Abstract
Mast-cell products can stimulate fibroblast proliferation, implying that these cells are key players in fibrosis. One mast-cell product, the serine protease tryptase, is known to activate protease-activated receptor 2 (PAR2) and cause proliferation of fibroblasts. We found that recombinant tryptase, human mast-cell (HMC-1) supernatant, which contains tryptase, and the PAR2-activating peptide SLIGKV exert fibroproliferative actions in human fibroblasts. Here we report insights into this action, which after activation of PAR2 leads to increased expression of cyclooxygenase 2 (COX2), a key enzyme in the biosynthesis of prostaglandins, and consequently to enhanced prostaglandin synthesis. Subsequent cell proliferation is mediated by the prostaglandin 15-deoxy-Δ12,14-prostaglandin J2, which acts via the nuclear peroxisome proliferator-activated receptor γ (PPARγ). Fibroblast proliferation induced by tryptase and PAR2 agonist peptide can be blocked by antagonists of COX2 and PPARγ, implying that the proliferative effect of tryptase is PAR2-initiated but depends on COX2, 15-deoxy-Δ12,14-prostaglandin J2, and PPARγ. This previously uncharacterized pathway could be of relevance for human fibrotic diseases. For instance, increased numbers of activated mast cells are correlated with fibrosis in testes of infertile men. In these cases all components of the signaling pathway of tryptase were detected as well as expression of COX2. Therefore, our study describes as-yet-unknown interactions between mast cells and fibroblasts, which could be relevant for human fibrotic diseases.
Numerous diseases and inflammatory reactions are associated with fibroproliferative tissue responses. Increased numbers of fibroblasts/myofibroblasts and deposition of extracellular matrix proteins are found, for example, in lung diseases including asthma (1), diseases of the liver and gastrointestinal tract (2), and renal diseases (3, 4) as well as in cardiac (5) and skin disorders (6). Mast cells usually are present in these cases (3, 4) and seem to be key players in fibrosis. Mast cells secrete a plethora of potent mediators (7–10), which may be involved in the pathogenesis of fibrosis. One mast-cell product, the serine protease tryptase, is of special interest and has been shown to exert fibroproliferative action, most likely via activation of the protease-activated receptor 2 (PAR2) (1, 11). How this ultimately leads to cell proliferation is not fully known.
Coculture studies of a human mast-cell (HMC) line and human orbital fibroblasts have indicated up-regulation of cyclooxygenase 2 [COX2, inducible form of the key enzyme in the synthesis of prostaglandins (PGs)] in fibroblasts (12) and production of PGE2. The mast-cell product responsible for this action was thought to be IL-4. Other reports indicated that IL-1β (13) or secretory phospholipase A (14) are involved in COX2 induction and PG synthesis. Importantly, it seems that PGs, including PGD2, can regulate fibroblast proliferation and type I collagen production (15, 16). Although details of this action are not well known, this result may imply a direct role for PGs in fibrosis.
In humans, fibrotic thickening in the wall of seminiferous tubules occurs in testes of men with impaired spermatogenesis (17). This frequent change is observed irrespective of the causes of male infertility and is regarded a hallmark of male infertility. However, neither the reasons for the fibrotic changes nor their precise consequences for the complex process of spermatogenesis are known.
Nevertheless this condition attracted our interest, because coinciding with fibrotic changes activated, tryptase-producing mast cells are increased in testes of subfertile and infertile men (18). We speculated that these events may be causally linked and that mast cells via tryptase may be able to initiate fibrosis. Previous studies in which drugs including ketotifen and tranilast, which seem to act by blocking degranulation of mast cells, improved sperm count in subfertile men (19–21) lend some support of this assumption.
In the present report we investigated a possible link between mast cells and fibroblasts. We describe details of the interaction of the mast-cell product tryptase and fibroblast proliferation. Unexpectedly, we observed that the proliferative action of mast-cell tryptase in fibroblasts depends on COX2 and PG synthesis. Because components of this pathway are present in human fibrotic diseases, our results are of likely relevance to the understanding of pathogenic events in fibroproliferative disorders.
Materials and Methods
Cell Culture.
Human fetal foreskin fibroblast cells (HFFF2s, European Collection of Cell Cultures, Salisbury, U.K.) were maintained in DMEM supplemented with 10% FCS (Sigma). For all experiments, cells were incubated in FCS-free medium for 24 h to synchronize the cell cycle. To drive G0 cells into cell cycle, we treated serum-starved HFFF2s with medium containing 2.5% FCS. After 20 h, when cells entered into S phase (22), they were incubated for 24 h with 12, 120, and/or 1,200 milliunits/ml tryptase (Promega), 10−5 M PAR2 agonist peptide SLIGKV (Genzentrum, University of Munich, Germany), 10−6 M meloxicam (Calbiochem), 10−6 M PGs [D2, E1, E2, F2α, and J2 (Sigma) and 15-deoxy-Δ12,14-PGJ2 (15d-J2, Cayman Chemical, Ann Arbor, MI)] or conditioned medium from HMC-1 kindly provided by J. H. Butterfield (Mayo Clinic, Rochester, MN). In some experiments we performed a 2 h pretreatment with 10−6 M bisphenol A diglycidyl ether (BADGE, Fluka) before the stimulation for 24 h with tryptase, PAR2 agonist peptide SLIGKV, and 15d-PGJ2.
Human Biopsies.
