Abstract
The causes of many sporadic diseases are unexplained; the contribution of recessive loci with reduced penetrance is one possibility that has been difficult to explore. We describe an approach to this problem by first searching for diseases with higher prevalence in populations with high rates of consanguinity, then determining whether disease cases are more commonly the product of consanguinous union than controls in such populations, followed by analysis of genetic linkage in consanguinous cases. We demonstrate the utility of this approach by investigation of congenital heart disease in Iran. We found that patent ductus arteriosus (PDA), a common congenital heart disease, accounts for a higher fraction of congenital heart disease in Iran (15%) than in the United States (2–7%). Moreover, Iranian PDA cases demonstrated a marked increase of parental consanguinity (63%), compared with the general Iranian population (25%) or control cases with tetralogy of Fallot (30%). The recurrence of PDA among siblings was 5%. A genomewide analysis of linkage in 21 unrelated consanguinous PDA cases demonstrated a multipoint logarithm of odds score of 6.27 in favor of linkage of PDA to a 3-centimorgan interval of chromosome 12q24, with 53% of kindreds linked. These findings together establish a recessive component to PDA and implicate a single locus, PDA1, in one third or more of all PDA cases in Iran; they further suggest a role for this locus in PDA worldwide. Finally, these results suggest a general approach to the identification of recessive contributions to sporadic diseases.
Keywords: linkage (genetics), patent ductus arteriosus, consanguinity, human chromosomes, pair 12
Many diseases occur sporadically within populations, and the factors accounting for this distribution are generally unknown. Although most of these recur within families more often than expected by chance alone, the pattern of familial recurrence is not compatible with simple Mendelian transmission; this modest familial clustering typically is presumed to reflect multifactorial determination with contributions from multiple genes and/or environmental factors. An alternative possibility is that the observed familial recurrence could be attributed to single loci with large effect but reduced penetrance such that within a given family nearly all affected subjects would harbor mutation at the same disease locus, but only a fraction of individuals inheriting the risk allele develop disease.
For dominant alleles with reduced penetrance, analysis of extended kindreds can be expected to identify multiplex families with affected members in multiple branches of the kindred. Analysis of linkage in such families is expected to reveal chromosome segments inherited identically by descent among all affected subjects. For several diseases such as breast cancer (1) and IgA nephropathy (2), analysis of linkage in such “loaded” kindreds has demonstrated the role of single loci with large effect, streamlining the path to identification of disease loci.
A part of the genetic space that heretofore has been largely unexplored is the search for recessive loci with reduced penetrance. In this instance, disease recurrence in multiple generations generally is not expected, and in populations with modest sibship sizes, recurrence among siblings is expected to be uncommon as well. As a result, such traits would seem to be sporadic in the population with relatively low recurrence among siblings. Moreover, the low recurrence rate among siblings for a disease of modest frequency in the population would make it extremely difficult to recruit sufficient multiplex kindreds for a linkage study with adequate power.
We have considered an approach to recognize diseases in which recessive loci with reduced penetrance play a large role. In this setting, we presume that disease occurrence should be increased in populations in which consanguinous union is common such that recessive alleles become homozygous with increased frequency; such differences in disease prevalence should be readily recognizable to trained clinicians with exposure to both populations. For diseases found to be more common in populations with high consanguinity, recessive contributions would be inferred by the finding that disease cases occur more commonly as a result of parental consanguinity than births in the general population. Diseases that pass these criteria can be pursued by genomewide analysis of linkage in consanguinous kindreds by using homozygosity mapping (3), in which a small number of kindreds with a single affected case can be sufficient to map a disease locus.
