Abstract
The effects of depot formulations of the luteinizing hormone-releasing hormone (LHRH) agonist Decapeptyl (25 μg/day) for 30 days or LHRH antagonist Cetrorelix pamoate (100 μg/day) for 30 days and daily injections of 100 μg of Decapeptyl for 10 days on the expression of mRNA for pituitary LHRH receptor (LHRH-R) and the levels of LHRH-R protein were evaluated in rats. Serum sex steroid concentrations and the weights of the reproductive organs were greatly reduced in all groups treated with analogs, demonstrating an efficient blockade of the pituitary–gonadal axis. Decapeptyl microcapsules elevated serum LH in female rats, but decreased it in male rats. LHRH-R mRNA expression in female pituitaries was reduced to 41% and 56–65% on days 10 and 30, respectively, whereas LHRH-R protein was 64% of control on day 10 and returned to pretreatment levels on day 30. Decapeptyl microcapsules reduced LHRH-R mRNA expression in male pituitaries to 58% on day 30 but not LHRH-R protein. Daily injections of Decapeptyl caused a desensitization of LH responses in female rats, while raising LHRH-R mRNA expression in female rats by 23% and LHRH-R protein levels by 119%. Cetrorelix pamoate reduced serum LH in female rats and diminished LHRH-R mRNA to 30% and 26% and LHRH-R protein to 57% and 48% on days 10 and 30, respectively. Elevated LHRH-R protein levels of ovariectomized rats were reduced after 10-day treatment with Cetrorelix or 100 μg/day Decapeptyl. Thus, changes in the mRNA expression after treatment with Cetrorelix, but not always Decapeptyl, paralleled those of LHRH-R protein. The inhibitory effect of Cetrorelix on serum LH, pituitary LHRH-R mRNA, and LHRH-R protein was greater than that of Decapeptyl.
The hypothalamic decapeptide luteinizing hormone-releasing hormone (LHRH) plays a central role in the control of reproduction by stimulating the release of pituitary luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which in turn promote gonadal functions and regulate sex steroid secretion (1–3). The effects of LHRH are mediated by high-affinity G protein-coupled LHRH-receptor (LHRH-R) on pituitary gonadotropes (4–7). The responses to LHRH vary under different conditions and critically depend on the regimens of administration and doses delivered to gonadotrope cells. An intermittent stimulation with low doses of LHRH or LHRH agonists, mimicking the normal pulsatile release of the neuropeptide from the hypothalamus, stimulates the release of gonadotropins, whereas a chronic exposure to LHRH agonists leads to an inhibition of pituitary responses and suppression of serum LH, FSH, and sex steroid levels (8–12). This desensitization of gonadotrope cells involves a down-regulation of pituitary receptors for LHRH and inhibition of gene expression of LHRH-R by mechanisms, some of which still remain to be clarified (5, 7, 13–15).
The agonistic analogs of LHRH such as Decapeptyl, leuprolide, Zoladex, and buserelin have well established clinical indications, including in vitro fertilization and embryo transfer programs and treatment of uterine leiomyomas, endometriosis, central precocious puberty, and sex hormone-dependent tumors (4–6, 12, 13, 16–20). These applications are based on selective medical hypophysectomy or on the reversible medical castration and the creation of a state of sex steroid deprivation, all induced by these analogs (4–6, 12, 13, 16–20). Potent antagonists of LHRH such as Cetrorelix, Ganirelix, and Abarelix (6–18, 21–24) also have been synthesized. Antagonists of LHRH compete with LHRH for the same receptor sites and cause an immediate inhibition of the release of gonadotropins and sex steroids (5, 12, 17, 24, 25). Depot formulations of LHRH antagonists such as Cetrorelix and Abarelix could become an important addition to the clinical armamentarium. The aim of the present study was to investigate and compare the long-term effects of depot formulations of LHRH agonist Decapeptyl and antagonist Cetrorelix on the mRNA for pituitary LHRH-R, LHRH-R protein, serum LH, and sex steroid levels and other endocrine parameters in rats.
Materials and Methods
Peptides.
The LHRH agonist [d-Trp6]LHRH (Decapeptyl, Triptorelin) was supplied by Debiopharm (Lausanne, Switzerland) as [d-Trp6]LHRH acetate. For injection, Decapeptyl was dissolved in distilled water containing 5% mannitol and given s.c. Microcapsules of [d-Trp6]LHRH were provided by Ipsen Pharma Biotech (Signes, France) as vials of Decapeptyl, containing 4.2 mg of [d-Trp6]LHRH dispersed in 160 mg of poly(dl-lactide-co-glycolide) and excipients including mannitol. For injection, the microspheres were suspended in diluent and administered i.m. A depot formulation of the LHRH antagonist Cetrorelix (21) was supplied by Zentaris (Frankfurt) as Cetrorelix pamoate (D20762) and contained Cetrorelix peptide-base and pamoic acid in a molar ratio of 2:1 and mannitol suspended in distilled water.
Animals.
Adult male and female Sprague–Dawley rats (200–220 g) (Charles River Breeding Laboratories) were used. The animals were allowed standard rat diet and tap water ad libitum and were maintained under controlled conditions (24°C, 12-h light, 12-h dark schedule). Vaginal smears were monitored daily and only rats showing two consecutive estrous cycles were used.