Archival diagnostic testicular biopsies from adult men with fertility problems (age range, 28–37 years) were assigned to the following groups: specimens from men with cases of idiopathic infertility revealing normal spermatogenesis without obvious alterations (normal group, n = 7); specimens from patients with Sertoli cell-only (SCO) syndrome (n = 7); specimens from patients with germ-arrest (GA) syndrome (n = 9); and specimens from patients with mixed-atrophy (MA) syndrome (n = 6). The etiology of testicular failure was heterogenic; the majority of patients presented cryptorchidism in early childhood or idiopathic infertility. Serum follicle-stimulating hormone levels ranged from 2.2 to 58 milliunits/ml, and testicular volume ranged from 5 to 20 ml. Because these studies were performed retrospectively, no data concerning atopic diathesis were available. The evaluation of human specimens was approved by the local ethics committee of the Technical University of Munich.
Western Blot and Dot Blot.
HFFF2 cells were plated on 60-mm dishes (Nunc) and incubated in the presence or absence of tryptase, PAR2 agonist peptide SLIGKV, and HMC-1-conditioned medium. We performed immunoblots as described (23) by using a polyclonal rabbit anti-human COX2 antiserum (1:1,000, Oxford Biomedical Research, Oxford, MI) and a monoclonal mouse anti-human tryptase (1:500, DAKO). Dot-blot assays were performed by using the mentioned tryptase antibody (24).
PGF2α and 15d-J2 Immunoassays.
Approximately 7.5 × 105 HFFF2 cells per well were plated on 24-well plates (Nunc) and incubated in the presence or absence of 120 ml/ml tryptase for 1 and 3 h. We determined PGF2α and 15d-PGJ2 in 100 μl of the incubation medium (total volume per well was 250 μl) by using commercially available kits (R & D Systems and Assay Designs, Ann Arbor, MI, respectively).
Proliferation Assays.
Approximately 7 × 103 HFFF2 cells per well were plated on 96-well plates (Nunc) and incubated for 24 h in the presence or absence of tryptase, HMC-1-conditioned medium, meloxicam, PAR2 agonist peptide, PGs, and BADGE. Cell proliferation was determined by using the CellTiter 96 AQueous One Solution cell-proliferation assay (Promega) (25). The specificity and sensibility of this method was evaluated previously in our lab by comparison with a [3H]thymidine incorporation assay (see ref. 25). Cell proliferation was analyzed also by immunostaining with a monoclonal mouse antibody against the human proliferating cell nuclear antigen (Oncogene Research Products, Cambridge, MA; for details see below). To evaluate whether some of the chemical drugs used induce apoptotic events, we used the terminal deoxynucleotide transferase-mediated dUTP nick-end labeling (TUNEL) method (26).
Histological, Immunohistochemical, and Immunofluorescence Assays.
Archival human testicular biopsies had been fixed in Bouin's fixative and were embedded in paraffin. We fixed the HFFF2 cells in 4% formalin (Sigma). Then we processed the paraffin sections and the fixed HFFF2 cells for hematoxylin staining (Sigma) and immunohistochemical (27) and immunofluorescence (25) studies. We used commercial antibodies and antisera [monoclonal mouse anti-human tryptase, 1:50, DAKO; monoclonal mouse anti-human peroxisome proliferator-activated receptor γ (PPARγ), 1:100, Santa Cruz Biotechnology; monoclonal mouse anti-human proliferating cell nuclear antigen, 1:80, Oncogene Research Products; polyclonal goat anti-human PAR2 1:100, Santa Cruz Biotechnology; and polyclonal rabbit anti-human COX2, 1:200, Oxford Biomedical Research]. For control purposes, the first antiserum was omitted, or incubation with normal nonimmune sera was carried out. In the case of PAR2, an additional control was performed by preabsorption of the antiserum with a four-times-concentrated specific blocking peptide (Santa Cruz Biotechnology) for 1 h at 37°C with gentle shaking.
Laser-Capture Microdissection (LCM) and Laser Pressure Catapulting (LPC) Techniques.
LCM and LPC from human testicular biopsies were performed as described (27). We used the focused nitrogen laser of the Robot-MicroBeam (P.A.L.M. Mikrolaser Technologie, Bernried, Germany) to circumscribe the target from surrounding material. Microdissected samples were ejected from the object slide directly into the cap of a microcentrifuge tube. RNA stabilization reagent (50 μl, RNEasy protect minikit, Qiagen, Hilden, Germany) was added into the cap. Finally, the samples were frozen at −70°C until RT-PCR assays were performed.
RT-PCR Analysis and PCR Amplification.
We identified gene expression in (i) HFFF2 cells, (ii) fixed and paraffin-embedded human testicular sections that were scratched from the slides as described (28), and (iii) material collected by LCM and LPC of hematoxylin-stained cells as described (27).
We extracted RNA by using the Purescript kit (Biozym, Hessisch Oldenburg, Germany). Then we performed reverse transcription followed by PCR amplification (27). For analysis of samples obtained from paraffin-embedded sections and LCM, a second PCR-amplification step, with nested primers, was used. Information about the oligonucleotide primers used and cDNAs isolated are given in Table 1. We designed oligonucleotide primers for COX2, PAR2, and PPARγ to be homologous to areas of different exons. Finally, we verified the identity of PCR products by sequencing with a fluorescence-based dideoxy-sequencing reaction and an automated sequence analysis on an ABI model 373A DNA sequencer.
Table 1.