We use this approach to demonstrate that persistent patency of the ductus arteriosus (patent ductus arteriosus, PDA) is commonly a recessive disease with incomplete penetrance. PDA is the second most common congenital heart disease, affecting ≈1/1,600–5,000 live births in the United States (4). In fetal life the ductus arteriosus, a muscular artery, shunts blood from the pulmonary artery to the aorta, bypassing the lungs. Its abrupt closure at birth establishes the mature circulatory pattern and represents a dramatic example of vascular remodeling. Failure of this normal process results in persistent PDA, which left untreated can result in pulmonary hypertension and heart failure (5). The closure of the ductus is a complex process involving smooth muscle cell migration into the subendothelial space and detachment of endothelial cells, leading to formation of subintimal cushions. Subsequent accumulation of extracellular matrix with smooth muscle contraction closes the vessel lumen and results in formation of the ligamentum arteriosum (6, 7). Aspects of this process are regulated by oxygen tension and a decrease in levels of hormones such as prostaglandin E2 (8–12); however, the molecular mechanisms underlying the remodeling process are unknown. Although PDA occurring in preterm infants often closes spontaneously or in response to inhibitors of prostaglandin biosynthesis (13), cases occurring at term delivery require surgical ligation or embolization (ref. 5; Fig. 1). Term PDA is one of the most common congenital heart diseases worldwide and accounts for 2–7% of congenital heart disease in the United States (14, 15).
Fig 1.
Cross-section of term PDA. A segment of a term PDA was obtained after therapeutic surgical ligation and resection. A Van Giessen stain is shown. The lumen is widely patent. The internal elastic lamina (lamina interna, LI) is disrupted (arrow), as occurs in the normal closure of the ductus; however, few smooth muscle cells have migrated into the intima (I), and intimal cushions have not formed. M, vessel media.
Term PDA typically has not been regarded as a genetic disease, because it most often occurs sporadically. Nonetheless, term PDA recurs among 5% of siblings of PDA cases (16, 17), suggesting a genetic component to disease pathogenesis that has typically been presumed to be multifactorial. That single genes can influence this trait is demonstrated by a mouse model of PDA resulting from disruption of the prostaglandin E2 receptor (18) and by very rare syndromic forms of PDA; for example, mutations in the transcription factor TFAP2B cause Char syndrome, a rare autosomal dominant syndromic form of PDA (19).
Methods
Ascertainment and Recruitment of PDA Cases.
The study protocol was approved by the Human Investigation Committee of the Yale University School of Medicine and the Committee for Ethics and Research Conduction of the Shahid-Rajai Cardiovascular Medical Center of Teheran. Based on our clinical observation suggesting that PDA was more common in Iran than the United States, discharge diagnoses from the Pediatric Cardiology service of the Shahid-Rajai Cardiovascular Medical Center for the years 1991–2000 were reviewed, and medical records of all admissions to the service from 1998 to 2000 were reviewed to identify cases of term PDA and tetralogy of Fallot. Records were reviewed for documentation of diagnosis, age of diagnosis, presenting clinical findings, treatment, family history and parental consanguinity. For this study, consanguinity was defined as union of second cousins or individuals more closely related.
Patients with nonsyndromic term PDA arising from consanguinous union were recruited during inpatient hospitalization at the Shahid-Rajai Cardiovascular Medical Center. Twenty-one unrelated cases were identified and recruited. In all cases the diagnosis had been established by echocardiography followed by angiography and surgical ligation of the ductus. Patients underwent a physical examination, and venous blood samples were collected. Blood samples were processed locally with the Puregene red cell lysis and cell lysis solution (Gentra Systems). DNA extraction was completed after transport to Yale University.
Genotyping and Analysis of Linkage.
Genomewide analysis of linkage was performed by using 382 highly polymorphic di- and tetranucleotide repeat markers spaced at an average distance of 10 centimorgans (cM) across all autosomes (20). Additional markers were typed in selected intervals to maximize informativeness; among these were six new polymorphic simple sequence-repeat markers in the 12q24 interval that were identified from genomic sequence (Table 1). Markers were genotyped by PCR using specific fluorescently labeled oligonucleotide primers and genomic DNA as template. The resulting amplified products were fractionated by electrophoresis on denaturing polyacrylamide gels by using an ABI377 or ABI3700 instrument and analyzed by using genescan 2.1 and genotyper1.1.1 software. All genotypes were scored independently by two investigators, and critical genotypes were repeated to ensure data accuracy. Analysis of linkage by homozygosity mapping (3) was performed on a Sun Blade 2000 with the GENEHUNTER linkage program (21). The disease locus was specified as an autosomal recessive trait, allowing for locus heterogeneity among families, with a disease allele frequency of 0.01, and no phenocopies. Marker loci were modeled as having four alleles of equal frequency, providing 75% heterozygosity. The order of the 36 marker loci typed in the 12q24 interval was established by their localization on draft and finished human genomic sequence from the National Center for Biotechnology Information (NCBI) www.ncbi.nlm.nih.gov web site. Allele frequencies for these marker loci were also estimated from observed frequencies in 22 unaffected, unrelated Iranian control subjects; the mean heterozygosity of markers in these subjects was 81%. These markers showed no significant evidence of marker–marker disequilibrium in control subjects.
