Abstract
Lipid rafts are important signaling platforms in T cells. Little is known about their properties in human CD8+ T cells. We studied polarization of lipid rafts by digital immunofluorescence microscopy in primary human T cells, using beads coated with anti-CD3 and anti-CD28 mAbs (CD3/28 beads). Unlike CD4+ T cells, CD8+ T cells did not polarize lipid rafts when stimulated with CD3/28 beads, when the anti-CD28 antibody was substituted with B7.2Ig, or if an anti-CD8 antibody was added to the CD3/28 beads. This phenomenon was also observed in human antigen-specific CD8+ T cells. On stimulation with CD3/28 beads, the T cell antigen receptor clustered at the cell/bead contact area in both CD4+ and CD8+ T cells. Examination of lipid rafts isolated by sucrose density gradient centrifugation revealed the constitutive expression of p56Lck in the raft fractions of unstimulated CD8+ T cells, whereas p56Lck was recruited to the raft fraction of CD4+ T cells only after stimulation with CD3/28 beads. Stimulation with CD3/28 beads induced marked calcium flux, recruitment of PKC-θ and F-actin to the cell/bead contact site, and similar proliferation patterns in CD4+ and CD8+ T cells. Thus, polarization of lipid rafts is not essential for early signal transduction events or proliferation of human CD8+ lymphocytes. It is possible that the lower stringency of CD8+ T cell activation obviates a requirement for raft polarization.
During T cell activation, the information exchange crucial for initiation of signal transduction events takes place in the immune synapse, a specialized contact area between T cell and antigen-presenting cell (APC). Receptor molecules segregate into supramolecular activation clusters (SMACs) (1), and lipid rafts recruit signal transduction molecules (2), transfer to the site of T cell antigen receptor (TCR) engagement (3), and facilitate lymphocyte signaling (4). During formation of the immune synapse, PKC-θ is integrated into both SMACs (1) and lipid rafts (5), indicating that the function of SMACs and membrane rafts might be overlapping. The polarization of lipid rafts is thought to be essential for T cell activation, as the dispersion of lipid-rich microdomains inhibits T cell activation (4). Most studies on synapse formation and lipid rafts have been performed in CD4+ T cells. Only recently was the formation of SMACs shown in CD8+ T cells (6).
Although CD4 and CD8 act as coreceptors during T cell activation, there are marked differences between CD4+ and CD8+ T cells. The affinity of CD8 for the MHC/TCR complex is much higher than that of CD4, leading to a more stable interaction of MHC class I and the TCR (7). Furthermore, the number of engaged TCRs required for activation of a CD4+ T cell is 200–300 receptors (8, 9), whereas CD8+ T cells can be activated by a single MHC-peptide molecule (10). CTLs also require only a brief period of antigenic stimulation (11), and do not require costimulation for proliferation and differentiation into cytotoxic effector cells (12).
We studied the polarization of lipid rafts in human CD8+ T cells by using beads as artificial antigen-presenting cells and show that, unlike CD4+ T cells, CD8+ T cells do not require the polarization of lipid rafts for early signal transduction pathways or for proliferation.
Methods
Cell Purification and Activation.
Peripheral blood lymphocytes were obtained from normal donors by leukopheresis and elutriation, and CD8+ T cells or CD4+ T cells were purified by negative selection as described (13). For purification of the CD8+ T cells, the OKT4 mAb (American Type Culture Collection) was substituted for the OKT8 mAb. To verify the purity of the T cell preparations, cells were stained with antibodies to CD3, CD8, CD4, CD16, CD56, and CD19 (BD PharMingen). Initial T cell preparations were >94% CD3+ CD8+ or CD3+ CD4+, respectively. For generation of phytohemagglutinin (PHA)-blasts, CD4+ or CD8+ T cells were cultured in RPMI 1640 (BioWhittaker) supplemented with 5% FBS, 20 mM l-glutamine, 100 units/ml penicillin and streptomycin (all from Sigma), and 20% HL-1 (BioWhittaker), and activated with 1 μg/ml PHA (Murex/Abbott, Abbott Park, IL) for 2 days. Long-term activated T cells were recultured in RPMI 1640 after the initial 2-day activation with PHA in the presence of 0.1 μg/ml PHA and 20 units/ml human IL-2 (Hoffman–La Roche). Human influenza (FLU)-specific CD8+ T cells were obtained as described (14). The cells were studied at day 43 of culture. Epstein–Barr virus (EBV)-transformed B cells from the same donor were pulsed overnight with FLU peptide and used as APCs.
Immunofluorescence Staining and Microscopy.