In Vivo Experiments.
Experiment 1.
Three groups of 20 normal female rats were treated as follows: Group 1 was injected i.m. with Cetrorelix pamoate (3 mg/0.2 ml), estimated to release 100 μg/day for 30 days. Group 2 received i.m. Decapeptyl microcapsules (0.75 mg/0.4 ml) releasing 25 μg/day for 30 days. Group 3 received an i.m. injection of the vehicle only. Blood samples were taken from the jugular vein under isoflurane anesthesia and on days 0, 4, 10, and 30 (or before decapitation). Serum was separated by centrifugation and stored at −20°C until assayed. Ten rats in group 1 and 2 were killed in the morning of the 10th day and the remaining rats, at the end of the 30-day treatment. Control rats in group 3 were killed in diestrus II phase of the cycle on the closest possible date to day 10 or 30. The ovaries and uterus were removed and weighed. Anterior pituitaries (four in each group) were homogenized in the TRI reagent (Sigma) and stored at −70°C for LHRH-R mRNA determination. The remaining pituitaries were frozen on dry ice and kept at −70°C for LHRH-R protein analysis.
Experiment 2.
Eight female and eight male rats were treated with Decapeptyl microcapsules (0.75 mg/0.4 ml). The eight control female and eight male animals received vehicle only. Blood samples were taken on days 0, 4, 10, and 30 (or before decapitation). Control female rats were killed in diestrus II phase of the cycle on the date closest to day 30. The pituitaries, ovaries and uterus or testes, seminal vesicles, and prostate were removed and weighed.
Experiment 3.
Eight female rats were treated with daily injections of Decapeptyl for 10 days (100 μg/day). The control animals received vehicle only. Blood samples were taken on days 1, 4, and 10 immediately before the administration of Decapeptyl and 2 h and 5 h after the injection. The animals were killed (the control animals in diestrus II), and the ovaries, uterus, and pituitaries were removed and weighed.
RIA.
Concentration of LH in the serum was determined by an RIA using materials provided by the National Institute of Diabetes and Digestive and Kidney Diseases, National Hormone and Pituitary Program, Torrance, CA (A. F. Parlow): rLH-RP-3 (AFP-7187B), rLH-I-10 (AFP11536B), and anti-rLH-RIA-11 (AFP C697071P). We determined estradiol, progesterone, and testosterone by using commercial kits (Diagnostic System Laboratories, Webster, TX, DSL-4800, DSL-3400, and DSL-4100).
RT-PCR Analysis.
Total RNA (1 or 2 μg) was reverse-transcribed and then amplified by using the reagents and protocol of the GeneAmp RNA PCR Core kit (Perkin–Elmer). Reverse transcriptase (RT) reaction and the PCR amplification were done as described (26) with the GeneAmp PCR System 2400 (Perkin–Elmer). The number of cycles was within the exponential range of PCR amplification (22–25 cycles). PCR products were electrophoresed on a 2% agarose gel at 4°C, stained with ethidium bromide, and visualized under UV light. Bands of PCR-amplified products were analyzed semiquantitatively by using a zoom digital camera (DC290) with EDAS 290 imaging system (Kodak). The levels of rat LHRH-R mRNA products were related to rat β-actin mRNA values and expressed as percentage of the vehicle-treated controls.
Immunodetection of LHRH-Rs.
Membrane fraction was prepared from rat pituitaries as described (27). Membrane proteins (5–25 μg) were solubilized in 20 mM Tris⋅HCl buffer (pH 8.0) containing 10% (vol/vol) glycerol, 1% (wt/vol) SDS, 1 mM EDTA, and 1 mM DTT, and heated for 10 min. Proteins were resolved by SDS/10% PAGE and then transferred to nitrocellulose sheets (Hoefer). The nitrocellulose sheet was soaked in Tris-buffered saline (10 mM Tris⋅HCl, pH 7.5/50 mM NaCl) containing 0.1% Tween-20 (TBST buffer). Excess protein-binding sites were saturated with TBST buffer containing 5% nonfat dried milk. The blotted membranes were incubated for 1 h at room temperature with goat polyclonal anti-human LHRH-R (Santa Cruz Biotechnology) antisera diluted 1/1,000 in the TBST buffer. The immunoreactive proteins were developed with peroxidase-conjugated anti-goat IgG Abs (Santa Cruz Biotechnology) and visualized by a chemiluminescent detection system (Pierce). The bands were analyzed with the imaging densitometer specified above, and the relative protein levels were quantified. Western blot analysis for LHRH-R protein was performed also on some of the pituitaries from experiments reported earlier (2), in which ovariectomized (OVX) rats were treated with depot formulation of Cetrorelix releasing 100 μg/day or with daily injections of 100 μg of Decapeptyl.
Analysis of Data.
Results are expressed as mean ± SEM. For statistical analysis of data the computer software sigmastat (Jandel, San Rafael, CA) was used. In vivo results were subjected to one-way ANOVA, followed by Bonferroni t test, and P < 0.05 was accepted as statistically significant.
Results
Effects of Cetrorelix Pamoate and Microcapsules of Decapeptyl in Female Rats After 10 and 30 Days.
