Abstract
Poly(A+) RNA was extracted from the temporal lobe (TL) of medically intractable epileptic patients which underwent surgical TL resection. Injection of this mRNA into Xenopus oocytes led to the expression of ionotropic receptors for γ-aminobutyric acid (GABA), kainate (KAI) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA). Membrane currents elicited by GABA inverted polarity at −15 mV, close to the oocyte's chloride equilibrium potential, were inhibited by bicuculline, and were potentiated by pentobarbital and flunitrazepam. These basic characteristics were also displayed by GABA currents elicited in oocytes injected with mRNAs isolated from human TL glioma (TLG) or from mouse TL. However, the GABA receptors expressed by the epileptic TL mRNA exhibited some unusual properties, consisting in a rapid current run-down after repetitive GABA applications and a large EC50 (125 μM). AMPA alone evoked very small or nil currents, whereas KAI induced larger currents. Nevertheless, upon cyclothiazide treatment, AMPA elicited substantial currents that, like the KAI currents, were inhibited by 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX). Furthermore, the glutamate receptor 5 (GluR5) agonist, ATPA, failed to evoke an obvious current although both RT-PCR and Western blot analyses showed GluR5 expression in the epileptic TL. Oocytes injected with mouse TL or human TLG mRNAs generated KAI and AMPA currents similar to those evoked in oocytes injected with epileptic TL mRNA but, in contrast to these, the mouse TL and human TLG oocytes were also responsive to ATPA. Our findings are in accord with the concept that both a depression of GABA inhibition and a dysfunction of the KAI-receptor system maintain a high neuronal excitability that results in epileptic seizures.
Epilepsy is a large group of heterogeneous neurological disorders characterized by recurrent seizures, with several types of currents activated simultaneously in many neurons. Specifically, in focal epilepsy (FE) the seizures develop in localized areas of a cerebral hemisphere. Most FE patients undergo substantial benefit following optimal treatments with anti-epileptic drugs. However, patients with medically intractable temporal lobe focal epilepsy (TLE) are seizure-free only after resective surgery. Several approaches, including histology, electrophysiology, molecular biology, immunohistochemistry, and genetics have been used extensively to decipher the pathogenesis of epilepsies, yielding the general view that a dysfunction of voltage-activated and/or ligand activated-receptor channels may be involved in epilepsies (1–5).
Taking advantage of the temporal lobectomy of patients afflicted with TLE, we have now “transplanted” transmitter-gated receptors from the human epileptic brain to Xenopus oocytes. It is well known that Xenopus oocytes injected with brain mRNAs of many species are able to express many types of functional neurotransmitter receptors, due to translation of the exogenous mRNA by the oocyte's own protein synthesizing machinery (6, 7). Here we give evidence that Xenopus oocytes injected with poly(A)+ RNA extracted from the human epileptic temporal lobe and hippocampus, express functional ionotropic γ-aminobutyric acid type A (GABAA) as well as glutamate receptors (GluRs) with properties worthy of further investigation. This study opens previously unexplored avenues toward the diagnosis and treatment of epileptic patients.
Materials and Methods
Patients Selection.
Patients with intractable TLE were selected for surgery on the basis of crisis frequency or impairment of the quality of life. Patients underwent surgery with two alternative surgical procedures: (i) the standard temporal lobectomy with removal of amygdala, hippocampus, parahippocampal gyrus up to the edge of the tentorium, and lateral cerebral cortex (4.5 cm behind the temporal tip in the dominant hemisphere or 6 cm in the nondominant one) and (ii) the amygdalohippocampectomy associated to the removal of the temporal tip. The human brain tissue (1–2 g weight in average) was frozen and processed for mRNA extraction 1 day after removal. The operation freezing time ranged between 30 s and 3 min. All procedures for human tissue were performed with the informed consent of the patients and were approved by the Ethics Committee of the Faculty of Medicine, University of Rome “La Sapienza.” The patients underwent presurgical diagnostic identification of the epileptogenic focus based on clinical, video-electroencephalogram (EEG) and MRI reports. Briefly, all patients were evaluated with neuropsychological and psychiatric tests and all patients underwent continuous long-term intensive video-EEG monitoring (Telefactor Instruments, Philadelphia). Medications were tapered down, and sleep deprivation was used to facilitate seizure occurrence during recording sessions. The video-EEG recording techniques, performed via colloid fixed scalp electrodes, were in agreement with international guidelines developed by the American Electroencephalographic Society. Scalp EEG recordings were performed by using bipolar longitudinal-transverse and referential montages and, using a high definition monitor and filter settings, they were visually evaluated to detect background abnormalities, interictal slow and epilepticform activity, ictal changes, and postictal slowing. During and after seizures, the patients were monitored to examine auras, language dysfunction, loss of contact, automatisms, and subtle motor and sensory symptoms possibly disrupted by epileptic discharges. Patients were also examined by using 0.5–1.5 T MRI, with T1- and T2-weigthed axial, coronal, and sagittal sections, and fluid-attenuated inversion recovery (FLAIR) images. Table 1 summarizes the clinical profiles of the 11 patients studied. All of these patients were seizure free when a follow up was done 2–6 months after surgery.
Table 1.