Oligonucleotide primers (first and second, nested set) for PCR amplification of cDNAs obtained after reverse transcription from HFFF2 cells, paraffin-embedded human testicular biopsies, and cells of the wall of seminiferous tubules collected after LCM
| Target
|
|
Primers | bp
|
|
|---|---|---|---|---|
| Sense | Antisense | |||
| COX2 | 1 | 5′-GCAAATCCTTGCTGTTCC-3′ | 5′-GGAGGAAGGGCTCTAGTA-3′ | 368 |
| 2 | 5′-CCATGTCAAAACCGTGG-3′ | 5′-TAGGAGAGGTTGGAGAA-3′ | 326 | |
| PAR2 | 1 | 5′-CATCCTGCTAGCAGCCTC-3′ | 5′-ACCTCTGCACACTGAGGC-3′ | 480 |
| 2 | 5′-GATGGCACATCCCACGTC-3′ | 5′-GGCATGTATGTGATAGGC-3′ | 288 | |
| PPARγ | 1 | 5′-TCTGGCCCACCAACTTTG-3′ | 5′-ACAAGCATGAACTCCATA-3′ | 356 |
| 2 | 5′-GATTACAAGTATGACCTG-3′ | 5′-GCTTTATCTCCACAGAC-3′ | 164 | |
Statistical Analysis.
The results obtained were analyzed statistically by using the computer program PRISM (GraphPad, San Diego). One-way ANOVA followed by the Student's t test for two comparisons or the Student–Newman–Keuls test for multiple comparisons was used. The data are expressed as means + SEM.
Results
Tryptase Acting via PAR2 Increases COX2, PG Production, and Fibroblast Proliferation.
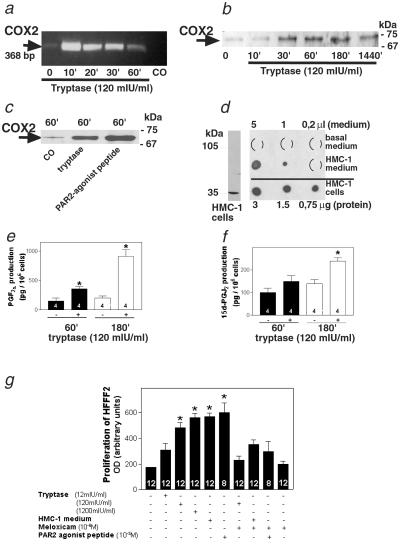
Tryptase increased both COX2 mRNA levels (within 10 min) and COX2 protein levels (30 min) in HFFF2 cells (Fig. 1 a and b). Action of human recombinant tryptase was concentration-dependent (not shown), with 120 milliunits/ml being the lowest tryptase concentration tested to cause a significant increase of COX2 protein levels. Tryptase action is mediated by its specific receptor PAR2, because the PAR2-activating peptide SLIGKV also increased COX2 protein levels in HFFF2s (Fig. 1c). HMCs contain and also secrete tryptase, as shown in Western and dot-blot immunoassays using HMC-1 and HMC-1-conditioned medium (Fig. 1d). As expected, a consequence of tryptase-induced COX2 up-regulation is PG synthesis, which was confirmed by measurement of two PGs, PGF2α and 15d-PGJ2, in cell culture medium of HFFF2s after 1 and 3 h of treatment with tryptase (Fig. 1 e and f). Human recombinant tryptase and conditioned medium from the HMC-1 line as well as the PAR2 agonist peptide SLIGKV significantly increased proliferation in HFFF2. This action of human recombinant tryptase was concentration-dependent, with 120 milliunits/ml being the lowest tryptase concentration tested to cause a significant increase on HFFF2 proliferation. Therefore, this concentration was used for most of the experiments. The proliferative action of tryptase, HMC-1-conditioned medium, and the PAR2 agonist peptide was blocked by the COX2 antagonist meloxicam, implying that PGs are crucially involved in this action (Fig. 1g).
Fig 1.
Trypase via PAR2 increases COX2 levels and stimulates PG synthesis and fibroblast proliferation. (a) Example of an RT-PCR analysis, showing rapid up-regulation of COX2 mRNA in HFFF2 cells after treatment with tryptase. mIU, milliunits. (b) Immunoblot depicting increased COX2 protein levels in HFFF2 cells from 10 to 1,440 min after treatment with tryptase (2.5 μg of total protein per lane). (c) Immunoblot showing that the PAR2 agonist peptide SLIGKV also increases COX2 protein levels (15 μg of total protein per lane). (d) HMCs (HMC-1) contain tryptase (see Western blot, Left, 15 μg of total protein). Dot blot shows that HMC-1 cells contain tryptase and secrete tryptase into the culture medium (Right, basal or HMC-1-conditioned medium, collected from 5 × 106 cells after 24 h of incubation, were loaded). (e and f) Tryptase increases PGF2α (e) and 15d-PGJ2 (f) production in HFFF2s. The data represent means ± SEMs of values obtained after 1 and 3 h of treatment with tryptase (n = 4 per treatment). *, P < 0.05 vs. the corresponding control group. (g) Tryptase stimulates fibroblast proliferation via PAR2, COX2 up-regulation, and PGJ2 production. Meloxicam (a COX2 antagonist) inhibits the stimulatory action of conditioned medium collected from HMCs (HMC-1), recombinant human tryptase, and the PAR2 agonist peptide SLIGKV on HFFF2 proliferation after 24 h of incubation. This figure shows results obtained from one of three experiments that yielded comparable results. The results shown are means + SEMs of n = 8–12 replicate wells per treatment. *, P < 0.05 vs. the corresponding control group.
Tryptase Stimulates Fibroblast Proliferation Indirectly via 15d-PGJ2 and PPARγ.