Cells were washed once with serum-free RPMI 1640 and incubated with Dynal Epoxy beads (Dynal Biotech, Lake Success, NY) that had been coated with anti-CD3 (OKT3; ref. 15) and anti-CD28 (9.3; ref. 16) mAbs or with anti-CD3 mAb and B7.2Ig (a generous gift of Beatriz Carreno, Genetics Institute, Boston) at a 1:3 bead to cell ratio for various times at 37°C. The cell/bead conjugates were then settled onto poly-l-lysine-coated coverslips, fixed in 3.7% formaldehyde and stained. For GM1 labeling, cholera toxin B-FITC (Sigma) or a polyclonal rabbit anti-GM1 antiserum (Matreya, State College, PA) was used. When staining with anti-GM1 antiserum, the coverslips were blocked with 5% normal donkey serum and then stained with donkey anti-rabbit Cy3 (both from Jackson ImmunoResearch). Staining for the TCR was performed using a TCR α/β antibody conjugated with FITC (Accurate Chemicals, West Point, NY). Staining for PKC-θ and F-actin was performed after permeabilization of the cells with 0.1% Triton X-100 (Sigma) for 10 min by using a polyclonal anti-PKC-θ (Santa Cruz Biotechnology) and phalloidin-FITC (Sigma), followed by donkey anti-goat Cy3 (Jackson ImmunoResearch). For the experiments with FLU-specific T cells, the cells were incubated with biotinylated CTB for 30 min at 37°C, then mixed with APCs and settled on coverslips. After fixation with 3.7% formaldehyde, the conjugates were stained with Phalloidin-FITC and Streptavidin Cy-3 as described above. After staining, the coverslips were mounted onto microscope glass slides (Fisher) with GelMount (Biomeda, Hayward, CA) and stored at 4°C until analysis.
Immunofluorescence and corresponding Nomarski images of the cells and cell/bead conjugates were recorded by a digital fluorescence microscopy system (Intelligent Imaging Innovations, Denver) consisting of a Zeiss Axioplan microscope fitted with a Xenon light source and a Sensicam CCD camera (Cooke, Auburn Hills, MI). slidebook software (Intelligent Imaging Innovations) was used for image analysis and 3D volume rendering. A constrained iterative deconvolution algorithm was used to remove out-of-focus haze. Within each experiment, all images were renormalized to the same range of intensity.
Sucrose Density Gradient Centrifugation and Western Blotting.
The isolation of lipid rafts by using sucrose density gradient centrifugation was performed as described (17). Briefly, 60 million CD4+ or CD8+ T cells were incubated with uncoated beads or CD3/28 beads, followed by lysis at 4°C in Mes-buffered saline containing 1% Triton X-100 and protease and phosphatase inhibitors. The lysates were then mixed with 80% sucrose in Mes-buffered saline, transferred to ultracentrifuge tubes and overlaid with 30% and 5% sucrose solutions, respectively. The Triton-insoluble fractions were separated from the cell lysates by ultracentrifugation for 18 h. Fractions (350 μl) were then removed sequentially starting from the top of the gradient. Thirty microliters of fractions 1–6, 8, 10, and 12 were subjected to 10% SDS/PAGE, followed by transfer to nitrocellulose by using anti-LAT (Upstate Biotechnology, Lake Placid, NY) or anti-p56LCK (Zymed) and HRPO-conjugated secondary antibodies (Jackson ImmunoResearch). Protein bands were detected using Super Signal (Pierce).
Recording of Calcium Flux.
Cells were labeled with the calcium-sensitive dye fura-2 (Molecular Probes) and settled onto cell culture dishes at 37°C for 20 min. The dishes were then transferred to a Zeiss Axioplan microscope equipped with a heated stage maintaining the cell culture dish temperature at 37°C throughout the experiments. Fluorescence emissions at 340 and 380 nm were recorded using a 63× water immersion lens. Image recording and analysis was performed using slidebook software (Intelligent Imaging Innovations). For activation of the cells, CD3/28-coated beads were added to the culture dish at t = 0 s, and pictures were recorded at regular intervals to capture calcium flux. As a positive control, ionomycin (Molecular Probes) at 1 μg/ml was added to the microscope dish at the end of the experiment.
Proliferation Assay.
Cells were labeled with CFSE (Molecular Probes) and either cultured in the presence of CD3/28 beads or left unstimulated for 3 days in the dark. The cells were then analyzed by flow cytometry using a FACScalibur flow cytometer (Becton Dickinson).
Results
CD8+ T Cells Do Not Polarize Lipid Rafts at the Site of TCR Engagement.
Before studying lipid raft polarization in CD8+ T cells, we confirmed by digital immunofluorescence microscopy and flow cytometry that CD4+ and CD8+ T cells express similar amounts of the ganglioside GM1, a marker for lipid rafts (see Fig. 10, which is published as supporting information on the PNAS web site, www.pnas.org).
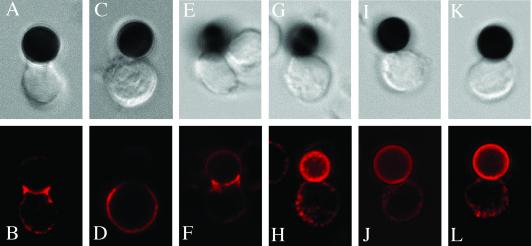
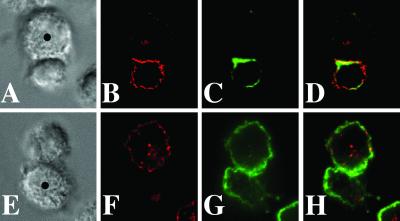
We then assayed raft polarization to the site of contact by stimulating primary human T cells with CD3/28 beads (16). Unlike in CD4+ T cells (Fig. 1B), no raft polarization could be observed in the CD8+ T cells after 30 min (Fig. 1D). The polarization of lipid rafts in CD4+ T cells depended on the amount of anti-CD3 antibody present on the beads. Beads with a ratio of anti-CD3 to anti-CD28 of 50:50 (Fig. 1B) and 15:85 (Fig. 1F) induced lipid raft polarization to the cell/bead interface, whereas beads with a ratio of 5:95 did not (Fig. 1J). Lipid raft polarization was also tested at additional time points (5, 15, and 40 min) and was observed in CD4+ T cells but not in CD8+ T cells (see Fig. 11, which is published as supporting information on the PNAS web site).
