Abstract
Thirty to forty percent of diffuse large B cell lymphomas (DLBCL) carry BCL-6 translocations that disrupt its 5′ regulatory region. This same region is also subject to somatic hypermutations, although only a small fraction of these mutations have a detectable effect on transcription. Here, we show that transcription of the BCL-6 gene is negatively self-regulated in multiple cell types. This mechanism operates by means of the interaction of two BCL-6-binding sites within exon 1 of the gene and the BCL-6 protein itself, which is a potent transcription repressor. Because the DLBCL-associated “activating mutations” specifically target these exon 1 binding sites, and because the entire exon 1 is usually removed in the BCL-6-translocated tumors, this autoregulation is bypassed in 30–40% of all DLBCL cases. Our results not only demonstrate an important mechanism governing the expression of BCL-6, but also explain how BCL-6 is deregulated in a large number of DLBCL patients, providing a better understanding of BCL-6-related lymphomagenesis.
Non-Hodgkin's lymphomas (NHL) are a group of very heterogeneous cancers mostly derived from germinal center (GC) B cells (1). One of the most common NHL subtypes is the diffuse large B cell lymphoma (DLBCL), which accounts for up to 80% of the total NHL mortality. The most frequent genetic abnormalities associated with DLBCL are chromosomal translocations and mutations of the BCL-6 gene, although the precise mechanism by which deregulated BCL-6 contributes to lymphomagenesis is poorly understood. Translocations disrupt BCL-6 in its 5′ flanking region often replacing the noncoding exon 1 with a heterologous promoter, leading to sustained high-level BCL-6 expression in tumor cells (2, 3). The same 5′ regulatory region, in particular the 5′ portion of intron 1, is also subjected to high-frequency somatic mutations in normal GC B cells and GC-derived NHL and Hodgkin's disease tumors (4–6). These mutations are thought to be generated by the Ig somatic hypermutation mechanism (7, 8). Recently, a small subset of BCL-6 mutations found in DLBCLs have been reported to cause transcriptional up-regulation of BCL-6 and have been called “activating mutations.” Still, because very little is known about how BCL-6 transcription is normally regulated, the precise mechanism underlying its deregulation in lymphomas remained elusive.
BCL-6 protein is a potent transcription repressor that binds to DNA with its carboxyl zinc fingers and represses transcription with its amino-terminal POZ domain and a segment of its middle region (9–11). BCL-6 is expressed predominantly in the GC B cells, yet certain T cell subsets, activated macrophages, and several nonhematopoietic cell types also express BCL-6 (12–14). Knock-out (KO) mouse studies demonstrated that BCL-6 plays a critical role in GC formation, Th1/Th2 differentiation, and generation of memory T cells (15–17). Two recent reports suggest that BCL-6 expression in DLBCL is associated with a better prognosis. In one study, the use of gene expression profiling identified two distinct DLBCL subtypes: one is “GC B-like” with favorable survival after chemotherapy; the other is “activated B-like DLBCL” with poor survival (18). As expected, only the first subtype expresses high levels of BCL-6. In the second study, BCL-6 expression as a single variable was found to strongly predict survival in DLBCL patients irrespective of any subclassification (19). Clearly, these tantalizing observations call for in-depth study of BCL-6 function in the biology of DLBCL.
Several BCL-6 target genes have been reported including the regulator of plasma cell differentiation Blimp-1 (20, 21). The link between Blimp-1 and BCL-6 is very intriguing for tumorigenesis because it suggests that terminal differentiation of GC B cells is normally blocked by BCL-6 until its down-regulation at the end of the GC reaction (22). Likewise, BCL-6 may facilitate transformation by inhibiting differentiation of lymphoma cells. Signals currently known to down-regulate BCL-6 include surface IgM engagement, which destroys the BCL-6 protein through an activated Ras/MAPK and ubiquitination/proteasome pathway (23), and the CD40–CD40L interaction, which down-regulates BCL-6 transcription (24). One important yet unsolved issue is how BCL-6 expression is normally maintained during GC reaction, and how such regulation is altered by genetic abnormalities in DLBCL.
In this study, we demonstrate that the BCL-6 gene is negatively self-regulated in multiple cell types, and that activating mutations in DLBCLs sustain BCL-6 expression by bypassing this regulation. Therefore, our work provides a unifying explanation as to how BCL-6 is deregulated by various genetic alterations in at least 30–40% of all DLBCLs.
Materials and Methods
Details of plasmid construction and PCR conditions are available on request.
Cell Cultures, Transfections, and Induction of Stable Transfectants.
3T3 and BT549 were maintained in DMEM with 10% FBS; all of the other human B cell lines were maintained in Iscove's modified Dulbecco's medium with 10% FBS. For transient transfections, we used Superfect (Qiagen, Valencia, CA) for 3T3, Mutu I, and Mutu III cells according to the manufacturer's instructions. For stable transfections, heavy metal inducible vector pMEP4-HA-BCL-6 or pMEP7-HA-BCL-6 were introduced into BT549 by using Superfect and B cells by electroporation. Transfectants were selected in Hygromycin B (Invitrogen) containing medium. Various combinations of CdCl2 and the antibiotic compound PF1070A (25) were used to induce transgene expression: 10 nM CdCl2 + 5 nM PF1070A for BT549; 1.0 μM and 0.7 μM CdCl2 for Ly7 and Ly1, respectively. The Ly cell lines have been described (26).