Table 1.
Primer sequences for designed markers from genomic sequence of chromosome 12q24
| Locus
|
Mb
|
Primer sequence | |
|---|---|---|---|
| Forward | Reverse | ||
| F1 | 125.5 | gaatgtgaggtgatagatctg | aggcatgagccactgtacctg |
| F3 | 125.8 | ctctccttcatttgtcctattc | agagttttctgaatgtccgcagc |
| F4 | 125.87 | catactggaagccactaaacc | gtgaacatctctaaacctctc |
| F8 | 125.9 | tcccagctactcaggaggctgag | cagtgctgttaactgtgaattaac |
| F12 | 126.9 | cactagataccagtgttgac | tctgctttcccatctgcaga |
| F9 | 127 | cgtagtgtta agctgttaggtg | caataacttggattggcctgt |
Mb, megabase distance from 12pter on NCBI map.
Candidate Gene Analysis.
Mutations in candidate genes in the 12q24 interval were sought by single-strand conformational polymorphism (SSCP). All coding regions and intron–exon junctions of these genes were amplified as 150- to 250-bp fragments by PCR using specific primers and fractionated under two nondenaturing conditions. Identified variants were eluted from gel, and the DNA sequence of both strands was determined.
Results
Evidence for Recessive Inheritance of PDA.
We investigated the occurrence of congenital heart disease in northern Iran, a population in which 25% of live births are the product of consanguinous union (22, 23). Review of discharge diagnoses from the inpatient pediatric cardiology service of the Shahid-Rajai Cardiovascular Medical Center in Tehran identified 13,300 patients with congenital heart disease from 1991 to 2000. Among these were 2,019 cases with term PDA. The proportion of PDA cases (15%) was striking and higher than that seen in the United States, where PDA accounts for 2–7% of cases of congenital heart disease (14).
A review of medical records for the period of 1998–2000 identified 358 term PDA cases; all were documented by angiography and had undergone surgical closure. Parental relationships were documented for 338 of these cases and revealed parental consanguinity in 212 (63%). This prevalence is markedly higher than in the general population (25%; P < 10−6) (22, 23). The prevalence of consanguinity among these PDA cases was also much higher than for another congenital heart disease, tetralogy of Fallot, seen at the same hospital over the same period (consanguinity in 59 of 196 cases (30%; P < 10−6, PDA vs. tetralogy of Fallot). In 24 PDA kindreds (7%) there was a history of two or more affected offspring, and none of the parents had a history of PDA. The overall recurrence rate of PDA among siblings of index cases was 5% and was not significantly different among consanguinous and nonconsanguinous kindreds. These findings together provide evidence for the influence of recessive loci with reduced penetrance.
Linkage of PDA to 12q24.
To search for recessive loci contributing to PDA, we recruited members of 21 consanguinous term PDA kindreds for further study. Affected subjects typically presented with tachypnea before age 2; congestive heart failure, cyanosis, or pneumonia were presenting findings in a subset of patients (Table 2). All were free of syndromic features.
Table 2.