Fig 1.
Primary CD8+ T cells do not polarize lipid rafts at the site of TCR engagement. CD4+ (B, F, and J) or CD8+ (D, H, and L) T cells were incubated with CD3/28 beads for 30 min and stained with anti-GM1 antiserum. The corresponding Nomarski images are shown in the top row. In E–H, cells were incubated with beads coated with a ratio of anti-CD3 to anti-CD28 of 15:85. In I–L, the ratio was 5:95. The data are representative of three experiments with different human donors.
It has been shown (19) that T cell activation induces the expression of GM1 on the plasma membrane of CD4+ T cells. To determine whether lipid raft polarization is restored in preactivated CD8+ T cells, we incubated the cells for 2 days with PHA and then analyzed CD3/28-induced lipid rafting. There was marked polarization of GM1 at the cell/bead contact site in CD4+ T cells, but there was no polarization of lipid rafts noted in PHA-activated CD8+ T cells (see Fig. 12, which is published as supporting information on the PNAS web site).
Previous studies have used CTB as a marker for lipid rafts (20, 21). In Fig. 2, we show that when using either CTB (Fig. 2 C and F) or anti-GM1 antiserum (Fig. 2 B and E), lipid raft polarization is detected in CD4+ T cells (Fig. 2 A–C), but not in CD8+ T cells (Fig. 2 D and E). For this experiment, cells were activated for 2 days with PHA alone and were then cultured for an additional 5 days in the presence of PHA and IL-2. CD8+ T cells also did not polarize lipid rafts when they were incubated with CD3/28 beads that had also been coated with anti-CD8 mAb (data not shown), indicating that the failure of CD8+ T cells to polarize lipid rafts was not due to absent costimulation through CD8.
Fig 2.
Long-term activated CD8+ T cells do not polarize lipid rafts. CD4+ (A–C) or CD8+ (D–F) T cells that had been activated with PHA and IL-2 for 7 days were incubated with CD3/28 beads for 20 min and stained with anti-GM1 antiserum (red, B and E) and cholera toxin B-FITC (green, C and F). Corresponding Nomarski images are shown in A and D. Data are representative of three different experiments.
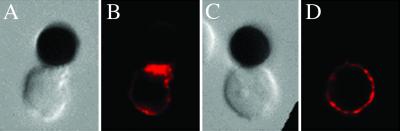
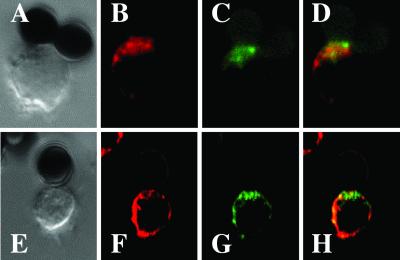
Lipid raft polarization was also not restored in PHA-activated CD8+ T cells when the anti-CD28 mAb on the beads was replaced by B7.2Ig, the natural ligand for CD28. Fig. 3B shows marked polarization of GM1 to the contact site in a CD4+ T cell stimulated with an anti-CD3/B7.2Ig-coated bead, whereas there was no polarization in a CD8+ T cell (Fig. 3D). In summary, lipid rafts do not polarize in primary or in vitro activated human CD8+ T cells.
Fig 3.
Replacement of the CD28 antibody with B7.2Ig on the beads does not reconstitute the polarization of lipid rafts in CD8+ T cells. CD4+ (A and B) or CD8+ (C and D) T cells that had been activated with PHA for 2 days were incubated with CD3/B7.2Ig beads for 30 min and stained with anti-GM1 antiserum. Corresponding Nomarski images are shown in A and C. The pictures are representative of four different experiments.
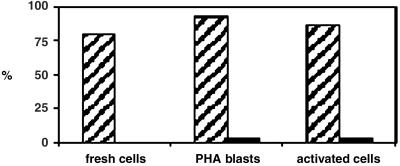
The above results indicate a striking difference between CD4+ and CD8+ T cells in the response to TCR ligation. To determine whether the differences were consistent, we screened thirty cell/bead conjugates for each activation condition; the results are summarized in Fig. 4. In primary cells, 80% of the conjugates with CD4+ T cells showed aggregation of GM1, whereas no conjugates with CD8+ T cells exhibited this phenotype. In cells activated with PHA, 93% of conjugates with CD4+ T cells, but only 3% of conjugates with CD8+ T cells, showed aggregation of GM1. This distribution was similar in PHA/IL-2-activated cells, with 87% for CD4+ and 3% for CD8+ conjugates.