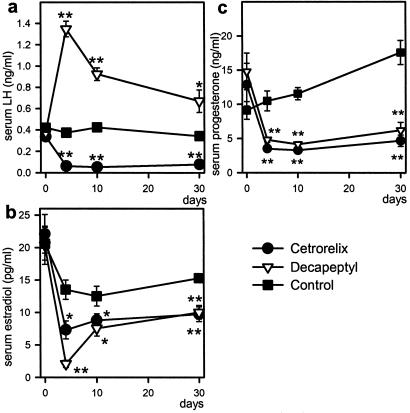
Rats treated with depot formulations of Cetrorelix (100 μg/day) or Decapeptyl (25 μg/day) remained in constant diestrus throughout the treatment. Serum LH was decreased in the Cetrorelix-treated group by >80% on day 4 and remained low during the 30-day treatment (P < 0.01). In the Decapeptyl group, a 3-fold elevation of serum LH was detected on day 4 (P < 0.01) and LH concentrations remained above the controls on days 10 and 30 (Fig. 1a). Serum levels of estradiol and progesterone were significantly lower in both the Cetrorelix and Decapeptyl groups (Fig. 1 b and c). In both groups, after 10 days, the weights of ovaries and uterus decreased similarly by ≈31% and 55%, respectively. After 30 days, the reduction in ovarian weights was ≈50% and uterine weights ≈70% in either group.
Fig 1.
Effects of Cetrorelix pamoate (releasing 100 μg/day) or Decapeptyl microcapsules (releasing 25 μg/day) on serum LH (a), serum estradiol (b), and serum progesterone (c) during the treatment in normal rats. *, P < 0.05; **, P < 0.01 vs. control.
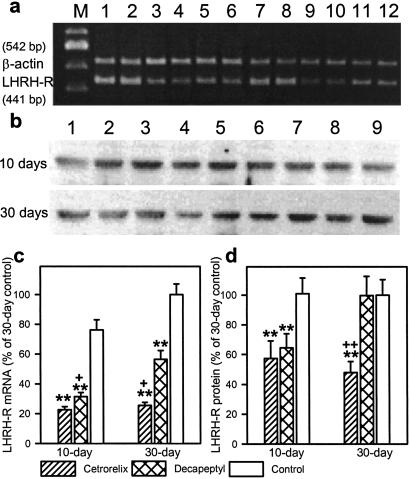
The expression of pituitary LHRH-R mRNA was strongly decreased by 70.3% and 58.7% on day 10 of treatment with Cetrorelix or Decapeptyl, respectively (P < 0.01) (Fig. 2 a and c). On day 30, LHRH-R mRNA in the control rats was slightly, but not significantly, higher than on day 10, whereas LHRH-R mRNA expression was reduced by 74.4% in Cetrorelix-treated rats (P < 0.01). The extent of reduction in LHRH-R mRNA in Decapeptyl-treated rats on day 30 was 43.6% (P < 0.01), significantly less than on day 10 (Fig. 2 a and c).
Fig 2.
Effects of Cetrorelix pamoate or Decapeptyl microcapsules on LHRH-R mRNA expression and LHRH-R protein levels in female rats after 10 and 30 days of treatment. (a) RT-PCR products of the pituitary LHRH-R mRNA and β-actin mRNA. Lanes: M, 100-bp DNA molecular weight marker; 1 and 2, 10-day control; 3 and 4, 10-day Cetrorelix; 5 and 6, 10-day Decapeptyl; 7 and 8, 30-day control; 9 and 10, 30-day Cetrorelix; and 11 and 12, 30-day Decapeptyl. (b) Western blot for LHRH-R protein. Lanes: 1–3, control; 4–6, Cetrorelix; 7–9, Decapeptyl. (c) LHRH-R mRNA levels after 10 and 30 days. (d) LHRH-R protein levels after 10 and 30 days. **, P < 0.01 vs. same-day control; +, P < 0.05; ++, P < 0.01 vs. 30-day Decapeptyl treated.
After 10 days, LHRH-R protein levels in pituitaries of the Cetrorelix- and Decapeptyl-treated rats were 43% and 36% lower (P < 0.05), respectively, than in the controls. However, on day 30, the LHRH-R protein levels returned to normal in the Decapeptyl-treated rats but decreased further to 48% of controls (P < 0.01) in the Cetrorelix group (Fig. 2 b and d).
Effects of Microcapsules of Decapeptyl in Male and Female Rats over a Period of 30 Days.