Gel Shift and Reporter Assays.
Procedures for nuclear extract preparation and gel shift analysis have been described (9). Sequences of the 20-bp gel shift probes are indicated in Fig. 2A. For reporter assays in 3T3 cells, 0.125 μg of the reporter, 0.04 μg of a CMV-β-gal plasmid, plus various amounts of pMT-2T-BCL-6 were used to transfect cells in each well of 24-well plates. For B cells, 2 μg of the reporter plasmid, 0.5 μg of a CMV-β-gal plasmid, plus various amount of pMT-2T-BCL-6 were used for 1.5 × 106 cells per transfection. All transfections were performed in duplicate and harvested 48 h later. Luciferase activities were analyzed with the Luciferase Assay System (Promega) and normalized by control readings from the β-gal enzyme. Pab-Luc and Pab-dm1-Luc plasmids were constructed by PCR/subcloning. The pLA/S5 series reporter constructs derived from lymphoma samples were obtained from R. Dalla-Favera (Columbia University).
Fig 2.
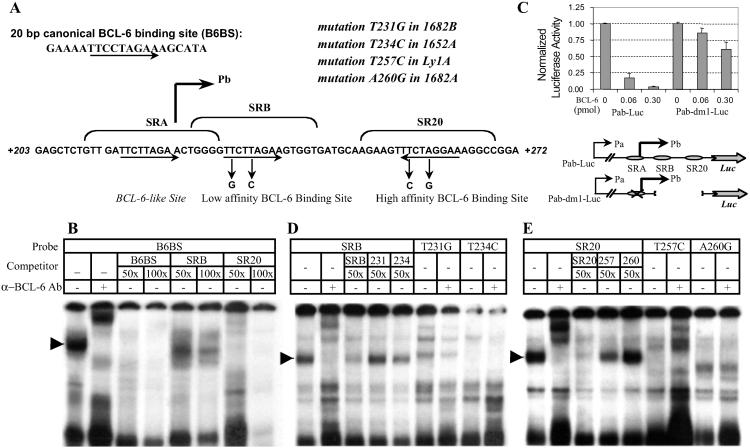
BCL-6 exon 1 contains two functional BCL-6 binding sites that are targeted by activating mutations in lymphomas. (A) Sequences surrounding the transcriptional start site of the major promoter (Pb) (2), showing the locations of the two BCL-6 binding sites (SRB and SR20), and the four activating mutations from three lymphoma cases 1682, 1652, and Ly1. The two alleles of 1682 each carry a mutation in this region. Arrows underlie the 9-bp core sequences of the motifs. The consensus B6BS is also given for comparison. (B) Gel shift analysis of the SRB and SR20 motifs. The 32P-labeled B6BS probe was used with nuclear extract from the BCL-6-positive Ly1 cells. Specificity of the major complex, indicated by an arrow, was verified by supershift assay with the anti-BCL-6 serum, 73-6. An excess amount of cold B6BS, SRB, and SR20 oligos was used to assess their relative affinity for BCL-6. (C) Reporter assay of Pab-Luc construct in 3T3 cells. Pab-Luc and Pab-dm1-Luc were transfected with increasing amounts of a BCL-6 expression plasmid. (D and E) Gel shift analysis of the mutated SRB and SR20 sites. Labels are used as described for B.
PCR, RT-PCR, and Northern Blot.
Total RNA samples were prepared from mouse tissues or cell lines with the Trizol reagent (Invitrogen). For RT-PCR, 1 μg of RNA was used for cDNA synthesis with the Superscript Reverse Transcriptase (Invitrogen). RT-PCR products were obtained with the primers 5′-GGGTTCTTAGAAGTGGTG-3′ (BCL6-1) and 5′-AGGGTTGATCTCAGGATC-3′ (BCL6-4) and directly sequenced with primer BCL6-4. BCL-6 genomic PCR products were obtained with the primers 5′-GGGTTCTTAGAAGTGGTGATGC-3′ (BCL6-e1) and 5′-TGGGACTAATCTTCGGCATT-3′ (BCL6-i2), and sequenced directly with BCL6-i2. For Northern blot, 10 μg of RNA per sample was separated on 0.9% formaldehyde-agarose gel, transferred, and hybridized by using standard methods. Full-length BCL-6 cDNA and a rat GAPDH probe were labeled with [α-32P]dCTP by using the Ready-to-go kit (Amersham Pharmacia). The filters were exposed to phospho-imaging screens and the results analyzed by the IMAGEQUANT software.
Chromatin Immunoprecipitation (ChIP) Assay.