Fig 4.
Quantitative analysis of cell/bead conjugates showing aggregation of GM1. For both CD4+ and CD8+ cells, 30 conjugates each were scored in primary cells, PHA blasts, and long-term activated cells after stimulation with CD3/28 beads for 30 min. The numbers indicate the percentage of conjugates scored that exhibit aggregation of GM1 at the cell/bead contact site. Data are compiled from three different experiments for each cell activation condition.
Human Antigen-Specific CD8+ T Cells Do Not Polarize Lipid Rafts at the Site of TCR Engagement.
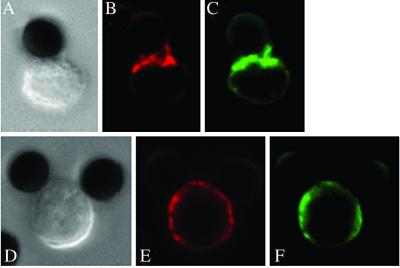
To study lipid raft polarization in a human antigen-specific system, we used FLU-specific CD8+ T cells (14) together with EBV-transformed B cells as APCs. Although FLU-specific CD8+ T cells do not polarize lipid rafts (Fig. 5B), they recruit F-actin to the APC/T cell contact area (Fig. 5C). When the APCs were not pulsed with FLU peptide, there was no accumulation of F-actin at the APC/T cell interface (Fig. 5G). We could visualize the polarization of lipid rafts in human FLU-specific CD4+ T cells by using tetramer technology in a cell/bead system (see Fig. 13, which is published as supporting information on the PNAS web site). These experiments extend our observations beyond a system of antibody-coated beads and confirm that the polarization of lipid rafts in CD8+ T cells is also absent in the context of antigen.
Fig 5.
Human antigen-specific CD8+ T cells do not polarize lipid rafts to the APC-T cell contact area, but recruit F-actin to the immunological synapse. FLU-specific CD8+ T cells were prelabeled with CTB-Bio and incubated with EBV-transformed B cells as APCs (•) for 30 min. In A–D, APCs were pulsed with FLU peptide overnight, and in E–H, APCs remained nonpulsed. CTB stain is shown in red (B and F) and F-actin stain is shown in green (C and G). (D and H) The overlay of both stains. Corresponding Nomarski images are shown in A and E. The pictures are representative of two different experiments. Ten conjugates were examined for each condition. We observed recruitment of F-actin to the immunological synapse in 8 of 10 conjugates.
TCRs Are Clustered at the Cell/Bead Interface in both CD4+ and CD8+ T Cells.
To study the fate of the TCR in our experimental model, we stimulated primary human T cells with CD3/28 beads and performed double staining for the TCR and GM1 (Fig. 6). As previously shown, unlike in CD4+ T cells (Fig. 6B), no raft polarization could be observed in the CD8+ T cells after 30 min (Fig. 6F). However, there was accumulation of TCRs at the cell/bead interface in both CD4+ (Fig. 6C) and CD8+ T cells (Fig. 6G). This finding indicates that in human CD8+ T cells the recruitment of TCRs to the immune synapse is independent of lipid raft polarization, and that receptors outside the lipid rafts can be successfully recruited to the cell/bead interface.
Fig 6.
Primary CD8+ T cells recruit the TCR to the cell–bead contact area. CD4+ (B–D) or CD8+ (F–H) T cells were incubated with CD3/28 beads for 30 min and stained with anti-GM1 antiserum (red, B and F) or anti-TCR antibody (green, C and G). (D and H) The overlay of both stains. The corresponding Nomarski images are shown in A and E. The pictures are representative of three different experiments.
p56Lck Is Constitutively Expressed in Lipid Rafts from CD8+ T Cells.
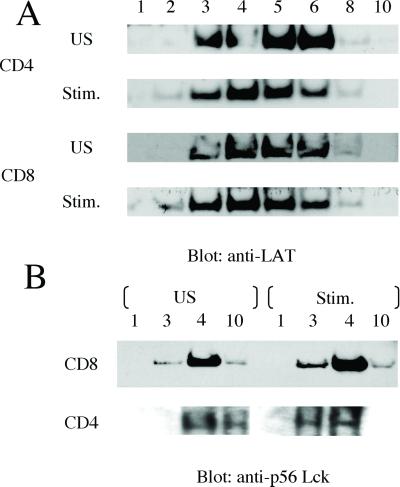
We examined lipid raft composition in CD4+ and CD8+ T cells by using sucrose density gradient centrifugation and first identified the gradient fractions containing lipid rafts by Western blotting and staining for LAT (Fig. 7A). LAT is known to exclusively partition to lipid rafts (17). LAT was present in fractions 3–6 in CD4+, as well as CD8+ T cells (Fig. 7A). The distribution of LAT was similar in unstimulated and stimulated T cells.
Fig 7.