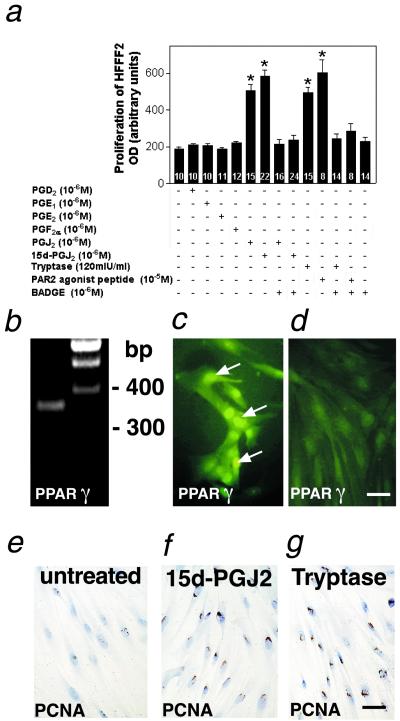
To examine which PGs and which receptors are involved in the proliferative role exerted by tryptase, we studied the effects of a variety of different PGs on HFFF2 proliferation. PGJ2 and its metabolite 15d-PGJ2 significantly increased HFFF2 proliferation (Fig. 2a), but we did not observe any proliferative effect when other PGs (D2, E1, E2, and F2α) were tested (Fig. 2a). HFFF2s express PPARγ, the specific receptor of 15d-PGJ2 (Fig. 2 b–d), and BADGE, an antagonist of PPARγ, blocked the stimulatory action of 15d-PGJ2 on HFFF2 proliferation (Fig. 2a). Moreover, BADGE also blocked the stimulatory action of tryptase and the PAR2 agonist peptide SLIGKV, indicating that the fibroproliferative role of trypase/PAR2 is exerted via 15d-PGJ2 and its binding to PPARγ (Fig. 2a). Immunocytochemical staining with the proliferation marker proliferating cell nuclear antigen confirmed the proliferative role of tryptase and 15d-PGJ2 on HFFF2 (Fig. 2 e–g). These treatments do not affect apoptosis as we determined by the terminal deoxynucleotide transferase-mediated dUTP nick-end labeling method (data not shown).
Fig 2.
Tryptase and the PAR2 agonist peptide SLIGKV stimulate fibroblast proliferation indirectly via 15d-PGJ2 and PPARγ. (a) PGJ2 and its metabolite 15d-PGJ2 stimulate HFFF2 proliferation, whereas PGs D2, E1, E2, and F2α have no effect. BADGE (a PPARγ antagonist) blocks the stimulatory action of recombinant human tryptase, PAR2 agonist peptide SLIGKV, PGJ2, and 15d-PGJ2 on HFFF2 proliferation. This figure shows results obtained from one of three experiments that yielded comparable results. The results shown are means + SEMs of n = 8–24 replicate wells per treatment. *, P < 0.05 vs. the corresponding control group. mIU, milliunits. (b) RT-PCR analysis showing PPARγ mRNA expression in HFFF2 cells (Left). (Right) Size markers. (c and d) Immunofluorescence staining showing PPARγ expression in the nucleus of HFFF2 cells (c). PPARγ immunostaining is not detected in a control section, in which the PPARγ antibody was replaced by normal nonimmune mouse serum (d). (Bar, 40 μm.) (e–g) Immunocytochemical staining of the proliferation marker proliferating cell nuclear antigen. After treatment with 15d-PGJ2 (f) and tryptase (g), more nuclei of HFFF2 cells are immunoreactive compared with basal conditions (e). (Bar, 40 μm.)
Identification of the Components Required for the Tryptase/COX2/15d-PGJ2 Pathway in Vivo in Human Tissues.
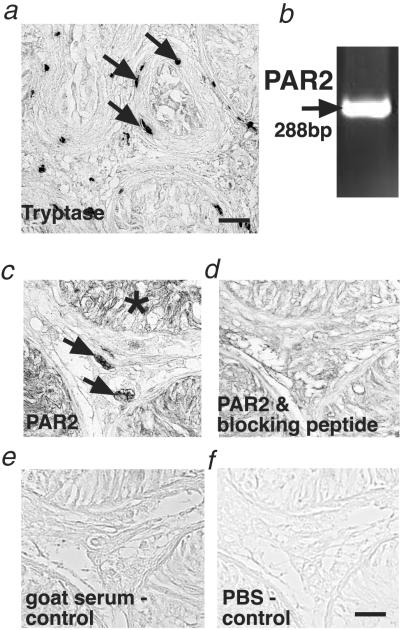
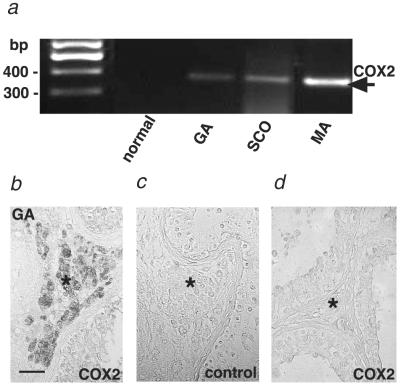
To examine whether this newly identified pathway may be of relevance to a human fibrotic disorder, we studied human testicular biopsies from men presenting normal, mildly, or severely impaired spermatogenesis (18). We studied this tissue, because it is well documented (18, 29–33) that interstitial and peritubular tryptase-positive mast cells are 2–3 times increased and show signs of activation/degranulation in these cases (Fig. 3a). Fibrotic changes of the tubular wall also are well documented and quantified (for further information, see our previous report in ref. 18). The specific tryptase receptor, PAR2, was detected in interstitial cells and the germinal epithelium (Fig. 3 b–f). Specific immunoreactions were observed in all samples examined (seven SCO syndrome, nine GA syndrome, six MA syndrome, and seven normal spermatogenesis samples). In contrast, evidence for PG synthesis initiated by COX2 induction was obtained only in pathological biopsies with impaired spermatogenesis (same sample as mentioned for PAR2) but not in biopsies showing normal spermatogenesis (control group, n = 7) (Fig. 4). COX2 was localized only in interstitial cells, i.e., the same testicular compartment, in which we also found PAR2.