ChIP was performed by using the ChIP assay kit (Upstate Biotechnology, Waltham, MA) according to the manufacturer's instruction with the following modifications: 10 × 106 cells were used for each reaction; chromatin was sheared to an average length of 600 bp; 2 μg of the anti-BCL-6 N3 antibody or normal rabbit IgG (both from Santa Cruz Biotechnology) or anti-Acetyl-histone H3 antibody (Upstate Biotechnology) was used. PCR products were amplified with the BCL6-e1 and BCL6-i2 primers and resolved on 1.5% agarose gels with or without EcoNI digestion. Gel images were analyzed by the IMAGEQUANT software.
Results
Elevated Expression of the Truncated BCL-6 mRNA in BCL-6 KO Mice.
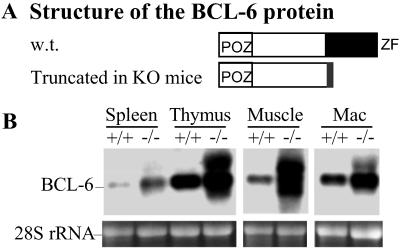
One of our targeting constructs used to generate the BCL-6 KO mice only deletes the DNA-binding zinc finger domain of BCL-6 (15). Although the mice are phenotypically null for BCL-6 function, they produce several BCL-6 transcripts as a result of alternative splicing with the inserted neo cassette. These transcripts encode a truncated and cytoplasmic BCL-6 protein incapable of DNA binding (Fig. 1A and data not shown). Unexpectedly, these alternative BCL-6 transcripts are notably overexpressed in the KO mice when compared with the wild-type BCL-6 mRNA in the littermate controls. Such overexpression is observed in whole spleen, thymus, bone marrow-derived macrophages, as well as skeletal muscle and skin (Fig. 1B and data not shown). In the spleen, we also detected more BCL-6 protein from the BCL-6−/− B220+IgD+ cells than in the control wild-type cells by flow cytometry (not shown).
Fig 1.
The truncated BCL-6 gene is overexpressed in BCL-6 KO mice. (A) Structure of the truncated BCL-6 protein made in the BCL-6 KO mice compared with the wild type. (B) Northern blot analysis of BCL-6 expression in multiple tissues of the KO mice. Ethidium bromide staining of the 28S rRNA is used as a loading control. Mac, macrophage.
BCL-6 Exon 1 Contains Two Functional BCL-6-Binding Sites.
The result in Fig. 1B can be explained by enhanced transcription or a more stabilized message due to fusion with neo. Because BCL-6 is a transcription repressor, we started to investigate the first possibility. Analysis of the BCL-6 exon 1 sequence revealed three motifs closely resembling the consensus BCL-6-binding site B6BS (Fig. 2A). Two of them, SRB and SR20, were recognized by nuclear BCL-6 protein in gel shift assays (Fig. 2 B, D, and E). Competition experiments with cold probes also indicated that BCL-6 can bind to SR20 with higher affinity than to SRB (Fig. 2B), consistent with the fact that SR20 matches perfectly to the 9-bp core of B6BS, whereas SRB has a one-base mismatch. The third BCL-6-like motif, SRA, did not bind BCL-6 in these assays (data not shown). To assess the functional importance of these motifs in vivo, we performed luciferase reporter assays using a 650-bp genomic fragment containing the immediate 5′ flanking sequences and the entire exon 1 (Pab-Luc, Fig. 2C). We also used a mutant construct, which had both the SRB and SR20 sites deleted, and SRA site mutated (Pab-dm1-Luc; Fig. 2C). In NIH 3T3 cells, the wild-type reporter was strongly repressed by BCL-6 in a dose-dependent manner, whereas the mutant version was largely resistant to BCL-6. These results are consistent with the notion that BCL-6 may repress its own transcription in a feedback loop.
Overexpression of Exogenous BCL-6 Protein Down-Regulates Endogenous BCL-6 Gene.
To address directly the question whether the endogenous BCL-6 gene is subjected to feedback control, we generated stably transfected cell lines that carry an inducible BCL-6 transgene. Series of transfectants were obtained from several mature B cell lines, including the Burkitt's lymphoma cell line Mutu I and the DLBCL cell line Ly7. Both cell lines have a germ-line BCL-6 gene with an unmutated exon 1 (not shown). Very similar results were obtained from these cell lines; data from Ly7 are shown in Fig. 3A. Within 3 h of induction, exogenous BCL-6 quickly accumulated from a previously undetectable level to a level approaching that of the endogenous gene. In an equally fast yet reciprocal response, the endogenous BCL-6 dropped to <40% of its steady-state level. Over the course of 24 h and in response to the gradual decline of the exogenous BCL-6, the endogenous mRNA started to rebound. Such specific change is only seen in BCL-6-transfected and not control pMEP4-transfected cells, demonstrating that the repression was caused by exogenous BCL-6 rather than the inducing agent. The fact that at the end of 24 h, the endogenous BCL-6 has not fully recovered is probably a result of delayed decline of exogenous protein compared with its mRNA. We also applied the same approach to study a breast cancer cell line BT549, which expresses low-level BCL-6. With BT549, we detected leakage expression of the transgene accompanied by decreased endogenous BCL-6 when compared with the control-transfected cells (Fig. 3B). Further induction of the transgene caused more substantial repression of endogenous BCL-6. Combined with the observation that the BCL-6 mRNA is overexpressed in several non-B cell types of the KO mice (Fig. 1B), our results indicate that negative autoregulation of BCL-6 is operative in multiple cell types in both human and mouse.