Identification of raft fractions and targeting of p56Lck to lipid rafts in human CD4+ and CD8+ T cells. Primary human T cells were either unstimulated (US) or stimulated with CD3/28 beads for 10 min, followed by lysis in Mes lysis buffer plus protease and phosphatase inhibitors. Lysates were subjected to sucrose density gradient centrifugation for lipid raft purification. Numbers denote gradient fractions. The gradient fractions were separated by SDS/PAGE , followed by detection of LAT (A) and Lck (B) with immunoblot analysis.
It has been suggested that the partitioning of Lck to lipid rafts is essential for successful activation and propagation of TCR signal transduction (22). We performed Western blotting for Lck in sucrose gradients from CD4+ and CD8+ T cells. In CD8+ T cells, Lck was present in lipid raft fractions 3 and 4 in unstimulated as well as stimulated cells (Fig. 7B), and the pattern of distribution was similar. In unstimulated CD4+ T cells, however, the majority of Lck was present in fractions 10 (nonraft) and 4 (raft), but not fractions 1 or 3. On stimulation of the CD4+ T cells, the Lck distribution shifted to raft fractions 3 and 4 (Fig. 7B). These results indicate that the composition of lipid rafts in CD8+ T cells is different from that in CD4+ T cells. The fact that lipid rafts from CD8+ T cells already contain important signal transduction molecules before activation of the cells might explain why CD8+ T cells do not polarize lipid rafts to the cell/bead or cell/cell contact site.
Early Signal Transduction Events Are Intact in CD8+ T Cells Activated with CD3/28 Beads.
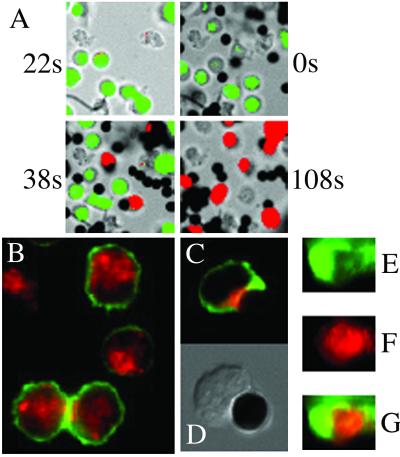
To study early activation events in our experimental system, CD4+ and CD8+ T cells were loaded with fura-2, and CD3/28-induced calcium flux was measured by using real-time imaging. Fig. 8A shows representative pictures from an experiment with CD8+ T cells. The four panels show a time course of the CD8+ T cells stimulated with the CD3/28 beads. The beads were added to the cell culture at t = 0 s. At 38 s, the cells in contact with beads show an increased intracellular calcium concentration (indicated by red), whereas the cells not in contact with beads remain in their resting state (indicated by blue or green). Sixty percent of all cells imaged showed calcium flux; 80% of the cells in contact with CD3/28 beads showed increased intracellular calcium concentration. On addition of ionomycin (t = 108 s), all cells imaged exhibit an increased intracellular calcium concentration. Incubation of the cells with uncoated beads did not induce calcium flux (data not shown). Activation of CD4+ T cells with the CD3/28 beads showed similar results (data not shown).
Fig 8.
Early signal transduction events are intact in CD8+ T cells activated with CD3/28 beads. (A) CD8+ T cells show intracellular calcium flux on stimulation with CD3/28 beads. Each picture shows the composition of a bright-field image overlaid with a transparent color scale of the ratio of fluorescence emissions at 340 and 380 nm of fura-2. The ratio correlates with intracellular calcium concentration: green, resting (low); red, high intracellular calcium concentration. The picture taken at t = −22 s shows CD8+ T cells in the resting state. At t = 0 s, the CD3/28 beads were added. At 108 s, ionomycin was added as a positive control. These data are representative of three experiments. (B–G) PKC-θ and F-actin are recruited to the cell/bead contact site in CD8+ T cells. In resting T cells (B), PKC-θ (in red) is distributed in the cytoplasm and F-actin (in green) is distributed evenly in the plasma membrane. After activation of the CD8+ T cells for 30 min with CD3/28 beads, PKC-θ and F-actin are recruited to the cell/bead contact site (C). (D) The Nomarski image of the cell/bead conjugate shown in C. Three-dimensional volume rendering shows distribution of F-actin in the peripheral SMAC (E) and PKC-θ in the central SMAC (F). (G) The overlay of pictures D and E. The data are representative of three different experiments.
It has recently been shown that PKC-θ (5) and F-actin (20) are recruited to the immune synapse during T cell activation. We asked whether this could also be demonstrated in our experimental system. In unstimulated CD8+ T cells, PKC-θ is localized throughout the cytoplasm, and F-actin is distributed evenly at the plasma membrane (Fig. 8B). On activation of CD8+ T cells with CD3/28 beads, there is recruitment of PKC-θ and F-actin to the cell/bead contact site (Fig. 8C). PKC-θ accumulates at the center of the bead/cell contact site (Fig. 8 C and F), whereas F-actin is located in a more peripheral region (Fig. 8 C and E). Similar results were obtained with CD4+ T cells (data not shown). The distribution of F-actin seen in our experiments is similar to that observed by Bunnell et al. (23) in a real-time imaging system using T cells plated onto anti-TCR-coated coverslips. Thus, although CD8+ T cells fail to exhibit lipid raft polarization at the cell/bead contact site, early signal transduction pathways are intact.