Fig 3.
Identification of tryptase-positive mast cells and PAR2 in human testes with fibrotic changes. (a) Immunohistochemical staining of tryptase-containing mast cells in the interstitium and the wall of the seminiferous tubules (arrows) in a human testicular biopsy from a patient showing subnormal spermatogenesis and tubular fibrosis. For complete and detailed quantitative evaluation of the observed changes, see ref. 18. (Bar, 60 μm.) (b) RT-PCR analysis showing PAR2 mRNA expression in human testes. (c–f) Immunohistochemical staining showing PAR2 expression. PAR2 in a human testicular biopsy from a patient with SCO syndrome in interstitial cells (arrow) and the germinal epithelium (asterisk) (c). In a consecutive section, the PAR2 antibody was incubated previously with a specific blocking peptide to show specificity (d). For control purposes, the PAR2 antiserum was replaced by normal goat serum (e) and PBS buffer (f). (Bar, 30 μm.) Note that PAR2 RNA expression and specific immunoreaction were detected in all biopsies examined (normal group, n = 7; SCO syndrome, n = 7; GA syndrome, n = 9; and MA syndrome, n = 6).
Fig 4.
COX2 expression in human testes with fibrotic changes. (a) RT-PCR analysis showing COX2 mRNA expression in testicular biopsies from patients with GA, SCO, and MA syndromes. COX2 was not found in normal testicular biopsies. Normal group, n = 7; SCO syndrome, n = 7; GA syndrome, n = 9; and MA syndrome, n = 6. First lane, size markers. (b–d) Immunohistochemical staining showing COX2 expression in testicular biopsies. (b) COX2 in a testicular biopsy from a patient with GA syndrome. (c) COX2 immunostaining is not detected in a control section in which the COX2 antiserum was replaced by normal rabbit serum. (d) COX2 was not observed in biopsies from men showing normal spermatogenesis. Asterisks indicate the interstitial compartment. (Bar, 100 μm.) Note that COX2 RNA expression and specific immunoreaction were not found in normal testicular biopsies (normal group, n = 7), whereas COX2 was detected in all pathological samples examined (SCO syndrome, n = 7; GA syndrome, n = 9; and MA syndrome, n = 6).
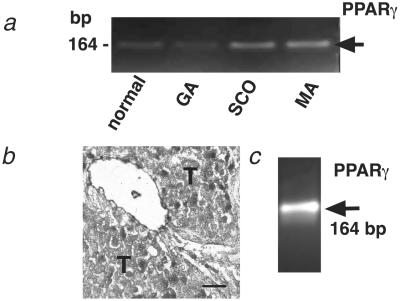
Normal biopsies, as well as biopsies from patients with impaired spermatogenesis, express PPARγ (Fig. 5a). The use of LCM and LPC techniques enabled us to detect a site of expression of PPARγ in the wall of testicular seminiferous tubules (Fig. 5 b and c).
Fig 5.
PPARγ expression in human testes. (a) RT-PCR analysis showing PPARγ mRNA expression in testicular biopsies from patients with normal spermatogenesis and GA, SCO, and MA syndromes. (b) A hematoxylin-stained human testicular biopsy after LCM of the wall of the seminiferous tubules. Material obtained was used for RT-PCR analysis. T, seminiferous tubule. (Bar, 20 μm.) (c) RT-PCR analysis indicating PPARγ mRNA expression in a sample of testicular tubular wall obtained by LCM and LPC. Note that PPARγ mRNA was detected in samples of all biopsies (normal group, n = 7; SCO syndrome, n = 7; GA syndrome, n = 9; and MA syndrome, n = 6).
Discussion
Our study reveals details of the interaction between mast cells and fibroblasts. The previously unknown signaling pathway linked to fibroblast proliferation involves the mast-cell product tryptase, its receptor PAR2, induction of COX2, and synthesis of 15d-PGJ2 and its action through PPARγ.
We report that conditioned medium from HMC-1, which among other proteins contains tryptase, stimulates proliferation of human fibroblasts. Although mast cells secrete a mixture of potent substances, which also may be able to affect fibroblast proliferation, our results imply that among these secretory factors, tryptase is of special importance. Thus, similar to HMC-1 medium, pure human recombinant tryptase exerts a fibroproliferative action. Moreover, the PAR2 agonist peptide SLIGKV mimicked the proliferative effect of tryptase on fibroblasts, indicating that it is initiated via activation of PAR2.
Effects of tryptase on fibroblast proliferation, chemotaxis, and collagen production were observed by other authors (1, 11, 15), identifying tryptase as a fibroblast growth factor. Unexpectedly, we now find that tryptase and the PAR2 agonist peptide SLIGKV induce the PG-synthesizing enzyme, COX2. Induction of both COX2 mRNA and protein are rapid events and occur within minutes. That the resulting COX2 is biologically active is indicated by increased levels of two classes of secreted PGs, PGF2α and 15d-PGJ2 from tryptase-induced fibroblasts, observed after 1–3 h in our study. To our knowledge there are few reports indicating that HMCs up-regulate COX2. One example is a study on coculture of HMCs and human orbital fibroblast, which caused PGE2 synthesis. The mast-cells product IL-4 was thought to mediate this action (12). In dermal fibroblasts, IL-1β (13) also was identified to increase COX2 (34). Thus, induction of COX2 and increased PG synthesis in fibroblasts are events that are not restricted to action of tryptase.