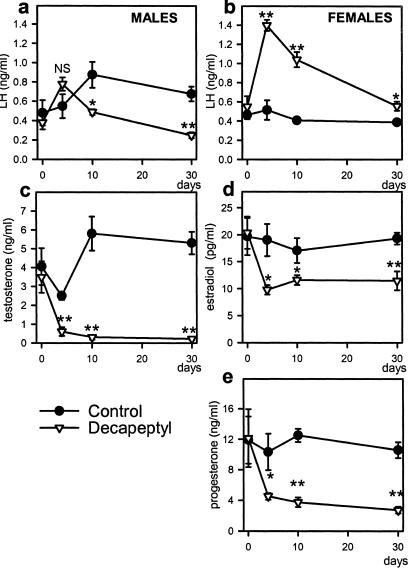
The female rats treated with Decapeptyl microcapsules (25 μg/day) remained in constant diestrus throughout the treatment. In male rats, after an initial rise, serum LH was decreased by 45% on day 10 and by 63% on day 30 (Fig. 3a). However, in female rats, Decapeptyl treatment caused again a nearly 3-fold elevation in serum LH on day 4 (P < 0.01) and LH concentrations remained significantly elevated on days 10 and 30 (Fig. 3b). Decapeptyl also produced a 76–95% decrease in serum testosterone in male rats (Fig. 3c) and a significant reduction of serum estradiol (32–41%) and progesterone (56–74%) in female rats (Fig. 3 d and e). The weights of the reproductive organs were reduced in the Decapeptyl-treated groups as compared with controls (ovaries from 93 ± 5 to 44 ± 3 mg, uterus from 405 ± 14 to 131 ± 9 mg, testes from 2,863 ± 65 to 1,003 ± 104 mg, seminal vesicles from 1,493 ± 157 to 131 ± 11 mg, and prostates from 1,064 ± 75 to 160 ± 16 mg, P < 0.01).
Fig 3.
Effect of microcapsules of Decapeptyl (releasing 25 μg/day) on serum hormone levels in male and female rats during the 30-day treatment. (a) Serum LH in male rats. (b) Serum LH levels in female rats. (c) Serum testosterone in male rats. (d) Serum estradiol in female rats. (e) Serum progesterone in female rats. *, P < 0.05; **, P < 0.01 vs. control.
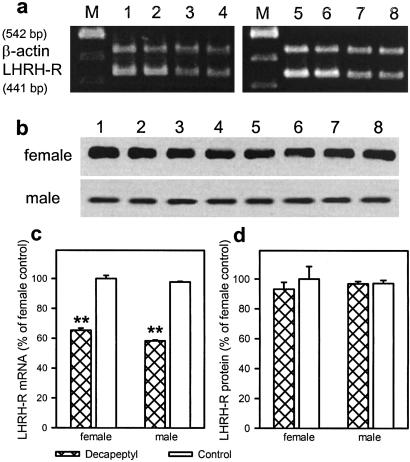
The mRNA expression for pituitary LHRH-R was decreased in females and males by 35% and 40%, respectively (P < 0.01) (Fig. 4 a and c). LHRH-R protein levels were also similar in the control male and female pituitaries. Despite the decrease in LHRH-R mRNA levels, the LHRH-R protein was not reduced in the treated rats after 30 days (Fig. 4 b and d).
Fig 4.
Effect of microcapsules of Decapeptyl on LHRH-R mRNA expression and LHRH-R protein levels in female and male rats after the 30-day treatment. (a) RT-PCR products of the pituitary LHRH-R mRNA and β- actin mRNA. Lanes: M, 100-bp DNA molecular weight marker; 1 and 2, female controls; 3 and 4, female Decapeptyl treated; 5 and 6, male controls; and 7 and 8, male Decapeptyl treated. (b) Western blot for LHRH-R protein. Lanes: 1–4, controls; 5–8: Decapeptyl. (c) LHRH-R mRNA levels after 30 days. (d) LHRH-R protein levels after 30 days. Values are expressed as percentage of the control levels in female rats; **, P < 0.01 vs. control.
Effects of Daily Injections of Decapeptyl for 10 Days in Female Rats.
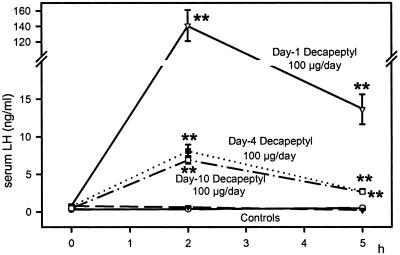
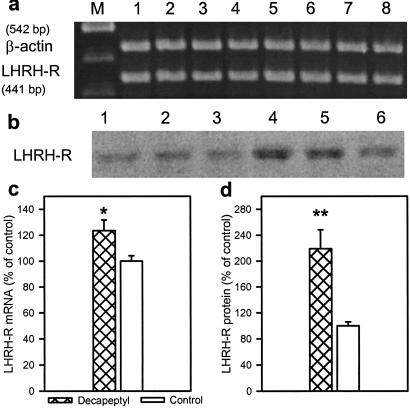
Repeated daily injections of 100 μg of Decapeptyl abolished the estrus cycle, and the rats remained in diestrus during the 10-day period. The weights of ovaries and uterus were decreased by 33% and 56% (P < 0.01), respectively, during this treatment as in the case of the depot preparations. In the Decapeptyl-treated rats, serum estrogen and progesterone levels on day 10 were reduced from 18.8 ± 1.7 to 12.8 ± 0.6 pg/ml (P < 0.05) and from 20.5 ± 0.8 to 4.8 ± 0.4 ng/ml (P < 0.01), respectively. Daily treatment with Decapeptyl also resulted in a desensitization of the LH responses (Fig. 5). The highest elevation in LH was obtained on day 1, and the responses on days 4 and 10 were greatly reduced. In contrast to depot preparations, daily Decapeptyl injections caused a 23% elevation in LHRH-R mRNA expression (P < 0.05) and a 119% rise in LHRH-R protein (P < 0.01) in the treated group at the end of the 10-day period (Fig. 6)
Fig 5.