Fig 3.
Overexpression of exogenous BCL-6 down-regulates the endogenous gene. The heavy-metal-inducible pMEP4-HA-BCL-6 and control pMEP4 plasmids were stably transfected into Ly7 (A) and BT549 (B) cells. Representative results from three independent inductions are shown. In each experiment, cells were sampled before and at various time points after induction and analyzed by Northern blot for expression of both the endogenous and exogenous BCL-6 transcripts. Results were quantitated and normalized by the GAPDH signal. Plotted in the bar graph are ratios of various BCL-6 signals vs. the endogenous BCL-6 mRNA in the control cells, which was set as 1.0. All signals were first normalized to GAPDH for loading.
DLBCL-Associated Activating Mutations Specifically Target Exon 1 BCL-6-Binding Sites.
Although most chromosomal translocations deregulate BCL-6 by promoter substitution, only a few BCL-6 mutations have measurable effect on transcription. These so-called “activating mutations” are preferentially found in DLBCL cases. When tested in reporter assays in B cells, they confer unique transcriptional advantage over the wild-type sequence or other “innocent mutations.”¶ In addition, the responsible mutations of four such alleles have all been mapped to exon 1. Thus, we asked the most obvious question: do these mutations target any transcription factor-binding site(s)? As illustrated in Fig. 2A, not only are these activating mutations clustered in exon 1, all four of them specifically target the core sequence of either SRB or SR20. On the basis of our previous studies (9), we predicted that these mutations would inactivate BCL-6 binding. This prediction is confirmed by gel shift experiments (Fig. 2 D and E) and further substantiated by ChIP analysis of BCL-6 binding in vivo (see below). In the gel shift experiments, BCL-6 bound readily to labeled wild-type SRB or SR20 probes but failed to recognize their mutated counterparts (T231G and T234C, T257C and A260G, respectively). In addition, the mutated sequences could not compete with the wild-type probes for BCL-6 binding.
DLBCL-Associated Activating Mutations Bypass Negative Autoregulation in B Cells.
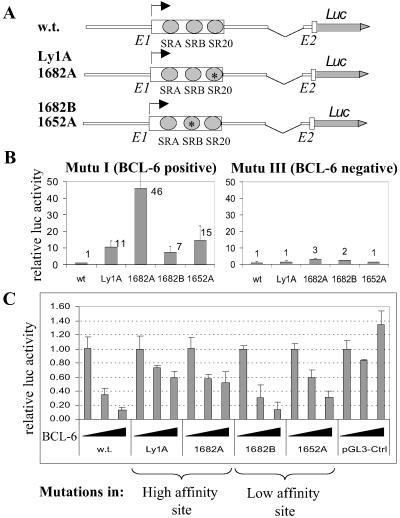
To investigate further the functional consequences of these activating mutations in autoregulation, we studied their responsiveness to BCL-6 repression in B cells. To mimic the endogenous BCL-6 gene more closely, we used a series of reporter plasmids derived from the four mutated alleles, each includes a 6-kb DNA fragment with extensive 5′ flanking sequences plus both exon 1 and the short exon 2 (Fig. 4A). In the BCL-6-positive Mutu I cells, the mutated constructs gave rise to 7- to 46-fold transactivation compared with the wild-type control; however, such transcriptional advantage largely disappeared when the same constructs were transfected into the isogenic, BCL-6-negative Mutu III cells (Fig. 4B). [The remaining minor difference might be caused by other transcription repressor(s) with similar DNA-binding specificity (28) in Mutu III.] This result strongly suggests that these mutations can confer resistance to repression by the endogenous BCL-6 in Mutu I. To confirm this further, we cotransfected these reporters into Mutu III cells with a BCL-6 expression plasmid. Although the wild-type reporter was repressed by BCL-6 in a dose-dependent manner, three of the four mutants displayed variable resistance to repression, with the two high-affinity-site mutants displaying strongest resistance (Fig. 4C). It is somewhat unexpected that the 1682B construct was as sensitive to BCL-6 as the wild-type control. It seems that the wild-type SR20 site on this allele is sufficient to mediate autoregulation. The overall effect of these mutations on BCL-6 expression in vivo is likely to be more complicated in nature. For example, in tumor cells from case 1682 with both the 1682A and 1682B alleles, at least one copy of the BCL-6 gene should be resistant to feedback repression even if the 1682B allele is only partially immune.
Fig 4.