The notion that PGs and their receptors regulate proliferation and type I collagen synthesis in fibroblasts was suggested by earlier studies (15, 16, 35). When we tested different PGs known to interact with different prostanoid receptors, we observed that only PGJ2 and its metabolite, 15d-PGJ2, acting via the nuclear receptor PPARγ, but not other members of the PG family increase HFFF2 proliferation. Levels of 15d-PGJ2 are increased in HFFF2s after tryptase treatment, as found in the present study. To evaluate whether tryptase and 15d-PGJ2 exert their proliferative role through independent mechanisms, we used meloxicam (a COX2 inhibitor) and BADGE (a PPARγ antagonist). Meloxicam completely abolishes the proliferative effects of tryptase and the PAR2 agonist peptide SLIGKV. Moreover, BADGE prevents not only the stimulatory action of 15d-PGJ2 but also the effect of tryptase and the PAR2 agonist peptide SLIGKV on HFFF2 proliferation. Thus, the signaling pathway of tryptase, which mediates fibroblast proliferation, involves induction of COX2, synthesis of 15d-PGJ2, and its action via PPARγ.
Fibroblasts are critical components of the inflammatory response and participate in normal and pathological tissue remodeling. Together with smooth muscle cells, fibroblasts are believed to mediate tissue fibrosis and collagen deposition (11, 36). A recent report links liver fibrosis to expression of COX2 (37) and furthermore shows that COX2 expression increased with the progression of fibrosis in the patients examined. The reasons for induction of COX2 were not addressed in this paper, and neither were mast cells nor their state of activation examined. Mast cells, however, are commonly increased in fibrotic tissues as has been well documented recently, for example, in renal interstitial fibrosis (3), IgA nephropathy (4), chronic pancreatitis (38), or cystic fibrosis (39). Whether mast cells are activated or whether COX2 is also expressed in these and other fibrotic processes has not been reported to our knowledge. It therefore remains to be shown whether the tryptase-signaling pathway is of importance in all or only some cases of fibrosis. We have started to address this question by evaluating human tissues in which both fibrotic tissue remodeling and increased numbers of activated mast cells have been well documented, namely testes from infertile men (18, 29–33).
Fibrosis of the wall of seminiferous tubules commonly occurs in human testes with defects of spermatogenesis (17). We described in detail in a previous study that in all these conditions testicular mast cells contain tryptase, are significantly increased in numbers, and present signs of degranulation and activation (18). The thickness of the tubular wall due to fibrotic remodeling is doubled in the biopsies from infertile men examined and correlates to the number of mast cells (for quantitative details, see ref. 18). Based on our results in HFFF2 cells, we have reexamined these biopsies and report that they contain virtually all the known components associated with the tryptase-induced signaling pathway, i.e., tryptase, its receptor PAR2, COX2, and PPARγ. Thus, we hypothesize that tryptase released from testicular mast cells can target interstitial PAR2-bearing cell populations, activate PAR2, and, consequently, up-regulate the expression of COX2 and stimulate PG synthesis. Importantly, we identified testicular COX2 expression only in testes showing abnormal spermatogenesis but not in normal testes. The wall of the seminiferous tubules is composed of fibroblasts and myofibroblasts possessing PPARγ, which may act as a receptor for 15d-PGJ2. The existence of PPARγ has been described in adipose tissue, colon, the immune system, and the retina (40) but also in fibroblasts (41) and in our study in the cells of the tubular wall. Thus, the fact that all components identified to be involved in tryptase-induced proliferation of HFFF2 cells are present in human testicular fibrotic tissues examined implies that mast-cell–fibroblast interactions, elucidated in HFFF2 cells, could be of relevance for human diseases. We suggest that they are of special relevance to those human diseases in which increased numbers of activated mast cells are associated with fibroproliferative disorders.
Our work, by elucidating details of how tryptase can stimulate fibroblast proliferation, identifies several potential therapeutic targets, namely tryptase, COX2, and PPARγ. Drugs targeting these factors are being developed or are already in clinical use for a variety of conditions. For instance, APC 366 (an antagonist of mast-cell tryptase) is being studied for use in asthma (42), and lactoferrin (a potent tryptase inhibitor) (43) is being tested for allergic treatments. PPARγ ligands including the specific antagonist BADGE, may have a potential therapeutic value in obesity (44), type 2 diabetes (45), colorectal cancer (46) but also in liver fibrosis (47). Antagonists of COX2 are commonly available, relatively safe drugs and currently are being used mainly in the treatment of inflammatory conditions (48, 49) but also as antifibrotic agents (50, 51). Thus, our work describes insights into the signaling pathway related to the interaction between mast cells and fibroblasts that may lead to new therapeutic approaches in human fibrotic disorders.
Acknowledgments
We thank Marlies Rauchfuss, Gabriele Terfloth, Barbara Zschiesche, Andrea Thalhammer, Andreas Mauermayer, and Annette Krieger of the Anatomical Institute for expert technical assistance and Prof. Manfred Gratzl for critical reading. We are grateful to Dr. J. H. Butterfield (Mayo Clinic, Rochester, MN) for providing the HMC-1 line. The work was supported by Deutsche Forschungsgemeinschaft Grant Ma1080/13/-1, Graduiertenkolleg 333 “Biology of Human Diseases,” Consejo Nacional de Investigaciones Científicas y Técnicas (Conicet), and in part by a grant from the German Ministry of Defense/Fraunhofer Gesellschaft.
