within the following age intervals: <25, 5-year age intervals (beginning with 25–29 and ending with 60–64) and >64. The plot was created by drawing a line (gray) between adjacent average values for the different age intervals.
To rigorously compare the sperm G1138A mutation results with the observed birth data we first used the sperm mutation frequencies of all donors between 18 and 59 years (only a small fraction of achondroplasia births come from fathers >59 years) to predict the frequency of sporadic achondroplasia births that might be expected for fathers in this 41-year age range. This critical comparison must fully account for the variation in count estimates both within and between individuals. Fig. 2 shows the median count value for each individual as well as the sample mean and 95% C.I. for the population mean for the members of each age group. Note the small inter-individual variation in counts found within each of the eight relevant age groups (the difference between the upper and lower bounds of the 95% C.I. is less than 2-fold). The fact that each age group is composed of many individuals reduces the significance of intra-individual variation in the PCR assay.
Our null hypothesis is that the probability that a father has a child with sporadic achondroplasia is proportional to his sperm G1138A mutation frequency at his age at conception. This prediction assumes that the sperm mutation properties of our donors are indistinguishable from fathers of children with achondroplasia. The age distribution of all sperm donors 18–59 years old and their G1138A counts were used to compute the age distribution of sperm donors expected to father children with sporadic achondroplasia. This distribution was then compared with the actual age distribution by using the data on sporadic achondroplasia from the studies reviewed by Risch et al. (7).
As shown in Table 1, the observed number of achondroplasia births to fathers under 30 is smaller than would be predicted by the G1138A mutation frequency, whereas the observed number of births to fathers >35 is more than predicted. To test the null hypothesis we performed a generalized likelihood ratio test (see Methods). The P values from this test took into account the variation in counts within each age group (including that caused by intra-individual variation). To approximate the P value of the log likelihood ratio we created a bootstrap sample of mutant counts and observed births (10,000 iterations each) giving 10,000 values of the log likelihood ratio. The proportion of these values that exceeded the value obtained from the real data were used to estimate the P value. The calculated log likelihood ratio (43.6132 = >10−12) is highly significant. We reject the null hypothesis that the chance that a father has a child with sporadic achondroplasia is proportional to his sperm G1138A mutation frequency at the time of conception. The same analysis was carried out by using only the USC or only the LLNL sperm donor data (see Table 3, which is published as supporting information on the PNAS web site, for the cohort-specific age distributions). In each case the null hypothesis is also rejected (log likelihood ratio for USC = 36.7392 = 1.98 × 10−6 and log likelihood ratio for LLNL = 33.5521 = 8.20 × 10−6).
Table 1.
Age distribution of 152 fathers of sporadic achondroplasia cases and predicted age distribution based on G1138A mutation frequencies
| Age group | Actual achondroplasia births, % | Sperm-based prediction of achondroplasia births, % | Sperm-based prediction of achondroplasia births (%) corrected |
|---|---|---|---|
| 18–24 | 5.26 | 18.16 | 15.35 |
| 25–29 | 13.16 | 24.60 | 24.26 |
| 30–34 | 24.34 | 22.25 | 22.40 |
| 35–39 | 18.42 | 15.44 | 15.15 |
| 40–44 | 22.37 | 9.38 | 11.08 |
| 45–49 | 9.87 | 6.45 | 7.48 |
| 50–59 | 6.58 | 3.73 | 4.27 |
The corrected prediction substitutes counts <15 with zero counts for donors <40 years old and counts <15 with 15 counts for donors ≥40 years.
Discussion
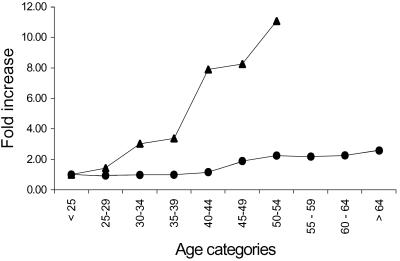
The lifelong spermatogonial stem cell replications have been suggested as an explanation for an increase in the frequency of mutant sperm that results in the increased incidence of sporadic achondroplasia with paternal age (1, 2, 4). A mathematical discrepancy between the cell replication model based on a linear equation to calculate the number of spermatogonial stem cell divisions (4, 26) and the exponential rise in sporadic achondroplasia with paternal age (1, 2, 4, 7, 10) has been pointed out (1, 2, 4, 7). Of course, other age-related mutation mechanisms that do not depend solely on premeiotic cell replications may be responsible for the birth data. However, no matter what the mutation mechanism, our direct measurement of the G1138A mutation frequency in sperm appears to rule out the idea that an age-dependent increase in sperm containing the GC-to-AT transition mutation at position 1138 explains the rapid rise in incidence of sporadic achondroplasia with paternal age (Fig. 3). Below, we discuss six possibilities that might account for this inconsistency.