“Activating mutations” confer resistance to BCL-6 repression in transient reporter assays. (A) Schematic representation of the reporter constructs used. (B) Activities of the wild-type and mutated reporters in Mutu I vs. Mutu III cells. (C) Reporter assays in Mutu III cells with increasing amount of cotransfected BCL-6 expression plasmid (0, 0.05, and 0.25 μg). Activity of the wild-type reporter was normalized to 1.0 for each cell type.
Exon 1 Mutation Causes Monoallelic Expression of BCL-6 in Ly1 Cells.
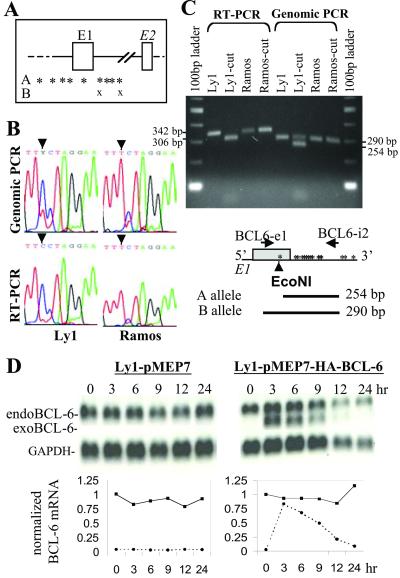
To determine whether expression of the endogenous BCL-6 gene could be altered by such activating mutations, we took advantage of the Ly1 cell line that carries one of the activating mutations (T257C) we have studied. Although both BCL-6 alleles in Ly1 have accumulated multiple mutations in the exon1/intron 1 boundary, only allele A has the T257C mutation in exon 1 which enables us to trace the allelic source of the mRNA transcripts (Fig. 5A). Fig. 5B shows the direct sequencing results of the BCL-6 RT-PCR and genomic PCR products from Ly1 cells and a wild-type control cell line Ramos. In the chromatogram, although the Ly1 genomic sequence is composed of both T and C at position 257, the mRNA pool is dominated by the mutated C signal. This result was further confirmed by digesting the PCR products with EcoNI enzyme, which only cuts the mutated allele A (Fig. 5C). All RT-PCR products from Ly1 were shortened by the EcoNI digestion, indicating that BCL-6 is exclusively expressed from the mutated A allele. We also used the inducible cell line approach to study the sensitivity of this allele to exogenous BCL-6. As illustrated in Fig. 5D, BCL-6 mRNA in Ly1 cells was nearly completely resistant to feedback repression. Our results demonstrate that a single-point mutation in the SR20 site is sufficient for the Ly1 A allele to bypass negative autoregulation.
Fig 5.
Monoallelic expression of BCL-6 in Ly1 cells. (A) Schematic representation of mutations in the two BCL-6 alleles in Ly1. Each “*” or “X” represents a single mutation. (B) PCR-direct sequencing analysis of the BCL-6 genomic and mRNA sequences. Ramos cell line was used as a germ-line control. Arrowheads indicate position 257 with respect to transcriptional start site. (C) Restriction analysis of the genomic and RT-PCR products corresponding to exon 1. EcoNI digestion shortens the mutated RT-PCR product from 342 to 306 bp, and its corresponding genomic PCR product from 290 to 254 bp. (D) Endogenous BCL-6 in Ly1 cells is resistant to expression of exogenous BCL-6. The experiment was performed as in Fig. 3.
Activating Mutation Abolishes BCL-6 Binding to Exon 1 in Vivo.
Monoallelic expression of BCL-6 in Ly1 also means that the B allele with wild-type exon 1 sequence is completely silenced. A negative autoregulation model would predict that this complete silencing is the result of direct BCL-6 binding to the B but not A allele. Still, other possibilities formally exist including allele-specific epigenetic silencing and the creation of a binding site for a novel transcription activator by the T257C mutation. This last possibility is rather unlikely because four different activating mutations all carry transcriptional advantage. Because the key issue that divides our hypothesis from alternative explanations is whether or not BCL-6 preferentially binds to the wild-type exon 1 sequence in vivo, we decided to use the ChIP technique (29) to study BCL-6 binding in Ly1 directly (Fig. 6). The specificity of our ChIP assay is demonstrated by the use of control rabbit IgG, which did not recover any exon 1 sequence either from Ly1 or control Ly7 cells. We also digested all PCR products with EcoNI to analyze their allelic origin. Quantitation of the wild-type and mutated bands from both the input and immunoprecipitated material revealed that BCL-6 preferentially binds to the wild-type B allele; whereas acetylated histone H3 is preferentially associated with the mutated A allele. This result is consistent with reports that BCL-6 can recruit histone deacetylase complexes (30, 31) and our own finding that only A allele is actively transcribed in Ly1 cells. Our data strongly suggest that the B allele with wild-type exon 1 is actively repressed by BCL-6 in Ly1 cells, whereas the A allele with mutated exon 1 is spared of BCL-6 binding and the subsequent feedback repression.
Fig 6.