Abbreviations
PAR2, protease-activated receptor 2
HMC, human mast cell
COX2, cyclooxygenase 2
PG, prostaglandin
HFFF2, human fetal foreskin fibroblast cell line
15d-PGJ2, 15-deoxy-Δ12,14-PGJ2
BADGE, bisphenol A diglycidyl ether
SCO, Sertoli cell only
GA, germ arrest
MA, mixed atrophy
PPARγ, peroxisome proliferator-activated receptor γ
LCM, laser-capture microdissection
LPC, laser pressure catapulting
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Akers I. A., Parsons, M., Hill, M. R., Hollenberg, M. D., Sanjar, S., Laurent, G. J. & McAnulty, R. J. (2000) Am. J. Physiol. 278, L193-L201. [DOI] [PubMed] [Google Scholar]
- 2.Albanis E. & Friedman, S. L. (2001) Clin. Liver Dis. 5, 315-334. [DOI] [PubMed] [Google Scholar]
- 3.Kondo S., Kagami, S., Kido, H., Strutz, F., Muller, G. A. & Kuroda, Y. (2001) J. Am. Soc. Nephrol. 12, 1668-1676. [DOI] [PubMed] [Google Scholar]
- 4.Kurusu A., Suzuki, Y., Horikoshi, S., Shirato, I. & Tomino, Y. (2001) Nephron 89, 391-397. [DOI] [PubMed] [Google Scholar]
- 5.MacKenna D., Summerour, S. R. & Villarreal, F. J. (2000) Cardiovasc. Res. 46, 257-263. [DOI] [PubMed] [Google Scholar]
- 6.Uitto J. & Kouba, D. (2000) J. Dermatol. Sci. 24, S60-S69. [DOI] [PubMed] [Google Scholar]
- 7.Galli S. J. (1990) Lab. Invest. 62, 5-33. [PubMed] [Google Scholar]
- 8.Metcalfe D. D., Baram, D. & Mekori, Y. A. (1997) Physiol. Rev. 77, 1033-1079. [DOI] [PubMed] [Google Scholar]
- 9.Ruoss S. J., Hartmann, T. & Caughey, G. H. (1991) J. Clin. Invest. 88, 493-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodbury R. G., Everitt, M. T. & Neurath, H. (1981) Methods Enzymol. 80, 588-609. [DOI] [PubMed] [Google Scholar]
- 11.Gruber B. L., Kew, R. R., Jelaska, A., Marchese, M. J., Garlick, J., Ren, S., Schwartz, L. B. & Korn, J. H. (1997) J. Immunol. 158, 2310-2317. [PubMed] [Google Scholar]
- 12.Smith T. J. & Parikh, S. J. (1999) Endocrinology 140, 3518-3525. [DOI] [PubMed] [Google Scholar]
- 13.Inoue H., Ohshima, H., Kono, H., Yamanaka, M., Kubota, T., Aihara, M., Hiroi, T., Yago, N. & Ishida, H. (1997) Biochem. Pharmacol. 53, 1941-1944. [DOI] [PubMed] [Google Scholar]
- 14.Tada K., Murakami, M., Kambe, T. & Kudo, I. (1998) J. Immunol. 161, 5008-5015. [PubMed] [Google Scholar]
- 15.Abe M., Kurosawa, M., Ishikawa, O. & Miyachi, Y. (2000) J. Allergy Clin. Immunol. 106, S78-S84. [DOI] [PubMed] [Google Scholar]
- 16.Okuda-Ashitaka E., Negishi, M., Sugama, K., Hatanaka, M. & Ito, S. (1990) Eicosanoids 3, 213-218. [PubMed] [Google Scholar]
- 17.de Krester D. M. & Baker, H. W. G. (1996) in Reproductive Endocrinology, Surgery, and Technology, eds. Adashi, E. Y., Rock, J. A. & Rosenwaks, Z. (Lippincott–Raven, Philadelphia), pp. 2031–2062.
- 18.Meineke V., Frungieri, M. B., Jessberger, B., Vogt, H. & Mayerhofer, A. (2000) Fertil. Steril. 74, 239-244. [DOI] [PubMed] [Google Scholar]
- 19.Hibi H., Kato, K., Mitsui, K., Taki, T., Yamada, Y., Honda, N., Fukatsu, H. & Yamamoto, M. (2001) Arch. Androl. 47, 107-111. [DOI] [PubMed] [Google Scholar]
- 20.Schill W. B., Schneider, J. & Ring, J. (1986) Andrologia 18, 570-573. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto M., Hibi, H. & Miyake, K. (1995) Fertil. Steril. 64, 1221-1223. [DOI] [PubMed] [Google Scholar]
- 22.Gilroy D. W., Saunders, M. A., Sansores-Garcia, L., Matijevic-Aleksic, N. & Wu, K. K. (2001) FASEB J. 15, 288-290. [DOI] [PubMed] [Google Scholar]
- 23.Schagger H. & von Jagow, G. (1987) Anal. Biochem. 166, 368-379. [DOI] [PubMed] [Google Scholar]
- 24.Meineke V., Moede, T., Gilbertz, K. P., Mayerhofer, A., Ring, J., Kohn, F. M. & Van Beuningen, D. (2002) Int. J. Radiat. Biol. 78, 577-583. [DOI] [PubMed] [Google Scholar]
- 25.Sommersberg B., Bulling, A., Salzer, U., Froehlich, U., Garfield, R. E., Amsterdam, A. & Mayerhofer, A. (2000) Biol. Reprod. 63, 1661-1668. [DOI] [PubMed] [Google Scholar]