Effect of daily injections of Decapeptyl (100 μg/day) for 10 days on serum LH in female rats during the treatment. Serum LH levels were measured for 5 h after the injection on days 1, 4, and 10. **, P < 0.01 vs. control.
Fig 6.
Effect of daily injections of Decapeptyl (100 μg/day) for 10 days on LHRH-R mRNA expression and LHRH-R protein levels in female rats. (a) RT-PCR products of the pituitary LHRH-R mRNA and β-actin mRNA. Lanes: M, 100-bp DNA molecular weight marker; 1–4, control; 5–8, Decapeptyl. (b) Immunodetection of LHRH-R. Lanes: 1–3, control; 4–6, Decapeptyl. (c) LHRH-R mRNA levels. (d) LHRH-R protein levels. *, P < 0.05; **, P < 0.01 vs. control.
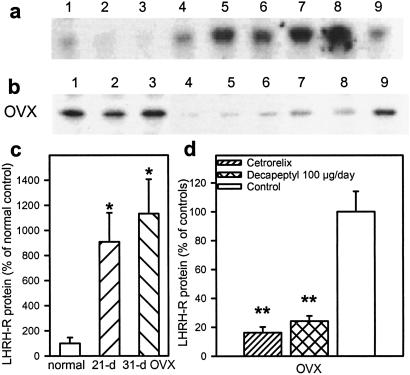
Changes in LHRH-R Protein After 10-Day Treatment with a Cetrorelix Depot Formulation or Daily Injections of 100 μg of Decapeptyl in OVX Female Rats.
The effects of these treatments on LH levels and LHRH-R mRNA expression were reported (28). In brief, Cetrorelix significantly decreased the LH levels, and daily injections of Decapeptyl reduced LH responses on days 4, 7, and 10 of the treatment in the OVX rats. Both Cetrorelix and the daily Decapeptyl injections reduced the expression of mRNA for pituitary LHRH-R in the OVX rats. Studies on levels of LHRH-R protein were performed. We found that the pituitaries of OVX control rats 21 and 31 days after the operation contained much higher levels of LHRH-R protein than those of normal rats. In these OVX rats, LHRH-R protein levels were decreased by 84% and 76% (P < 0.01) after depot Cetrorelix or daily Decapeptyl administration, respectively (Fig. 7).
Fig 7.
Western blots for pituitary LHRH-R protein. (a) Normal controls (lanes 1–3) and untreated OVX rats 21 days (lanes 4–6) and 31 days (lanes 7–9) after ovariectomy. (b) OVX rats: Controls (lanes 1–3) and rats treated with Cetrorelix (lanes 4–6) or daily injections of Decapeptyl for 10 days (lanes 7–9) starting 21 days after ovariectomy. (c) LHRH-R protein levels in normal and untreated OVX rats 21 and 31 days after ovariectomy. (d) LHRH-R protein levels in OVX rats after 10-day treatment. Values are expressed as percentage of untreated OVX rats. *, P < 0.05; **, P < 0.01 vs. control.
Discussion
Our results show that long-acting formulations of the LHRH agonist Decapeptyl and LHRH antagonist Cetrorelix inhibit the reproductive system in rats to similar extents. However, there are various differences in the effects of the two types of these analogs on serum LH levels and the expression of LHRH-R gene and levels of LHRH-R protein. During the 1-mo period after a single injection of depot preparations of either analog, female rats were in a state of constant diestrus, and at the end of the experiment the weights of uterus and ovaries were greatly reduced to a comparable degree. In female rats the levels of estradiol and progesterone were markedly diminished with either analog. Decapeptyl also had a powerful inhibitory effect on testosterone levels in male rats and decreased the weight of testes and accessory sex organs. Cetrorelix reduced serum LH levels in female rats; however, during Decapeptyl treatment we observed consistently a 2- to 3-fold rise in LH. This elevation in LH levels in female rats was significant during the entire 30-day treatment with Decapeptyl microcapsules, but was far smaller than that seen after daily injections of 100 μg of Decapeptyl on days 1, 4, and even 10. When Decapeptyl microcapsules were given to male rats, the LH levels fell below control levels after an initial rise. Thus, in this aspect there was a sex-related difference in the responses of pituitary cells to the agonists of LHRH. Sex-related differences in LH secretion were reported previously by one of us (J.E.H.) in the in vitro pituitary superfusion system after long-term (6 h) stimulation with LHRH (29, 30), showing that sex-related factors may play a role in the fine regulation of the gonadotropes. This finding indicates that the results on LH obtained in male animals might not be fully applicable to females. Despite the sex-related differences, a similar reduction in the mRNA expression, but no decrease in LHRH-R protein, was detected at 30 days, in both female and male rats after treatment with microcapsules of Decapeptyl. In our previous in vivo studies in male rats, we found a decrease in serum LH levels after long-term administration of [d-Trp6]LHRH preparations (31–33). However, Roth et al. (34) found elevated serum LH accompanied by lowered estradiol values in female rats treated with Decapeptyl for 12 days.