Fathers of children with sporadic achondroplasia could form a subgroup with distinct mutation properties (because of genetic or environmental factors) compared with our sperm donors. To address this possibility, we studied sperm DNA from four men who fathered a child with achondroplasia. When matched for age to the appropriate sperm donor age group, two (ages 31 and 32) fell within the observed 95% C.I., a third (age 35) had counts that exceeded, by 2-fold, the upper bound of the 95% C.I., whereas the oldest father (age 51) had counts below the lower bound of the 95% C.I. Although the data suggest that fathers of sporadic cases are representative of our sperm donors, the sample size is small. Additional studies will be needed before we can exclude the possibility that population heterogeneity is the explanation for the discrepancy between our mutation data and the achondroplasia paternal age effect.
As yet unappreciated ascertainment biases in the population studies may have overestimated the magnitude of the age effect for the fathers of sporadic achondroplasia cases.
An age-related sperm donor sampling bias could underestimate an age trend in the G1138A frequency data although we can already exclude racial background as a source of bias between young and old donors in both study groups.
Our PCR assay is clearly biased toward overestimating the number of mutants in individuals with <15 counts (Fig. 1). Among the 118 sperm donors, 24 had <15 counts. To assess the impact of this bias, the age distribution of sperm donors expected to father children with sporadic achondroplasia was again compared with the actual age distribution after making a correction to the counts of all individuals with <15 G1138A mutants (Table 1). This correction favored the null hypothesis because it lowered the counts in the youngest age groups and increased the counts in the older age groups. Thus, for ages <40 we set counts <15 equal to 0, while for ages >40 we set counts <15 equal to 15. Despite this severe correction the null hypothesis was still rejected (log likelihood ratio = 20.7268 = 0.0033). The data from the USC and LLNL cohorts were also examined individually by using the same correction (see Table 3). Despite the lower sample size, the USC cohort gave a log likelihood ratio = 25.1466 (= 3.21 × 10−4). The LLNL cohort gave a log likelihood ratio = 10.6348 (= 0.100). Our rather extreme correction is exacerbated in the LLNL cohort because the 18- to 24-year-old category contains only six individuals and all but one of them have <15 counts.
Our results would be formally consistent with the null hypothesis if there was an age-dependent exponential increase in the formation of germ-line premutagenic lesions (16) at the G1138A site that are neither converted to a full mutation or repaired before fertilization (27). One obvious candidate for such a premutagenic lesion is an unrepaired G/T mismatch resulting from deamination of 5-methyl cytosine (16). The cytosine at base pair 1138 is highly methylated in sperm (data not shown and ref. 28). A single sperm with a G/T mismatch would produce PCR product in our assay. However, the observed counts from a population of such sperm would be half of that produced by the same number of sperm carrying A/T transition mutations. A second possible premutagenic lesion contains an apyrimidinic (AP) site on one strand caused by removal of a thymine at a GT mismatch by a glycosylase (16). Taq polymerase is known to pause significantly opposite an abasic site (29) and primers with an internal abasic site can be extended but the extension products are poorly copied during PCR (30). Also, exposure to high temperatures during PCR may lead to strand breaks at abasic sites. It is likely therefore that after the first two steps of our assay, the products from sperm containing a G/AP lesion would be far less than that from an equal number of mutant sperm carrying the AT bp leading to a significant underestimate of the frequency of this premutagenic lesion. Is there any evidence that premutagenic lesions in sperm can lead to achondroplasia? If unrepaired immediately after zygote formation, sperm carrying a G/T lesion would produce a mosaic embryo (+/+ and +/G1138A) after the first cell division. If a G/AP lesion is converted to a G/T or G/G mismatch after zygote formation the embryo can become mosaic for an G1138A or G1138C mutation, respectively, after the first cell division. The G1138C mutation is found in ≈2% of all sporadic achondroplasia cases (14). Because individuals with the achondroplasia phenotype that have been reported to be mosaic for the G1138A mutation are exceedingly rare (reviewed in ref. 31), cases of sporadic achondroplasia caused by the above repair patterns of premutagenic lesions are likely to be infrequent. On the other hand, sperm carrying a G/T premutagenic lesion could lead to achondroplasia if the G was replaced by an A immediately after zygote formation. The other immediate repair alternative would lead to a WT embryo. Virtually nothing is known about the relative likelihood of the repair alternatives before the first zygotic cell division in early mammalian embryos.