- 26.Mochizuki H., Nakamura, N., Nishi, K. & Mizuno, Y. (1994) Neurosci. Lett. 170, 191-194. [DOI] [PubMed] [Google Scholar]
- 27.Frungieri M. B., Calandra, R. S., Lustig, L., Meineke, V., Köhn, F. M., Vogt, H. J. & Mayerhofer, A. (2002) Fertil. Steril. 78, 298-306. [DOI] [PubMed] [Google Scholar]
- 28.Mayerhofer A., Frungieri, M. B., Fritz, S., Bulling, A., Jessberger, B. & Vogt, H. J. (1999) J. Androl. 20, 341-347. [PubMed] [Google Scholar]
- 29.Maseki Y., Miyake, K., Mitsuya, H., Kitamura, H. & Yamada, K. (1981) Fertil. Steril. 36, 814-817. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal S., Choudhury, M. & Banerjee, A. (1987) Int. J. Fertil. 32, 283-286. [PubMed] [Google Scholar]
- 31.Hashimoto J., Nagai, T., Takaba, H., Yamamoto, M. & Miyake, K. (1988) Urol. Int. 43, 129-132. [DOI] [PubMed] [Google Scholar]
- 32.Nagai T., Takaba, H. & Miyake, K. (1992) Fertil. Steril. 57, 1331-1336. [PubMed] [Google Scholar]
- 33.Jezek D., Banek, L., Hittmair, A., Pezerovic-Panijan, R., Goluza, T. & Schulze, W. (1999) Andrologia 31, 203-210. [DOI] [PubMed] [Google Scholar]
- 34.Dubois R. N., Abramson, S. B., Crofford, L., Gupta, R. A., Simon, L. S., Van De Putte, L. B. & Lipsky, P. E. (1998) FASEB J. 12, 1063-1073. [PubMed] [Google Scholar]
- 35.Altiok S., Xu, M. & Spiegelman, B. M. (1997) Genes Dev. 11, 1987-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powell D. W., Mifflin, R. C., Valentich, J. D., Crowe, S. E., Saada, J. I. & West, A. B. (1999) Am. J. Physiol. 277, C183-C201. [DOI] [PubMed] [Google Scholar]
- 37.Cheng J., Imanishi, H., Iijima, H., Shimomura, S., Yamamoto, T., Amuro, Y., Kubota, A. & Hada, T. (2002) Hepatol. Res. 23, 185-195. [DOI] [PubMed] [Google Scholar]
- 38.Esposito I., Friess, H., Kappeler, A., Shrikhande, S., Kleeff, J., Ramesh, H., Zimmermann, A. & Buchler, M. W. (2001) Hum. Pathol. 32, 1174-1183. [DOI] [PubMed] [Google Scholar]
- 39.Hubeau C., Puchelle, E. & Gaillard, D. (2001) J. Allergy Clin. Immunol. 108, 524-529. [DOI] [PubMed] [Google Scholar]
- 40.Kersten S., Desvergne, B. & Wahli, W. (2000) Nature 405, 421-424. [DOI] [PubMed] [Google Scholar]
- 41.Simomin M. A., Bordji, K., Boyault, S., Bianchi, A., Gouze, E., Becuwe, P., Dauca, M., Netter, P. & Terlain, B. (2002) Am. J. Physiol. 282, C125-C133. [DOI] [PubMed] [Google Scholar]
- 42.Krishna M. T., Chauhan, A., Little, L., Sampson, K., Hawksworth, R., Mant, T., Djukanovic, R., Lee, T. & Holgate, S. (2001) J. Allergy Clin. Immunol. 107, 1039-1045. [DOI] [PubMed] [Google Scholar]
- 43.Elrod K. C., Moore, W. R., Abraham, W. M. & Tanaka, R. D. (1997) Am. J. Respir. Crit. Care Med. 156, 375-381. [DOI] [PubMed] [Google Scholar]
- 44.Wright H. M., Clish, C. B., Mikami, T., Hauser, S., Yanagi, K., Hiramatsu, R., Serhan, C. N. & Spiegelman, B. M. (2000) J. Biol. Chem. 275, 1873-1877. [DOI] [PubMed] [Google Scholar]
- 45.Yamauchi T., Waki, H., Kamon, J., Murakami, K., Motojima, K., Komeda, K., Miki, H., Kubota, N., Terauchi, Y., Tsuchida, A., et al. (2001) J. Clin. Invest. 108, 1001-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta R. A., Brockman, J. A., Sarraf, P., Willson, T. M. & DuBois, R. N. (2001) J. Biol. Chem. 276, 29681-29687. [DOI] [PubMed] [Google Scholar]
- 47.Miyahara T., Schrum, L., Rippe, R., Xiong, S., Yee, H. F., Jr., Motomura, K., Anania, F. A., Willson, T. M. & Tsukamoto, H. (2000) J. Biol. Chem. 275, 35715-35722. [DOI] [PubMed] [Google Scholar]
- 48.Hawker C. J. (1999) Lancet 353, 307-314. [DOI] [PubMed] [Google Scholar]
- 49.Hawkey C. J. (2001) Gastroenterol. Clin. North Am. 30, 921-936. [DOI] [PubMed] [Google Scholar]
- 50.Poon R., Smits, R., Li, C., Jagmohan-Changur, S., Kong, M., Cheon, S., Yu, C., Fodde, R. & Alman, B. A. (2001) Oncogene 20, 451-460. [DOI] [PubMed] [Google Scholar]
- 51.Miyajima A., Ito, K., Asano, T., Seta, K., Ueda, A. & Hayakawa, M. (2001) J. Urol. 166, 1124-1129. [PubMed] [Google Scholar]