It has also been reported that free gonadotropin α-subunit levels are elevated after treatment with LHRH agonists in rats and humans (35, 36). The LH antiserum from the National Hormone and Peptide Program used by us in the RIA of LH has <0.01% cross-reaction with FSH and thyroid-stimulating hormone (TSH). This means that the detected rise in LH could be because of the presence of immunoreactive β-subunits of LH. Lahlou et al. (36) found that in girls suffering from precocious puberty treated with microcapsules of Decapeptyl, the LH levels were significantly elevated when determined by RIA, but not when monoclonal immunoradiometric assay was used. They suggested that this discrepancy was due to the secretion of unusual LH molecules that are recognized by RIA, but not by monoclonal immunoradiometric assay (36). Because the serum levels of sex steroids in our study were greatly decreased, despite the elevated LH levels as measured by RIA, this elevation could have been caused by immunologically active, but biologically inactive, LH molecules.
Various in vivo studies on the effects of LHRH agonists and antagonists on the expression of mRNA for pituitary LHRH-R have been reported (26, 28, 33, 35, 37, 38). Kaiser and coworkers (37) reported that ovariectomy increased mRNA levels for LHRH-R in rat pituitaries. In vivo treatment with leuprolide lowered LH levels and markedly decreased mRNA for LHRH-R in sheep pituitaries (38). Administration of microcapsules of [d-Trp6]LHRH into rats was also reported to cause a decrease in the levels of mRNA for pituitary LHRH-R for at least 30 days (35). In the present study, the mRNA expression of pituitary LHRH-R and LHRH-R protein was greatly decreased 10 days after administration of depot formulations of Cetrorelix or Decapeptyl, but the relative extent of the reduction in LHRH-R protein was smaller. At 30 days, both the LHRH-R mRNA and the LHRH-R protein levels in the Cetrorelix-treated group continued to show a decrease. In contrast, in rats given depot Decapeptyl, the extent of the reduction in the LHRH-R mRNA expression declined and the LHRH-R protein levels returned to the control values, whereas LH levels were lower than on day 10. These results suggest that a reduction in the LHRH-R gene expression might contribute to the effects seen in the initial phase of the treatment, but other postreceptor mechanisms could be involved.
In rats treated with daily injections of Decapeptyl, there was a small increase in LHRH-R mRNA levels after 10 days. The extent of this increase was smaller than the 2.5-fold rise in LHRH-R mRNA reported (28). However, in the previous study (28), the control rats were killed in estrus as compared with controls in diestrus II in this experiment. There is no discrepancy between our present and previous findings because it has been reported that in normal rats in diestrus II, the LHRH-R mRNA levels are 2–3 times higher than in estrus (39, 40). The elevation of LHRH-R mRNA in the normal female rats after the daily Decapeptyl treatment found in this study was accompanied by a 2.2-fold rise of LHRH-R protein levels. However, in OVX rats the daily treatment with Decapeptyl or Cetrorelix pamoate for 10 days produced a decrease in the LHRH-R protein levels, elevated 9- to 11-fold after ovariectomy. Thus, in normal rats the daily Decapeptyl treatment stimulates the LHRH-R expression, but in OVX rats, where the gonadotropes are overstimulated, there is no further stimulation.
Decapeptyl and other LHRH agonists are stimulatory superactive analogs of LHRH with important clinical applications, and their effects depend on the dose and mode of administration. An acute injection of agonists of LHRH causes a marked release of LH and FSH, but continuous administration of these substances produces an inhibition of the hypophyseal–gonadal axis through the process of down-regulation of pituitary LHRH-R, a decrease in the expression of LHRH-R gene, and a suppression of circulating levels of LH and sex steroids. The molecular and cellular basis of the LHRH action on the pituitary and signal transduction pathways of LHRH receptors have been reviewed (12, 15, 19). The effects of prolonged administration of LHRH agonists on the sex steroid levels and gonads are similar to those of antagonists. However, LHRH antagonists produce a competitive blockade of LHRH-R, cause an immediate inhibition of the release of gonadotropins and sex steroids, and thus reduce the time of the onset of therapeutic effects. The principal mechanism of action of LHRH antagonists was thought to be based on a competitive receptor occupancy of LHRH-R. However, we were able to demonstrate that chronic administration of Cetrorelix also produces down-regulation of pituitary LHRH receptors and a decrease in the levels of mRNA for LHRH receptors in rats (26, 28, 33, 41, 42). Recently we showed that LHRH antagonists suppress the LHRH-R gene expression by counteracting the stimulatory effect of endogenous LHRH (26, 28). In vitro, in systems that lack LHRH, Cetrorelix does not cause any change in LHRH-R gene expression, but prevents the up-regulation of the receptor mRNA expression induced by exogenous LHRH (26, 28). In the present study Cetrorelix exerted a steady, negative effect at all of the stages of pituitary–gonadal regulation and decreased the LHRH-R mRNA and LHRH-R protein levels, as well as serum LH and sex steroids, and this effect resulted in a profound reduction in the weights of reproductive organs.
The results reported in this paper as well as previous studies (4, 6, 12, 13, 26, 28, 34, 41) demonstrate that large doses of Cetrorelix such as 0.3–0.5 mg per kg per day down-regulate pituitary LHRH-R in rats. Such a down-regulation could take place in a clinical setting in patients with prostate cancers treated with large doses of LHRH antagonists (6, 12). Clinical findings also suggest that a down-regulation of pituitary receptors for LHRH may have occurred in male volunteers treated with large doses (10 mg/day) of Cetrorelix for 5 days (43).