CD4+ and CD8+ T Cells Proliferate at a Similar Rate When Activated with CD3/28 Beads.
To compare the efficiency of cell cycle activation of the T cell subsets, primary CD4+ and CD8+ T cells were labeled with CFSE, incubated with CD3/28 beads for 3 days, and analyzed by flow cytometry. As shown in Fig. 9, CD4+ T cells (Fig. 9A) and CD8+ T cells (Fig. 9B) exhibit a similar rate of cell division. In some experiments, the progression through several rounds of the cell cycle was actually enhanced in CD8+ T cells, as more CD8+ T cells had undergone three cell divisions compared with CD4+ T cells. These data indicate that, although CD8+ T cells do not polarize lipid rafts on anti-CD3/anti-CD28 engagement, they proliferate in response to the same stimulus. Therefore, lipid raft polarization is not essential for the proliferation of CD8+ T cells.
Fig 9.
CD8+ and CD4+ T cells show a similar proliferation profile after activation with CD3/28 beads. CD4+ (A) and CD8+ (B) T cells were labeled with CFSE, activated with CD3/28 beads for 3 days, and analyzed by flow cytometry. Unstimulated cells that were labeled with CFSE were used as controls. Data are representative of two different experiments.
Discussion
We show here that human CD8+ T cells do not require the polarization of lipid rafts for activation or proliferation. Our experimental model employs an artificial antigen-presenting cell to mimic the signals provided by Ag/APC activation. This bead system has been previously used for rafting studies in human T cells (3). The polarization of lipid rafts to the cell/bead contact site was not observed in primary CD8+ T cells. This observation was consistent, as it was also extended to short-term PHA-activated cells and to CD8+ T cells cultured long-term in the presence of PHA and IL-2. Importantly, this observation was confirmed in an experimental system using human antigen-specific CD8+ T cells. Our observation is intriguing in that it has been postulated to date that lipid raft formation is essential for immune cell activation (4, 23–25). However, many of these experiments used the method of cholesterol depletion with methyl-β-cyclodextrin (MCD) to study the effect of raft disruption on cellular activation. We studied the polarization of lipid rafts in the presence of MCD and found that this compound severely affected T cell morphology and rapidly induced cell death at concentrations that affected lipid raft polarization (data not shown). Thus, MCD might have other effects besides disruption of lipid rafts that could impact the proper functioning of signal transduction pathways in these cells.
Our data support the thesis that the activation requirements of CD8+ T cells are different from those of CD4+ T cells. It seems that CD8+ T cells are more efficient and require fewer cell resources for activation. This has been attributed to the fact that the affinity of CD8 for the MHC/TCR complex is much higher than that of CD4 (7). Potter et al. (6) have shown that a mutation in MHC class I, which affects the binding of CD8, ablated the ability of APCs to form conjugates with CD8+ T cells, underlining the importance of CD8 in formation of the immune synapse.
CD8+ T cells also require as few as one MHC-peptide molecule for activation (10), whereas in CD4+ T cells, many more TCR receptors must be triggered for activation. It is thought that, on activation of T cells, lipid microdomains in the cell membrane form larger lipid rafts (24). Apart from this change in size, lipid rafts also recruit the TCR and essential signaling molecules (2, 26–29). We evaluated the composition of lipid rafts in CD8+ T cells by using sucrose density gradient centrifugation and Western blot analysis and showed that, unlike in CD4+ T cells, Lck is constitutively expressed in the raft fractions of CD8+ T cells. This may further contribute to the higher “efficiency” of CD8+ T cells. Furthermore, these cells may only require a small number of these signaling molecules at the site of activation and therefore do not aggregate lipid microdomains into large rafts. In contrast, CD4+ T cells, which require triggering of a larger number of TCRs for activation, might require the presence of a larger number of signaling molecules at the contact site. In this case, lipid rafts might serve as shuttle vessels and would help CD4+ T cells overcome the activation threshold by concentrating the requisite number of TCRs and signaling molecules. The results of a recent study published by Arcaro et al. (30) are in concert with this model. The authors elegantly show that CD8β, which has a high affinity for Lck, is partitioned in lipid rafts through palmitoylation. CD8β also mediates the constitutive association of CD8 with TCR/CD3, thus priming the CD8+ T cells for efficient activation. These authors did not study the composition of lipid rafts in CD8+ and CD4+ T cells; however, the fact that CD4 is excluded from lipid rafts before activation (31), whereas CD8 is constitutively expressed in lipid rafts (30), suggests a more effective means of activating CD8+ cells that does not require the aggregation of smaller membrane domains into larger lipid rafts.