Features of affected members of 21 PDA kindreds
| Kindred | Age at dx, years | Parental relationship | Clinical presentation | lod score at PDA1 |
|---|---|---|---|---|
| K126 | 2.0 | 2nd cousin | Tachypnea | 1.60 |
| K109 | 2.0 | 2nd cousin | Fatigue | 1.46 |
| K107 | 2.0 | 1st cousin | Tachypnea, CHF | 1.16 |
| K108 | 0.9 | 1st cousin | Tachypnea | 1.16 |
| K115 | 2.0 | 1st cousin | Growth delay | 1.16 |
| K111 | 1.5 | 1st cousin | Tachypnea, CHF, cyanosis | 1.16 |
| K134 | 1.5 | 1st cousin | Tachypnea | 1.16 |
| K117 | 1.0 | 1st cousin | Fainting | 1.15 |
| K139 | 2.0 | 1st cousin | Tachypnea | 1.15 |
| K122 | 1.9 | 2nd cousin | Growth delay | −0.41 |
| K123 | 1.5 | 2nd cousin | Pneumonia | −0.41 |
| K124 | 0.9 | 1st cousin | Hypotension | −0.42 |
| K125 | 0.9 | 2nd cousin | Hypotension | −0.42 |
| K131 | 13.0 | 1st cousin | Tachypnea | −0.43 |
| K116 | 6.0 | 2nd cousin | Cyanosis, CHF | −0.44 |
| K121 | 0.9 | 2nd cousin | Tachypnea | −0.72 |
| K114 | 0.9 | 1st cousin′ | Pneumonia | −0.65 |
| K113 | 3.0 | 1st cousin | Murmur only | −0.92 |
| K133 | 0.7 | 1st cousin | Tachypnea | −0.94 |
| K112 | 1.0 | 1st cousin | Pneumonia | −0.96 |
| K135 | 2.0 | 2nd cousin | Tachypnea | −0.98 |
lod, the multipoint lod score at zero recombination with marker D12S395; dx, diagnosis; 1st cousin′, first cousin once removed; CHF, congestive heart failure.
We performed a genomewide analysis of linkage by using homozygosity mapping and allowing for locus heterogeneity (see Methods). No chromosome segment was homozygous in all affected subjects. Under models allowing for locus heterogeneity, three intervals (12q24, 1q32, and 10q11) yielded initial multipoint logarithm of odds (lod) scores greater than 1.0. After typing of 5–10 additional markers in each of these intervals to increase informativeness, the lod score remained greater than 1.5 in only one interval, 12q24.
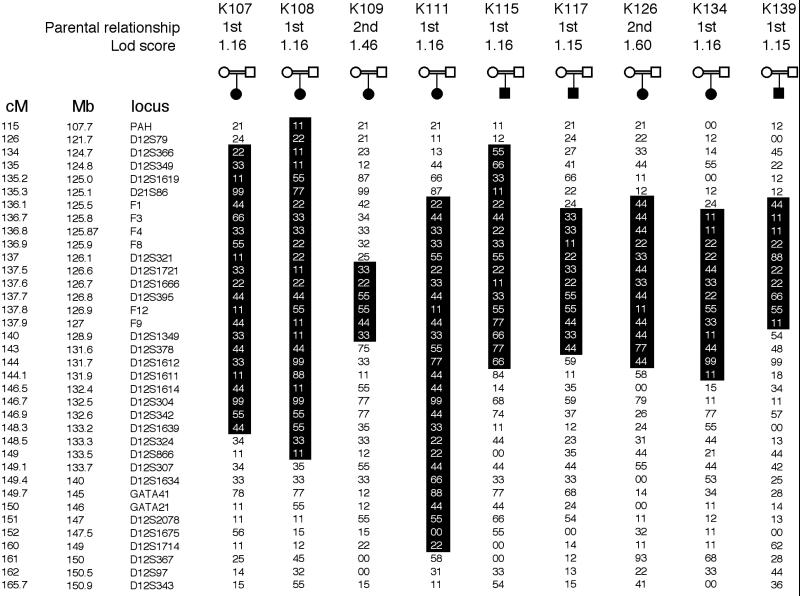
A panel of 36 highly polymorphic microsatellite markers spanning a 51-cM interval of 12q24 then was genotyped. In nine kindreds, overlapping segments of homozygosity were found, with 6–26 consecutive homozygous markers spanning chromosome segments ranging from 4 to 34 cM in length (Fig. 2); lod scores for linkage of this interval to the disease were greater than 1.0 in each of these kindreds and near their theoretical maximum under the specified parametric model (marker loci specified as having four alleles with equal frequencies, trait locus specified as recessive with disease allele frequency 0.01, and allowing locus heterogeneity with no phenocopies). Multipoint analysis of linkage with all typed markers in all families revealed a lod score of 6.27 in favor of linkage to 12q24 (odds ratio of 1.86 × 106:1 in favor of linkage), with an estimated 53% of kindreds linked. The lod score peak lies at zero recombination with locus D12S1721 (Fig. 3). The lod-1 interval spans a 3-cM interval (containing 2.8 million base pairs) flanked by loci D12S321 and D12S1349. These results far exceed the threshold for significant linkage with locus heterogeneity (lod score 3.3).