Finally, the discrepancy between the observed G1138A mutation frequency and the achondroplasia paternal age effect might be explained by selection. The G1138A mutation leads to an increased tyrosine kinase activity of FGFR3 protein and influences downstream signal transduction mediated by the Ras-mitogen-activated protein kinase-dependent and/or STAT1 signaling pathways, resulting in a variety of possible biological consequences (32). FGFR3 protein is found in all adult human male germ cells except elongating spermatids (33). The germ cells of the fetal, immature, and adult rat testis exhibit cell- and stage-specific localization of FGFs and FGFRs (including FGFR3 IIIc), which has been taken to imply that signaling via FGF ligands and receptors is spatially and temporally regulated in this organ (34). Although highly speculative, mature sperm derived from cells carrying the FGFR3 achondroplasia mutation may have a selective advantage for sperm motility or capacitation in utero. The molecular mechanisms involved in capacitation are not well known, but protein phosphorylation on tyrosine residues appears to be important (35) and relevant given FGFR3 function. To explain the paternal age effect, any selective advantage would have to increase with age perhaps in association with known changes that occur in the male reproductive system during normal aging (36).
Fig 3.
Age-dependent fold increase in the frequency of the G1138A mutations in sperm DNA (•) and sporadic achondroplasia cases (9, 11) (▴). Birth values are based on the observed-to-expected ratio of sporadic achondroplasia cases for fathers in different age groups (7). In each plot, the value for the youngest age interval is taken as 1 and divided into the values for the remaining age intervals to calculate the fold change. The plot was constructed by drawing a line between adjacent points.
All population studies of achondroplasia have documented a paternal age effect (1, 2, 4, 7, 9–11). The most recent detailed statistical analysis of sporadic achondroplasia (7) confirmed the paternal age effect but could not eliminate an additional influence of maternal age. An effect of the mother's age is unexpected because it has been proven that the mutations originate in the father's germ line (17). A maternal influence could be explained by selection. Embryos carrying G1138A might have an advantage for implantation in older mothers, perhaps in response to age-related changes in hormone levels. Examination of the pregnancy records of unaffected women married to affected men might be used to test this idea.
A particularly striking parallel to the achondroplasia mutation is found in Apert syndrome. This dominantly inherited condition is caused by either one of two specific nucleotide substitutions in the FGFR2 gene that arise almost exclusively in males by de novo mutation. The magnitude of the Apert syndrome paternal age effect is almost the same as achondroplasia (1, 2, 7, 37–39).
Apert syndrome and achondroplasia share an unusual feature that is independent of any paternal age effect. Based on the population incidence of sporadic cases (10, 18–20, 38), both conditions have exceptionally high mutation frequencies (1/15,000–1/70,000) when compared with what is known about other single nucleotide substitutions at CpG sites (40). This high rate led to the original idea of a DNA mutation hot spot (14, 15, 37, 41). Selection for cells carrying these mutations during the germ-line mitotic divisions was also mentioned as an alternative possibility for the high mutation frequencies (41, 42). Indirect support for selection comes from contrasting the virtually exclusive male origin of the de novo Apert syndrome and achondroplasia mutations with the relatively small (1.7- to 5-fold) male bias in nucleotide substitutions seen in neutral regions during primate evolution (3, 8).
The particular biochemical functions of FGFR genes make it easier to understand how certain mutations might provide a selective advantage in fertilization, implantation, or mitotic germ-line divisions. Some de novo gain-of-function mutations affecting other biochemical pathways might also provide a selective advantage under similar circumstances although these might be missed if selection did not vary with either parental age or sex.