Lipid rafts have been extensively studied in CD4+ T cells (3, 19, 32), whereas less information is available on their properties in CD8+ T cells. Villalba et al. (20) showed that Vav/Rac-dependent cytoskeleton reorganization is required for lipid raft clustering in T cells. For some of those experiments, the lymphocytic choriomeningitis virus (LCMV) mouse model was used and the polarization of lipid rafts in CD8+ T cells was visualized by microscopy after 2 h of incubation with peptide-pulsed antigen-presenting cells. In our analyses of polarization of lipid rafts, we did not see accumulation of GM1 in at the cell/bead contact site at 2, 2.5, and 3 h time points in either CD4+ or CD8+ T cells (see Fig. 14, which is published as supporting information on the PNAS web site). This finding might indicate that there is a difference with respect to the time course of lipid raft polarization in human and mouse T cells. It is known that the composition of lipid rafts in mouse cells is not identical to that in human cells. Whereas GM1 is the major ganglioside in mouse lipid microdomains, GM3 represents a major fraction of gangliosides in the human cell membrane (33, 34). Although we and others (3) have shown that GM1 is a reliable indicator for raft formation in human T cells, it is possible that other gangliosides that are not routinely studied may play a role as well. Mice that lack GM1 expression (35) exhibit defective proliferation and IL-2 secretion (36), although the properties of lipid rafts and their function have not yet been studied in this model. It is also possible that the kinetics of lipid raft polarization is influenced by the affinity of interaction between TCR and its ligand.
It is interesting to speculate whether CD8 expression gives the cell a “dominant-negative phenotype” with regard to lipid raft polarization. It has been shown that CD4 and CD8 double-positive thymocytes do not polarize lipid rafts (37). However, this difference could reflect events that occur during differentiation, and might not be comparable to our results obtained in mature T cells. Alternatively, as suggested by Balamuth et al. (32) for CD4+ mouse Th1 cells, CD4 may facilitate lipid raft formation.
In summary, we have made the surprising observation that, although early signal transduction pathways and proliferation are comparable in CD4+ and CD8+ T cells, there is a striking absence of lipid raft polarization in CD8+ T cells. Prior work has suggested that lipid raft polarization and synapse formation are essential for downstream events of TCR signal transduction. Intriguingly, Lee et al. (38) have recently shown that TCR signaling can precede immunological synapse formation. In the future, it will be necessary to define the function of lipid rafts in different subsets of human lymphocytes.
Supplementary Material
Acknowledgments
We thank Ben Freiberg of Intelligent Imaging Innovations for assistance with real-time imaging and Dennis DeSimone for help with SDS/PAGE and Western blotting. This research was supported in part by the Abramson Family Cancer Research Institute. T.H.F. is supported by National Institutes of Health Grants RO1 AI 35513 and RO1 AI 30575 and the Joseph L. Hollander Chair of Pediatric Rheumatology. B.K. is the recipient of a Howard Hughes Medical Institute Postdoctoral Fellowship for Physicians and an Amgen Rheumatology Fellowship Award. M.V.M. is supported in part by National Institutes of Health Training Grant DK07748.
Abbreviations
APC, antigen-presenting cell
TCR, T cell antigen receptor
SMAC, supramolecular activation cluster
PHA, phytohemagglutinin
FLU, influenza
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Monks C. R. F., Freiberg, B. A., Kupfer, H., Sciaky, N. & Kupfer, A. (1998) Nature 395, 82-86. [DOI] [PubMed] [Google Scholar]
- 2.Cherukuri A., Dykstra, M. & Pierce, S. K. (2000) Immunity 14, 657-660. [DOI] [PubMed] [Google Scholar]
- 3.Viola A., Schroeder, S., Sakakibara, Y. & Lanzavecchia, A. (1999) Science 283, 680-682. [DOI] [PubMed] [Google Scholar]
- 4.Xavier R., Brennan, T., Li, Q., McCormack, C. & Seed, B. (1998) Immunity 8, 723-732. [DOI] [PubMed] [Google Scholar]
- 5.Bi K., Tanaka, Y., Coudronniere, N., Sugie, K., Hong, S., van Stipdonk, M. J. B. & Altman, A. (2001) Nat. Immunol. 2, 556-562. [DOI] [PubMed] [Google Scholar]
- 6.Potter T. A., Grebe, K., Freiberg, B. & Kupfer, A. (2001) Proc. Natl. Acad. Sci. USA 98, 12624-12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia K. C., Scott, K. A., Brunmark, A., Carbone, F. R., Peterson, P. A., Wilson, I. A. & Teyton, L. (1996) Nature 384, 577-581. [DOI] [PubMed] [Google Scholar]