However, this down-regulation is unlikely to occur in the in vitro fertilization and embryo transfer procedures and the treatment of endometriosis, leiomyomas, and benign prostatic hyperplasia in which lower doses of Cetrorelix are used and in which the purpose of therapy is a temporary blocking of the pituitary LH surges (in vitro fertilization and embryo transfer) or incomplete inhibition of pituitary–gonadal axis and partial decrease in sex steroid levels. These important clinical applications support the merit of an evaluation of the effect of smaller doses of Cetrorelix on the pituitary–gonadal axis in rats.
Acknowledgments
We thank Beata Katona and Elena Glotser for the technical assistance, Ipsen Pharma Biotech for gifts of microcapsules of Decapeptyl, and Zentaris for Cetrorelix pamoate depot. We are grateful to Drs. Marc Roger and Najiba Lahlou for helpful suggestions. The work described in this paper was supported by the Medical Research Service of the Veterans Affairs Department (to A.V.S.) and by a grant from Zentaris to Tulane University School of Medicine (to A.V.S.). The materials for RIA of LH from the National Hormone and Peptide Program of the National Institute of Diabetes and Digestive and Kidney Diseases are greatly appreciated. Tulane University holds patents on the LHRH antagonist Cetrorelix, and A.V.S. is a coinventor on those patents.
Abbreviations
LH, luteinizing hormone
LHRH, LH-releasing hormone
LHRH-R, LHRH receptor
FSH, follicle-stimulating hormone
OVX, ovariectomized
References
- 1.Schally A. V., Arimura, A., Kastin, A. J., Matsuo, H., Baba, Y., Redding, T. W., Nair, R. M., Debeljuk, L. & White, W. F. (1971) Science 173, 1036-1038. [DOI] [PubMed] [Google Scholar]
- 2.Clayton R. N. & Catt, K. J. (1981) Endocr. Rev. 2, 186-209. [DOI] [PubMed] [Google Scholar]
- 3.Clayton R. (1989) J. Endocrinol. 120, 11-19. [DOI] [PubMed] [Google Scholar]
- 4.Schally A. V., Comaru-Schally, A. V., Nagy, A., Kovacs, M., Szepeshazi, K., Plonowski, A., Varga, J. L. & Halmos, G. (2001) Front. Neuroendocrinol. 22, 248-291. [DOI] [PubMed] [Google Scholar]
- 5.Schally A. V., Halmos, G., Rekasi, Z. & Arencibia, J. M. (2001) in Infertility and Reproductive Medicine Clinics of North America, ed. Devroey, P. (Saunders, Philadelphia), Vol. 12, pp. 17–44. [Google Scholar]
- 6.Schally A. V. & Comaru-Schally, A. M. (2000) in Cancer Medicine, eds. Holland, J. F., Frei, E., Bast, R. C., Kufe, D. E., Pollock, R. E. & Weichselbaum, R. R. (Dekker, New York), pp. 715–729.
- 7.Conn P. M., Huckle, W. R., Andrews, W. V. & McArdle, C. A. (1987) Recent Prog. Horm. Res. 43, 29-61. [DOI] [PubMed] [Google Scholar]
- 8.Belchetz P. E., Plant, T. M., Nakai, Y., Keugh, E. J. & Knobil, E. (1978) Science 202, 631-633. [DOI] [PubMed] [Google Scholar]
- 9.Loumaye E. & Catt, K. J. (1982) Science 215, 983-985. [DOI] [PubMed] [Google Scholar]
- 10.Shupnik M. A. (1990) Mol. Endocrinol. 4, 1444-1450. [DOI] [PubMed] [Google Scholar]
- 11.Haisenleder D. J., Ortolano, G. A., Yasin, M., Dalkin, A. C. & Marshall, J. C. (1993) Endocrinology 132, 1292-1296. [DOI] [PubMed] [Google Scholar]
- 12.Schally A. V. (1999) Peptides 20, 1247-1262. [DOI] [PubMed] [Google Scholar]
- 13.Schally A. V., Comaru-Schally, A. M., Gonzalez-Barcena, D., Reissmann, T. & Engel, J. (1997) in Endometriosis Today: Advances in Research and Practice, eds. Minaguchi, H. & Sugimoto, O. (Parthenon, Carnforth, U.K.), pp. 401–413.