Supplementary Material
Acknowledgments
We acknowledge helpful discussions with Russ Higuchi, Bob Watson, Jeff Strathern, Gary Bellus, and Jim Crow and the assistance of Sharon Kidd (donor recruitment) and Eddie Sloter (preparation and storage) for the University of California, Berkeley/LLNL sperm samples.
Abbreviations
FGFR, fibroblast growth factor receptor
USC, University of Southern California
LLNL, Lawrence Livermore National Laboratory
kt-PCR, kinetic PCR
C.I., confidence interval
References
- 1.Vogel F. & Rathenberg, R. (1975) Adv. Hum. Genet. 5, 223-318. [DOI] [PubMed] [Google Scholar]
- 2.Vogel F. & Motulsky, A. G., (1997) Human Genetics: Problems and Approaches (Springer, Berlin).
- 3.Bohossian H. B., Skaletsky, H. & Page, D. C. (2000) Nature 406, 622-625. [DOI] [PubMed] [Google Scholar]
- 4.Crow J. F. (2000) Nat. Rev. Genet. 1, 40-47. [DOI] [PubMed] [Google Scholar]
- 5.Huttley G. A., Jakobsen, I. B., Wilson, S. R. & Easteal, S. (2000) Mol. Biol. Evol. 17, 929-937. [DOI] [PubMed] [Google Scholar]
- 6.Hurst L. D. & Ellegren, H. (1998) Trends Genet. 14, 446-452. [DOI] [PubMed] [Google Scholar]
- 7.Risch N., Reich, E. W., Wishnick, M. M. & McCarthy, J. G. (1987) Am. J. Hum. Genet. 41, 218-248. [PMC free article] [PubMed] [Google Scholar]
- 8.Makova K. D. & Li, W. H. (2002) Nature 416, 624-626. [DOI] [PubMed] [Google Scholar]
- 9.Murdoch J. L., Walker, B. A., Hall, J. G., Abbey, H., Smith, K. K. & McKusick, V. A. (1970) Ann. Hum. Genet. 33, 227-244. [DOI] [PubMed] [Google Scholar]
- 10.Orioli I. M., Castilla, E. E., Scarano, G. & Mastroiacovo, P. (1995) Am. J. Med. Genet. 59, 209-217. [DOI] [PubMed] [Google Scholar]
- 11.Stevenson A. C. (1957) Am. J. Hum. Genet. 9, 81-91. [PMC free article] [PubMed] [Google Scholar]
- 12.Walter C. A., Intano, G. W., McCarrey, J. R., McMahan, C. A. & Walter, R. B. (1998) Proc. Natl. Acad. Sci. USA 95, 10015-10019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rousseau F., Bonaventure, J., Legeai-Mallet, L., Pelet, A., Rozet, J. M., Maroteaux, P., Le Merrer, M. & Munnich, A. (1996) Horm. Res. 45, 108-110. [DOI] [PubMed] [Google Scholar]
- 14.Bellus G. A., Hefferon, T. W., Ortiz de Luna, R. I., Hecht, J. T., Horton, W. A., Machado, M., Kaitila, I., McIntosh, I. & Francomano, C. A. (1995) Am. J. Hum. Genet. 56, 368-373. [PMC free article] [PubMed] [Google Scholar]
- 15.Shiang R., Thompson, L. M., Zhu, Y. Z., Church, D. M., Fielder, T. J., Bocian, M., Winokur, S. T. & Wasmuth, J. J. (1994) Cell 78, 335-342. [DOI] [PubMed] [Google Scholar]
- 16.Friedberg E., Walker, G. & Siede, W., (1995) DNA Repair and Mutagenesis (Am. Soc. Microbiol. Press, Washington, DC).