Fig 2.
Genotypes at 12q24 in PDA kindreds. Genotypes of 36 marker loci at 12q24 are shown for nine consanguinous PDA kindreds that show evidence of linkage. At the top, each kindred number is shown, followed by the parental relationship (1st denotes first cousins, etc.), followed by the multipoint lod score at the position of locus D12S395. Genotypes of 36 marker loci spanning a 51-cM interval of 12q24 are shown in their chromosomal order for each affected individual; the positions of each marker on the genetic and physical maps are indicated in cM and Mb, respectively. Homozygous chromosome segments within the linked interval are enclosed in boxes.
Fig 3.
Multipoint analysis of linkage of PDA to 12q24. The multipoint lod score for linkage of PDA across a 60-cM segment of chromosome 12 for all 21 kindreds is shown. The positions of 36 marker loci are shown at the top, and the multipoint lod scores calculated by using homozygosity mapping with the GENEHUNTER program are shown. The lod score peak occurs at zero recombination with marker D12S1721. The lod-1 interval for the location of PDA1 is represented as a thick bar. PAH, a tetranucleotide repeat STS in intron 3 of the phenylalanine hydroxylase gene.
These significant linkage results are robust to alternative recessive models of the trait locus and estimates of marker and disease allele frequencies. For example, analysis of linkage specifying marker allele frequencies as those observed in 22 unrelated Iranian control subjects yielded a lod score of 6.9 at the same chromosomal position under the same model of the trait locus. Similarly, varying estimates of the disease allele frequency (from 0.02 to 0.001), phenocopy rate (from 0 to 0.0005), and penetrance (from 0.3 to 1.0) all yielded lod scores greater than 6.0. Examination of genotypes and haplotypes in the linked interval does not reveal significant evidence for linkage disequilibrium between linked alleles or haplotypes and disease, providing no suggestion of a founder mutation. We refer to this first locus for nonsyndromic term PDA as PDA1.
Examination of genomic sequence in the vicinity of PDA1 identified several candidate genes for PDA including neuronal NO synthase (nNOS), paxillin, and PTPN11. nNOS is believed to play a role in regulation of ductus arteriosus tone (24), and NO regulates the posttranscriptional increase in fibronectin synthesis required for smooth muscle cell motility (25). Paxillin links extracellular matrix proteins and the actin cytoskeleton (26). PTPN11 is the gene mutated in Noonan syndrome, which has PDA as a variable feature (27). Accordingly, we searched for mutations in each of these genes among all 21 PDA kindreds. No variants in any of these genes were found that segregate with and show specificity for the disease (data not shown). We conclude that mutations in none of these three genes account for PDA in these kindreds. No other compelling candidate genes among 34 genes/hypothetical genes in the region have been identified.
Discussion
The findings in this study establish the common role of a recessive locus in nonsyndromic term PDA, one of the most common congenital heart diseases, and localize PDA1 to a 3-cM interval of 12q24. This interval spans 2.8 million bp and contains 28 known genes and 7 hypothetical genes. Whether the apparent reduced penetrance reflects a requirement for additional environmental or genetic factors or is stochastically determined is presently unknown.