- 14.Hawes B. E., Barnes, S. & Conn, P. M. (1993) Endocrinology 132, 2124-2130. [DOI] [PubMed] [Google Scholar]
- 15.Stojilkovic S., Reinhart, J. & Catt, K. (1994) Endocr. Rev. 15, 462-499. [DOI] [PubMed] [Google Scholar]
- 16.Schally A. V. & Comaru-Schally, A. M. (1997) Adv. Drug Delivery Rev. 28, 157-169. [DOI] [PubMed] [Google Scholar]
- 17.Schally A. V. (1999) Gynecol. Endocrinol. 13, 401-409. [DOI] [PubMed] [Google Scholar]
- 18.Reissmann T., Schally, A. V., Bouchard, P., Riethmuller, H. & Engel, J. (2000) Hum. Reprod. Update 6, 322-331. [DOI] [PubMed] [Google Scholar]
- 19.Conn P. M. & Crawley, W. F. (1991) N. Engl. J. Med. 324, 93-103. [DOI] [PubMed] [Google Scholar]
- 20.Emons G. & Schally, A. V. (1994) Reproduction 9, 1364-1379. [DOI] [PubMed] [Google Scholar]
- 21.Bajusz S., Csernus, V. J., Janaky, T., Bokser, L., Fekete, M. & Schally, A. V. (1988) Int. J. Pept. Protein Res. 32, 425-435. [DOI] [PubMed] [Google Scholar]
- 22.Molineaux C. J., Sluss, P. M., Bree, M. P., Gefter, M. L., Sullivan, L. M. & Garnick, M. B. (1998) Mol. Urol. 2, 265-268. [Google Scholar]
- 23.Nelson L. R., Fujimoto, V. Y., Jaffe, R. B. & Monroe, S. E. (1995) Fertil. Steril. 63, 963-969. [DOI] [PubMed] [Google Scholar]
- 24.Reissmann T., Engel, J., Kutscher, B., Bernd, M., Hilgard, P., Peukert, M., Szelenyi, I., Reichert, S., Gonzales-Barcena, D., Nieschlag, E., et al. (1994) Drugs Future 19, 228-237. [Google Scholar]
- 25.Klingmuller D., Schepke, M., Enzweiler, C. & Bidlingmaier, F. (1993) Acta Endocrinol. 128, 15-18. [DOI] [PubMed] [Google Scholar]
- 26.Kovacs M., Schally, A. V., Csernus, B. & Rekasi, Z. (2001) Proc. Natl. Acad. Sci. USA 98, 1829-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karande A. A., Rajeshwari, K., Schol, D. J. & Hilgers, J. H. M. (1995) Mol. Cell. Endocrinol. 114, 51-56. [DOI] [PubMed] [Google Scholar]
- 28.Kovacs M. & Schally, A. V. (2001) Proc. Natl. Acad. Sci. USA 98, 12197-12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horvath J. E., Keri, G., Seprodi, A., Teplan, I. & Flerko, B. (1992) J. Neuroendocrinol. 4, 565-573. [DOI] [PubMed] [Google Scholar]
- 30.Horvath J. E., Helyes, Z. & Flerko, B. (1996) Acta Biol. Hung. 47, 195-205. [PubMed] [Google Scholar]
- 31.Mason-Garcia M., Vigh, D., Comaru-Schally, A. M., Redding, T. W., Somogyvari-Vigh, A., Horvath, J. & Schally, A. V. (1985) Proc. Natl. Acad. Sci. USA 82, 1547-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redding T. W. & Schally, A. V. (1990) J. Endocrinol. 126, 309-315. [DOI] [PubMed] [Google Scholar]
- 33.Pinski J., Lamharzi, N., Halmos, G., Groot, K., Jungwirth, A., Vadillo-Buenfil, M., Kakar, S. S. & Schally, A. V. (1996) Endocrinology 137, 3430-3436. [DOI] [PubMed] [Google Scholar]
- 34.Roth C., Leonhardt, S., Seidel, C., Luft, H., Wuttke, W. & Jarry, H. (2000) Pediatr. Res. 48, 468-474. [DOI] [PubMed] [Google Scholar]
- 35.Lerrant Y., Kottler, M. L., Bergametti, F., Moumni, M., Blumberg-Tick, J. & Counis, R. (1995) Endocrinology 136, 2803-2808. [DOI] [PubMed] [Google Scholar]
- 36.Lahlou N., Roger, M., Chaussain, J. L., Feinstein, M. C., Sultan, C., Toublanc, J. E., Schally, A. V. & Scholler, R. (1987) J. Clin. Endocrinol. Metab. 65, 946-953. [DOI] [PubMed] [Google Scholar]
- 37.Kaiser U. B., Jakubowiak, A., Steinberger, A. & Chin, W. W. (1993) Endocrinology 133, 931-934. [DOI] [PubMed] [Google Scholar]
- 38.Wu J. C., Sealfon, S. C. & Miller, W. L. (1994) Endocrinology 134, 1846-1850. [DOI] [PubMed] [Google Scholar]
- 39.Funabashi T., Brooks, P. J., Weesner, G. D. & Pfaff, D. W. (1994) J. Neuroendocrinol. 6, 261-266. [DOI] [PubMed] [Google Scholar]
- 40.Kakar S. S., Grantham, K., Musgrove, L. C., Devor, D., Sellers, J. C. & Neill, J. D. (1994) Mol. Cell. Endocrinol. 101, 151-157. [DOI] [PubMed] [Google Scholar]
- 41.Halmos G., Schally, A. V., Pinski, J., Vadillo-Buenfil, M. & Groot, K. (1996) Proc. Natl. Acad. Sci. USA 93, 2398-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halmos G. & Schally, A. V. (2002) Proc. Natl. Acad. Sci. USA 99, 961-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Behre H. M., Kliesch, S., Puhse, G., Reissmann, T. & Nieschlag, E. (1997) J. Clin. Endocrinol. Metab. 82, 1403-1408. [DOI] [PubMed] [Google Scholar]