BCL-6 preferentially binds to the wild-type exon 1 in Ly1 cells. Both Ly1 and the control Ly7 cells were analyzed for association of BCL-6 and acetylated H3 with the exon 1 sequence. Location of the PCR primers is given with respect to exon 1 and the surrounding mutations. Various amounts of recovered genomic DNA as well as a fraction of the total chromatin input were used in PCR reactions with the BCL-6-e1 and BCL-6 i2 primer pairs. IgG, normal rabbit IgG used as control antibody; N3, polyclonal anti-BCL-6 antibody; N.C., negative control PCR reaction with no template.
Discussion
Genetic studies in humans and mice have suggested important functions for BCL-6 in both normal lymphoid system and lymphomagenesis. Therefore, mechanisms involved in regulation and deregulation of BCL-6 become very important as they determine the outcome of these biological processes. During the course of our study, Kikuchi et al. reported that a 14-bp sequence in BCL-6 exon 1 can be recognized by BCL-6 protein in vitro and mediate transcription repression by BCL-6 in transient reporter assays (32). This site is the high-affinity BCL-6-binding site SR20. Although the authors suggested that BCL-6 might be autoregulated, the importance of this site in regulating the endogenous BCL-6 gene was not investigated. In this study, we have established that the BCL-6 gene is negatively self-regulated in multiple cell types in vivo. Furthermore, we also present both in vitro and in vivo evidence to show that the DLBCL-associated “activating mutations” allow lymphoma cells to bypass this feedback loop, highlighting the importance of our findings in understanding a major pathological mechanism of DLBCL.
Activating mutations that map to the exon 1 BCL-6-binding sites can be found in 11% (4 of 35) of DLBCLs (R. Dalla-Favera, personal communication). All four of such mutations tested in this study abolished BCL-6 binding in vitro and provided resistance to BCL-6 repression in transient transfection assays. Results shown in Figs. 5 and 6 also strongly suggest that the T257C mutation in Ly1 spares allele A of BCL-6 binding and caused the monoallelic expression of BCL-6 in this cell line. It is worth noting here that somatic mutations of BCL-6 are the result of a physiological process occurring in GC B cells (5, 6). The fact that these activating mutations are enriched only in DLBCL cells but not other GC-derived normal or NHL B cells suggests a specific need by DLBCL to escape BCL-6 autoregulation. In addition, 30–40% of DLBCL cases carry BCL-6 translocations leading to constitutive BCL-6 expression from the translocated alleles, whereas the germ-line allele is transcriptionally silent, as revealed by Northern blot and RNase protection analyses (2, 33–35). Because exon 1 is removed in ≈70% of these cases (36), these translocated alleles can also be expected to escape the autoregulation, whereas the untranslocated alleles are silenced by the same mechanism. In this respect, we have investigated a BCL-6-translocated cell line Val. As expected, the endogenous BCL-6 mRNA in Val was resistant to feedback repression (data not shown). In addition, lymphomas carrying deletions in the BCL-6 exon 1/intron 1 region have been reported (37, 38). These alterations also have the potential to evade autoregulation, although their prevalence has not been well documented. In summary, we estimate autoregulation of BCL-6 is bypassed in 30–40% of all DLBCL cases. Still, it remains to be investigated how BCL-6 is deregulated in the translocated cases that have spared exon 1.
Negative autoregulation mechanisms are very commonly used in a variety of biological systems and organisms to achieve homeostasis in metabolism and to execute developmental programs in an orderly fashion (39, 40). This regulatory mode offers the most stability because it can resist random fluctuations from either internal or external sources. As “master switch” of cellular responses, many transcription factors are negatively self-regulated. Many examples of transcription factors/oncogenes regulated in negative feedback fashion exist. Well-known examples include c-Myc (41), neu (42), and E2F1 (43). GC is a very dynamic cellular environment in which B cells are constantly subjected to multiple stimuli from cytokines and cell–cell interactions, and undergo very intense proliferation and cell death (27). From this perspective, it is not surprising that BCL-6, which is maintained at very high and stable level in GC B cells, also adopts this regulatory mode. Functional importance of the exon 1 BCL-6-binding sites is underscored by the fact that they are embedded in a 120-bp domain with complete sequence identity between human and mice. In addition, the high-affinity SR20 site is present in the 5′ UTR of both frog and zebra fish BCL-6 mRNA, suggesting that the feedback mechanism of BCL-6 expression is as evolutionarily conserved as the structure of the protein itself (not shown).
BCL-6 is known to be rapidly up-regulated when B cells enter GC and abruptly down-regulated when they exit GC (12, 13). How could the negative autoregulation respond to such dramatic developmental changes? Our results indicate that the feedback loop is operational in both BCL-6 high and BCL-6 low cells suggesting existence of cell type-specific factor(s) that can modulate the feedback threshold. These factors might alter the ability of BCL-6 to bind DNA or to recruit co-repressors by covalently modifying BCL-6 (e.g., acetylation∥), or forming complexes with BCL-6. Alternatively, the BCL-6 locus may undergo cell-type or stage-specific epigenetic modifications, which in turn adjust the accessibility of the two exon 1 BCL-6 sites. Because proper expression of BCL-6 is critical to its function, it will be very important to understand how this autoregulation mechanism is modulated during normal B cell differentiation and malignant transformation.