We infer that PDA-1 contributes to a substantial fraction of PDA cases in Iran. From the proportion of nonsyndromic term PDA cases that result from consanguinous union in the Iranian population (63%) and the estimated proportion of consanguinous cases that are linked to PDA1 (53%), we estimate that PDA1 plays a role in the pathogenesis of at least a third of all term PDA cases in this population. This may represent an underestimate, because it assumes that none of the cases in outbred kindreds are attributable to this same locus; the fact that recurrence of term PDA in siblings is comparable with consanguinous and outbred kindreds in Iran is consistent with the same mechanism underlying disease in both. The sibling recurrence rate is also similar in outbred populations around the world (16, 17), raising the possibility that PDA1 contributes to this disease in all populations. Consistent with this notion, a prior study in India has also found an increase in parental consanguinity among term PDA cases (28). Identification of the PDA1 gene will permit this hypothesis to be tested directly.
Finally, these findings demonstrate the utility of a general approach to the identification of recessive loci contributing to the risk of sporadic disease. Heretofore, related strategies have largely started with population isolates with a limited number of founders. For example, understanding the genetics of Hirschsprung's disease was facilitated greatly by the recognition of its increased occurrence among the Amish (29). The extension of this approach to larger populations has the potential to identify many more such diseases. Such an approach seems particularly well motivated given the great difficulty in identifying loci imparting small effects on disease risk (29); the recognition of diseases with large effects attributable to single genes has the capacity to expedite progress in these disease areas. Exchanges of well trained physicians between outbred and inbred populations can provide opportunities to rapidly recognize diseases with increased prevalence in populations with high consanguinity; these candidate diseases can be assessed quickly for increased consanguinity within the population. Putative recessive alleles can be readily mapped from a small number of affected individuals born from consanguinous union, and the genes can be identified eventually by positional cloning. Such an approach may prove relevant to the understanding of a wide range of diseases not recognized presently to have large genetic contributions.
Acknowledgments
We are deeply grateful to the patients and families studied for their participation. We thank Miguel Reyes-Mugica for cutting and staining the PDA section. R.P.L. is an investigator of the Howard Hughes Medical Institute. A.M. is a recipient of a KO8 award from National Institutes of Health.
Abbreviations
PDA, patent ductus arteriosus
cM, centimorgan
lod, logarithm of odds
References
- 1.Hall J. M., Lee, M. K., Newman, B., Morrow, J. E., Anderson, L. A., Huey, B. & King, M. C. (1990) Science 250, 1684-1689. [DOI] [PubMed] [Google Scholar]
- 2.Gharavi A. G., Yan, Y., Scolari, F., Schena, F. P., Frasca, G. M., Ghiggeri, G. M., Cooper, K., Amoroso, A., Viola, B. F., Battini, G., et al. (2000) Nat. Genet. 26, 354-357. [DOI] [PubMed] [Google Scholar]
- 3.Lander E. S. & Botstein, D. (1987) Science 236, 1567-1570. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell S. C., Korones, S. B. & Berendes, H. W. (1971) Circulation 43, 323-332. [DOI] [PubMed] [Google Scholar]
- 5.Park M. K., (1996) Left-to-Right Shunt Lesions (Mosby, St. Louis), pp. 142–145.