- 17.Wilkin D. J., Szabo, J. K., Cameron, R., Henderson, S., Bellus, G. A., Mack, M. L., Kaitila, I., Loughlin, J., Munnich, A., Sykes, B., et al. (1998) Am. J. Hum. Genet. 63, 711-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camera G. & Mastroiacovo, P. (1988) Basic Life Sci. 48, 11-15. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Frias M. L., Bermejo, E., Cereijo, A., Sanchez, M., Lopez, M. & Gonzalo, C. (1991) Am. J. Med. Genet. 38, 626-629. [DOI] [PubMed] [Google Scholar]
- 20.Stoll C., Dott, B., Roth, M. P. & Alembik, Y. (1989) Clin. Genet. 35, 88-92. [DOI] [PubMed] [Google Scholar]
- 21.Higuchi R. & Watson, R. (1999) in PCR Methods Manual, eds. Innis, M. A., Gelfand, D. H. & Sninsky, J. J. (Academic, San Diego), pp. 263–284.
- 22.Germer S., Holland, M. J. & Higuchi, R. (2000) Genome Res. 10, 258-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cupples C. G. & Miller, J. H. (1989) Proc. Natl. Acad. Sci. USA 86, 5345-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barany F. & Gelfand, D. H. (1991) Gene 109, 1-11. [DOI] [PubMed] [Google Scholar]
- 25.Landegren U., Kaiser, R., Sanders, J. & Hood, L. (1988) Science 241, 1077-1080. [DOI] [PubMed] [Google Scholar]
- 26.Drost J. B. & Lee, W. R. (1995) Environ. Mol. Mutagen. 25, 48-64. [DOI] [PubMed] [Google Scholar]
- 27.Favor J. (1999) Mutat. Res. 428, 227-236. [DOI] [PubMed] [Google Scholar]
- 28.El-Maarri O., Olek, A., Balaban, B., Montag, M., van der Ven, H., Urman, B., Olek, K., Caglayan, S. H., Walter, J. & Oldenburg, J. (1998) Am. J. Hum. Genet. 63, 1001-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel P. H., Kawate, H., Adman, E., Ashbach, M. & Loeb, L. A. (2001) J. Biol. Chem. 276, 5044-5051. [DOI] [PubMed] [Google Scholar]
- 30.Stump M. D., Cherry, J. L. & Weiss, R. B. (1999) Nucleic Acids Res. 27, 4642-4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henderson S., Sillence, D., Loughlin, J., Bennetts, B. & Sykes, B. (2000) J. Med. Genet. 37, 956-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kannan K. & Givol, D. (2000) IUBMB Life 49, 197-205. [DOI] [PubMed] [Google Scholar]
- 33.Steger K., Tetens, F., Seitz, J., Grothe, C. & Bergmann, M. (1998) Histochem. Cell Biol. 110, 57-62. [DOI] [PubMed] [Google Scholar]
- 34.Cancilla B., Davies, A., Ford-Perriss, M. & Risbridger, G. P. (2000) J. Endocrinol. 164, 149-159. [DOI] [PubMed] [Google Scholar]
- 35.Jaiswal B. & Eisenbach, M. (2002) in Fertilization, ed. Hardy, D. M. (Academic, San Diego), pp. 57–117.
- 36.Hermann M., Untergasser, G., Rumpold, H. & Berger, P. (2000) Exp. Gerontol. 35, 1267-1279. [DOI] [PubMed] [Google Scholar]
- 37.Moloney D. M., Slaney, S. F., Oldridge, M., Wall, S. A., Sahlin, P., Stenman, G. & Wilkie, A. O. (1996) Nat. Genet. 13, 48-53. [DOI] [PubMed] [Google Scholar]
- 38.Wilkie A. O., Slaney, S. F., Oldridge, M., Poole, M. D., Ashworth, G. J., Hockley, A. D., Hayward, R. D., David, D. J., Pulleyn, L. J., Rutland, P., et al. (1995) Nat. Genet. 9, 165-172. [DOI] [PubMed] [Google Scholar]
- 39.Erickson J. D. & Cohen, M. M., Jr. (1974) Ann. Hum. Genet. 38, 89-96. [DOI] [PubMed] [Google Scholar]
- 40.Nachman M. W. & Crowell, S. L. (2000) Genetics 156, 297-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilkie A. O. (1997) Hum. Mol. Genet. 6, 1647-1656. [DOI] [PubMed] [Google Scholar]
- 42.Oldridge M., Lunt, P. W., Zackai, E. H., McDonald-McGinn, D. M., Muenke, M., Moloney, D. M., Twigg, S. R., Heath, J. K., Howard, T. D., Hoganson, G., et al. (1997) Hum. Mol. Genet. 6, 137-143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.