- 8.Demotz S., Grey, H. M. & Sette, A. (1990) Science 249, 1028-1030. [DOI] [PubMed] [Google Scholar]
- 9.Harding C. V. & Unanue, E. R. (1990) Nature 346, 574-581. [DOI] [PubMed] [Google Scholar]
- 10.Sykulev Y., Joo, M., Vturina, I., Tsoides, T. J. & Eisen, H. N. (1996) Immunity 4, 565-571. [DOI] [PubMed] [Google Scholar]
- 11.Van Stipdonk M. J. B., Lemmens, E. E. & Schoenberger, S. P. (2001) Nat. Immunol. 2, 423-429. [DOI] [PubMed] [Google Scholar]
- 12.Want B., Maile, R., Greenwood, R., Collins, E. J. & Frelinger, J. A. (2000) J. Immunol. 164, 1216-1222. [DOI] [PubMed] [Google Scholar]
- 13.June C. H., Ledbetter, J. A., Gillespie, M. M., Lindsten, T. & Thompson, C. B. (1987) Mol. Cell. Biol. 7, 4472-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maus M. V., Thomas, A. K., Leonard, D. G. B., Allman, D., Addya, K., Schlienger, K., Riley, J. L. & June, C. H. (2002) Nat. Biotechnol. 20, 143-148. [DOI] [PubMed] [Google Scholar]
- 15.Kung P., Goldstein, G., Reineherz, E. L. & Scholssman, S. F. (1979) Science 206, 347-349. [DOI] [PubMed] [Google Scholar]
- 16.Jung G., Ledbetter, J. A. & Muller-Eberhard, H. J. (1987) Proc. Natl. Acad. Sci. USA 84, 4611-4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boerth N. J., Sadler, J. J., Bauer, D. E., Clements, J. L., Gheith, S. M. & Koretzky, G. A. (2000) J. Exp. Med. 192, 1047-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine B. L., Bernstein, W. B., Connors, M., Craighead, N., Lindsten, T., Thompson, C. B. & June, C. H. (1997) J. Immunol. 159, 5921-5929. [PubMed] [Google Scholar]
- 19.Tuosto L., Parolini, I., Schroeder, S., Sargiacomo, M., Lanzavecchia, A. & Viola, A. (2001) Eur. J. Immunol. 31, 345-349. [DOI] [PubMed] [Google Scholar]
- 20.Villalba M., Bi, K., Rodriguez, F., Tanaka, Y., Schoenberger, S. & Altman, A. (2001) J. Cell Biol. 155, 331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung J. B., Baumeister, M. A. & Monroe, J. G. (2001) J. Immunol. 166, 736-740. [DOI] [PubMed] [Google Scholar]
- 22.Patel V. P., Moran, M., Low, T. A. & Miceli, C. A. (2001) J. Immunol. 166, 754-764. [DOI] [PubMed] [Google Scholar]
- 23.Bunnell S. C., Kapoor, V., Trible, R. P., Zhang, W. & Samelson, L. E. (2001) Immunity 14, 315-329. [DOI] [PubMed] [Google Scholar]
- 24.Langlet C., Bernard, A.-M., Drevot, P. & He, H.-T. (2000) Curr. Opin. Immunol. 12, 250-255. [DOI] [PubMed] [Google Scholar]
- 25.Montixi C., Langlet, C., Bernard, A. M., Thimonier, J., Dubois, C., Wurbel, M. A., Chauvin, J. P., Pierres, M. & He, H. T. (1998) EMBO J. 17, 5334-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drake D. R. & Braciale, T. J. (2001) J. Immunol. 166, 7009-7013. [DOI] [PubMed] [Google Scholar]
- 27.Janes P. W., Ley, S. C., Maggee, A. I. & Kabouridis, P. S. (2000) Semin. Immunol. 12, 23-34. [DOI] [PubMed] [Google Scholar]
- 28.Yang H. & Reinherz, E. L. (2001) J. Biol. Chem. 276, 18775-18785. [DOI] [PubMed] [Google Scholar]
- 29.Torgersen K. M., Vaage, J. T., Rolstad, B. & Tasken, K. (2001) Cell. Signalling 13, 213-220. [DOI] [PubMed] [Google Scholar]
- 30.Arcaro A., Gregoire, C., Bakker, T. R., Baldi, L., Jordan, M., Goffin, L., Boucheron, N., Wurn, F., van der Merve, P. A., Malissen, B. & Luescher, I. F. (2001) J. Exp. Med. 194, 1485-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millan J., Qaidi, M. & Alonso, M. A. (2001) Eur. J. Immunol. 31, 467-473. [DOI] [PubMed] [Google Scholar]
- 32.Balamuth F., Leitenberg, D., Unternaehrer, J., Mellman, I. & Bottomly, K. (2001) Immunity 15, 729-738. [DOI] [PubMed] [Google Scholar]
- 33.Yoasa H., Scheinberg, D. A. & Noughton, A. N. (1990) Tissue Antigens 36, 47-56. [DOI] [PubMed] [Google Scholar]
- 34.Kiguchi K., Henning-Chubb, C. B. & Huberman, E. (1990) J. Biochem. 107, 8-14. [DOI] [PubMed] [Google Scholar]
- 35.Sheikh K. A., Sun, J., Lio, Y., Kawai, H., Crawford, T. O., Proia, R. L., Griffin, J. W. & Schnaar, R. L. (1999) Proc. Natl. Acad. Sci. USA 96, 7532-7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao J., Furukawa, K., Fukumoto, S., Okada, M., Furugen, R., Miyzaki, H., Takamiya, K., Aizawa, S., Shiku, H., Matsuyama, T. & Furukawa, K. (1999) J. Biol. Chem. 274, 13744-13747. [DOI] [PubMed] [Google Scholar]
- 37.Ebert P. J. R., Baker, J. F. & Punt, J. A. (2000) J. Immunol. 165, 5435-5442. [DOI] [PubMed] [Google Scholar]
- 38.Lee K.-H., Holdorf, A. D., Dustin, M. L., Chan, A. C., Allen, P. M. & Shaw, A. S. (2002) Science 295, 1539-1542. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.