Acknowledgments
We are grateful to R. Dalla-Favera for sharing unpublished data on the DLBCL-specific activating mutations and the pLA/S5 reporter constructs. We thank A. Martin for helpful suggestions with the allelic analysis and J. Yu and A. Miller for technical support. This work was supported by research grants from the Lauri Strauss Leukemia Foundation and by National Institutes of Health Grant RO1 CA85573 (to B.H.Y.).
Abbreviations
DLBCL, diffuse large B cell lymphoma
NHL, non-Hodgkin's lymphoma
GC, germinal center
ChIP, chromatin immunoprecipitation
KO, knock-out
Bereschenko, O. R., Gu, W. & Dalla-Favera, R. (2000) Blood 96, 702a (abstr.).
Pasqualucci, L., Migliazza, A., Ye, B., Cattoretti, G. & Dalla-Favera, R. (1999) Blood 94, 58a (abstr.).
References
- 1.Harris N. L., Jaffe, E. S., Stein, H., Banks, P. M., Chan, J. K., Cleary, M. L., Delsol, G., De Wolf-Peeters, C., Falini, B. & Gatter, K. C. (1994) Blood 84, 1361-1392. [PubMed] [Google Scholar]
- 2.Ye B. H., Chaganti, S., Chang, C. C., Niu, H., Corradini, P., Chaganti, R. S. & Dalla, F. R. (1995) EMBO J. 14, 6209-6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W., Iida, S., Louie, D. C., Dalla-Favera, R. & Chaganti, R. S. (1998) Blood 91, 603-607. [PubMed] [Google Scholar]
- 4.Migliazza A., Martinotti, S., Chen, W., Fusco, C., Ye, B. H., Knowles, D. M., Offit, K., Chaganti, R. S. & Dalla-Favera, R. (1995) Proc. Natl. Acad. Sci. USA 92, 12520-12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasqualucci L., Migliazza, A., Fracchiolla, N., William, C., Neri, A., Baldini, L., Chaganti, R. S., Klein, U., Kuppers, R., Rajewsky, K. & Dalla-Favera, R. (1998) Proc. Natl. Acad. Sci. USA 95, 11816-11821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen H. M., Peters, A., Baron, B., Zhu, X. & Storb, U. (1998) Science 280, 1750-1752. [DOI] [PubMed] [Google Scholar]
- 7.Storb U., Shen, H. M., Michael, N. & Kim, N. (2001) Philos. Trans. R. Soc. London B 356, 13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zan H., Li, Z., Yamaji, K., Dramitinos, P., Cerutti, A. & Casali, P. (2000) J. Immunol. 165, 830-839. [DOI] [PubMed] [Google Scholar]
- 9.Chang C. C., Ye, B. H., Chaganti, R. S. & Dalla-Favera, R. (1996) Proc. Natl. Acad. Sci. USA 93, 6947-6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seyfert V. L., Allman, D., He, Y. & Staudt, L. M. (1996) Oncogene 12, 2331-2342. [PubMed] [Google Scholar]
- 11.Deweindt C., Albagli, O., Bernardin, F., Dhordain, P., Quief, S., Lantoine, D., Kerckaert, J. P. & Leprince, D. (1995) Cell Growth Differ. 6, 1495-1503. [PubMed] [Google Scholar]
- 12.Cattoretti G., Chang, C. C., Cechova, K., Zhang, J., Ye, B. H., Falini, B., Louie, D. C., Offit, K., Chaganti, R. S. & Dalla-Favera, R. (1995) Blood 86, 45-53. [PubMed] [Google Scholar]
- 13.Onizuka T., Moriyama, M., Yamochi, T., Kuroda, T., Kazama, A., Kanazawa, N., Sato, K., Kato, T., Ota, H. & Mori, S. (1995) Blood 86, 28-37. [PubMed] [Google Scholar]
- 14.Bajalica-Lagercrantz S., Piehl, F., Farnebo, F., Larsson, C. & Lagercrantz, J. (1998) Biochem. Biophys. Res. Commun. 247, 357-360. [DOI] [PubMed] [Google Scholar]
- 15.Ye B. H., Cattoretti, G., Shen, Q., Zhang, J., Hawe, N., de Waard, R., Leung, C., Nouri-Shirazi, M., Orazi, A., Chaganti, R. S., et al. (1997) Nat. Genet. 16, 161-170. [DOI] [PubMed] [Google Scholar]
- 16.Dent A. L., Shaffer, A. L., Yu, X., Allman, D. & Staudt, L. M. (1997) Science 276, 589-592. [DOI] [PubMed] [Google Scholar]
- 17.Ichii H., Sakamoto, A., Hatano, M., Okada, S., Toyama, H., Taki, S., Arima, M., Kuroda, Y. & Tokuhisa, T. (2002) Nat. Immunol. 3, 558-563. [DOI] [PubMed] [Google Scholar]