- 6.Jarkovska D., Janatova, T., Hruda, J., Ostadal, B. & Samanek, M. (1992) Physiol. Res. 41, 323-330. [PubMed] [Google Scholar]
- 7.Gittenberger-de Groot A. C. & Strengers, J. L. (1988) Int. J. Cardiol. 19, 153-166. [DOI] [PubMed] [Google Scholar]
- 8.Challis J. R., Dilley, S. R., Robinson, J. S. & Thorburn, G. D. (1976) Prostaglandins 11, 1041-1052. [DOI] [PubMed] [Google Scholar]
- 9.Clyman R. I., Wong, L., Heymann, M. A. & Rudolph, A. M. (1978) Prostaglandins 15, 325-331. [DOI] [PubMed] [Google Scholar]
- 10.Clyman R. I., Mauray, F., Roman, C., Rudolph, A. M. & Heymann, M. A. (1980) J. Pediatr. (Berlin) 97, 455-461. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell M. D., Brunt, J., Clover, L. & Walker, D. W. (1980) J. Reprod. Fertil. 58, 283-287. [DOI] [PubMed] [Google Scholar]
- 12.Piper P. J., Vane, J. R. & Wyllie, J. H. (1970) Nature 225, 600-604. [DOI] [PubMed] [Google Scholar]
- 13.Ramsay J. M., Murphy, D. J., Vick, G. W., III, Courtney, J. T., Garcia-Prats, J. A. & Huhta, J. C. (1987) Am. J. Dis. Child. 141, 294-297. [DOI] [PubMed] [Google Scholar]
- 14.Ferencz C., Rubin, J. D., McCarter, R. J., Brenner, J. I., Neill, C. A., Perry, L. W., Hepner, S. I. & Downing, J. W. (1985) Am. J. Epidemiol. 121, 31-36. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman J. I. & Christianson, R. (1978) Am. J. Cardiol. 42, 641-647. [DOI] [PubMed] [Google Scholar]
- 16.Polani P. E. & Campbell, M. (1960) Ann. Hum. Genet. 24, 343-357. [DOI] [PubMed] [Google Scholar]
- 17.Lamy M., de Grouchy, J. & Schweisguth, O. (1957) Am. J. Hum. Genet. 9, 17-41. [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen M., Camenisch, T., Snouwaert, J. N., Hicks, E., Coffman, T. M., Anderson, P. A., Malouf, N. N. & Koller, B. H. (1997) Nature 390, 78-81. [DOI] [PubMed] [Google Scholar]
- 19.Satoda M., Zhao, F., Diaz, G. A., Burn, J., Goodship, J., Davidson, H. R., Pierpont, M. E. & Gelb, B. D. (2000) Nat. Genet. 25, 42-46. [DOI] [PubMed] [Google Scholar]
- 20.Mansfield T. A., Simon, D. B., Farfel, Z., Bia, M., Tucci, J. R., Lebel, M., Gutkin, M., Vialettes, B., Christofilis, M. A., Kauppinen-Makelin, R., et al. (1997) Nat. Genet. 16, 202-205. [DOI] [PubMed] [Google Scholar]
- 21.Kruglyak L., Daly, M. J., Reeve-Daly, M. P. & Lander, E. S. (1996) Am. J. Hum. Genet. 58, 1347-1363. [PMC free article] [PubMed] [Google Scholar]
- 22.Akbari M. T., Papiha, S. S., Roberts, D. F. & Farhud, D. D. (1986) Z. Morphol. Anthropol. 76, 197-217. [PubMed] [Google Scholar]
- 23.Farhud D. (1991) Iran. J. Med. Health 20, 1-15. [Google Scholar]
- 24.Rairigh R. L., Storme, L., Parker, T. A., Le Cras, T. D., Markham, N., Jakkula, M. & Abman, S. H. (2000) Am. J. Physiol. 278, L105-L110. [DOI] [PubMed] [Google Scholar]
- 25.Mason C. A., Chang, P., Fallery, C. & Rabinovitch, M. (1999) FASEB J. 13, 1423-1434. [DOI] [PubMed] [Google Scholar]
- 26.Turner C. E. (2000) J. Cell Sci. 113, 4139-4140. [DOI] [PubMed] [Google Scholar]
- 27.Tartaglia M., Mehler, E. L., Goldberg, R., Zampino, G., Brunner, H. G., Kremer, H., van der Burgt, I., Crosby, A. H., Ion, A., Jeffery, S., et al. (2001) Nat. Genet. 29, 465-468. [DOI] [PubMed] [Google Scholar]
- 28.Gnanalingham M. G., Gnanalingham, K. K. & Singh, A. (1999) Acta Paediatr. 88, 473-474. [DOI] [PubMed] [Google Scholar]
- 29.Gabriel S. B., Salomon, R., Pelet, A., Angrist, M., Amiel, J., Fornage, M., Attie-Bitach, T., Olson, J. M., Hofstra, R., Buys, C., et al. (2002) Nat. Genet. 31, 89-93. [DOI] [PubMed] [Google Scholar]