- 18.Alizadeh A. A., Eisen, M. B., Davis, R. E., Ma, C., Lossos, I. S., Rosenwald, A., Boldrick, J. C., Sabet, H., Tran, T., Yu, X., et al. (2000) Nature 403, 503-511. [DOI] [PubMed] [Google Scholar]
- 19.Lossos I. S., Jones, C. D., Warnke, R., Natkunam, Y., Kaizer, H., Zehnder, J. L., Tibshirani, R. & Levy, R. (2001) Blood 98, 945-951. [DOI] [PubMed] [Google Scholar]
- 20.Shaffer A. L., Yu, X., He, Y., Boldrick, J., Chan, E. P. & Staudt, L. M. (2000) Immunity 13, 199-212. [DOI] [PubMed] [Google Scholar]
- 21.Reljic R., Wagner, S. D., Peakman, L. J. & Fearon, D. T. (2000) J. Exp. Med. 192, 1841-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fearon D. T., Manders, P. & Wagner, S. D. (2001) Science 293, 248-250. [DOI] [PubMed] [Google Scholar]
- 23.Niu H., Ye, B. H. & Dalla-Favera, R. (1998) Genes Dev. 12, 1953-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carbone A., Gaidano, G., Gloghini, A., Larocca, L. M., Capello, D., Canzonieri, V., Antinori, A., Tirelli, U., Falini, B. & Dalla-Favera, R. (1998) Blood 91, 747-755. [PubMed] [Google Scholar]
- 25.Asahi I., Miura, N., Yamabe, Y., Toyoda, H., Imura, N., Koyama, M. & Naganuma, A. (1999) Biochemistry 38, 10415-10423. [DOI] [PubMed] [Google Scholar]
- 26.Tweeddale M., Jamal, N., Nguyen, A., Wang, X. H., Minden, M. D. & Messner, H. A. (1989) Blood 74, 572-578. [PubMed] [Google Scholar]
- 27.Liu Y. J., de Bouteiller, O. & Fugier-Vivier, I. (1997) Curr. Opin. Immunol. 9, 256-262. [DOI] [PubMed] [Google Scholar]
- 28.Okabe S., Fukuda, T., Ishibashi, K., Kojima, S., Okada, S., Hatano, M., Ebara, M., Saisho, H. & Tokuhisa, T. (1998) Mol. Cell. Biol. 18, 4235-4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orlando V. (2000) Trends Biochem. Sci. 25, 99-104. [DOI] [PubMed] [Google Scholar]
- 30.Dhordain P., Albagli, O., Lin, R. J., Ansieau, S., Quief, S., Leutz, A., Kerckaert, J. P., Evans, R. M. & Leprince, D. (1997) Proc. Natl. Acad. Sci. USA 94, 10762-10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.David G., Alland, L., Hong, S. H., Wong, C. W., DePinho, R. A. & Dejean, A. (1998) Oncogene 16, 2549-2556. [DOI] [PubMed] [Google Scholar]
- 32.Kikuchi M., Miki, T., Kumagai, T., Fukuda, T., Kamiyama, R., Miyasaka, N. & Hirosawa, S. (2000) Oncogene 19, 4941-4945. [DOI] [PubMed] [Google Scholar]
- 33.Kerckaert J. P., Deweindt, C., Tilly, H., Quief, S., Lecocq, G. & Bastard, C. (1993) Nat. Genet. 5, 66-70. [DOI] [PubMed] [Google Scholar]
- 34.Miki T., Kawamata, N., Arai, A., Ohashi, K., Nakamura, Y., Kato, A., Hirosawa, S. & Aoki, N. (1994) Blood 83, 217-222. [PubMed] [Google Scholar]
- 35.Ohno H., Kerckaert, J. P., Bastard, C. & Fukuhara, S. (1994) Jpn. J. Cancer Res. 85, 592-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye B. H. (2000) Cancer Invest. 18, 356-365. [DOI] [PubMed] [Google Scholar]
- 37.Bernardin F., Collyn-d'Hooghe, M., Quief, S., Bastard, C., Leprince, D. & Kerckaert, J. P. (1997) Oncogene 14, 849-855. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura Y. (2000) Leuk. Lymphoma 38, 505-512. [DOI] [PubMed] [Google Scholar]
- 39.Little J. W., Shepley, D. P. & Wert, D. W. (1999) EMBO J. 18, 4299-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Becskei A. & Serrano, L. (2000) Nature 405, 590-593. [DOI] [PubMed] [Google Scholar]
- 41.Grignani F., Lombardi, L., Inghirami, G., Sternas, L., Cechova, K. & Dalla-Favera, R. (1990) EMBO J. 9, 3913-3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao X. Y. & Hung, M. C. (1992) Mol. Cell. Biol. 12, 2739-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson D. G., Ohtani, K. & Nevins, J. R. (1994) Genes Dev. 8, 1514-1525. [DOI] [PubMed] [Google Scholar]