Clinical characteristics and neuropathological findings of patients with temporal lobe epilepsy
|
|
Patient no. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| Age (yr)/sex | 21/M | 28/M | 17/F | 37/F | 37/M | 31/F | 26/F | 40/F | 11/F | 38/M | 21/M |
| Febrile seizures | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes |
| Seizure frequency (1/days) | 1/30 | 1/1 | 1/30 | 1/30 | 1/7 | 1/7 | 1/7 | 1/7 | 1/7 | 1/7 | 1/7 |
| Seizure type | CPS | CPS SGTCS | CPS | CPS SGTCS | CPS SGTCS | CPS | CPS | CPS | CPS | CPS | CPS |
| Side resected | L | L | R | L | L | R | R | R | R | R | L |
| Pathology | MTS | MTS | MTS | MTS | MTS | MTS | MTS | MTS | TL ganglio glioma | Gliosis | Gliosis |
MTS, mesial temporal sclerosis; CPS, complex partial seizures; SGTCS, secondary generalized tonic-clonic seizures; L, left; R, right; M, male; F, female.
Extra-hippocampus.
For comparative purposes, we used temporal lobe oligodendrogliomas (Grade II, TLG) from two not epileptic patients (not included in Table 1) that underwent surgery for removal of the tumor and thin nervous tissue regions around it, and we used the TL from 10 mice.
Protein Extracts and Immunoblot Analysis.
Neocortical tissues from patients that underwent brain surgery were homogenized in ice-cold lysis buffer (50 mM Tris⋅HCl, pH 8/5 mM EDTA, pH 8/250 mM NaCl/0.1% Triton X-100/50 mM NaF/0.1 mM sodium orthovanadate/1 mM phenylmethylsulfonylfluoride). The cell lysates were cleared by centrifugation at 15,000 rpm for 20 min at 4°C in a Microfuge R (Beckman), and protein concentration was determined by BCA assay (Pierce). For immunoblots, 40 μg of total protein were loaded on a 10% SDS/polyacrylamide gel and transferred onto nitrocellulose membranes. Blots were probed with primary antibodies against GluR5 and GluR6/7 receptors (Santa Cruz Biotechnology), and specific signals were detected with horseradish peroxidase secondary antibodies (Sigma) by a chemiluminescence reaction (ECL, Amersham Pharmacia).
mRNA Preparation and RT-PCR.
About 1 g of temporal neocortex frozen specimens, from TLE patients, were quickly homogenized in lysis buffer by using an Ultra-Turrex T8 homogenizer (IKA, Germany), and mRNA was extracted by using a Fast Track poly(A)+ RNA isolation kit (Invitrogen). The poly(A)+ RNA was dissolved in water (≅1 μg/μl) and used directly or stored at −80°C. The same experimental procedures were followed to extract mRNA from human TLG (two patients) and from mouse TL (10 DBA mice). First-strand cDNA, synthesized by using 150 ng of poly(A)+ RNA (First Strand cDNA Synthesis kit, Amersham Pharmacia Biosciences), was used for PCR amplification with specific primers designed to amplify the coding regions of GluR5–7 receptor subunits.
Oocyte Injection.
Preparation of Xenopus laevis oocytes and mRNA injection procedures were detailed elsewhere (8). Oocytes were injected with 50–100 ng of poly(A)+ RNA and maintained in modified Barth's solution at 16°C until electrophysiological recordings were performed. As control, some oocytes were injected with 100 nl of water.
Voltage-Clamp Recordings.
Membrane currents were recorded 5–7 days after injection, using two microelectrodes filled with 3 M KCl (9). Unless otherwise indicated, the oocyte membrane potential was held at −80 mV and perfused continuously, 10–11 ml/min, with oocyte Ringer's solution (82.5 mM NaCl/2.5 mM KCl/2.5 mM CaCl2/1 mM MgCl2/5 mM Hepes, adjusted to pH 7.4 with NaOH) at room temperature (20–22°C). To obtain dose/response relations, the neurotransmitters were applied, at 5 min intervals, to the oocytes held at −60 mV. The time for 10% decay (T0.1) of the membrane current, that is the time taken for the current to decay from its peak to 90%, was used to estimate the rate of receptor desensitization. The half dissociation constant (EC50) of GABA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), kainic acid (KAI), and the half-inhibitory concentration (IC50) of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) were estimated by fitting the data to Hill equations, using least-square routines (see ref. 8).
Solution exchange was achieved by using electromagnetic valves and a computer controlled perfusion system (Biologique, France). Current–voltage relationships were constructed stepping the membrane potential for 2–5 min to the desired value before applying the transmitter. Drugs were applied by adding them to the superfusing fluid, and all were from Sigma, except for AMPA, KAI, 2-amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl)propanoic acid (ATPA), cyclothiazide (CTZ), and bicuculline (Bic) (Tocris Neuramin, Bristol, U.K.).
Results
Functional Expression of Human Neurotransmitter Receptors.
Injections of Xenopus oocytes with mRNAs isolated from the temporal lobe of 9 (nos. 1–9 in Table 1) out of 11 patients studied caused the synthesis and oocyte membrane incorporation of several types of transmitter-activated receptor channels. These human mRNA-injected oocytes were responsive to GABA, AMPA, and KAI, but they did not generate currents when exposed to either serotonin or nicotine. Injections of mRNA derived from the brain of the two remaining patients (nos. 10 and 11 of Table 1) did not lead to the expression of functional receptors. Interestingly, both patients showed extended gliosis, although the possibility of some RNA degradation during the preparative procedures cannot be excluded.
GABA Currents.
Application of GABA (1 mM) to oocytes injected with TLE mRNA elicited inward membrane currents, whereas control (noninjected) oocytes showed no obvious responses to GABA. The GABA currents decayed with T0.1 ranging between 0.2 and 1.57 s (T0.1 = 0.89 ± 0.13; mean ± SEM.; 13 oocytes, 4 donors, 13/4) and their peak amplitude decreased after repetitive applications. Compared to oocytes injected with human TLG and mouse TL mRNAs, the GABA currents from oocytes injected with TLE mRNA showed a similar decay (mouse TL, T0.1 = 1.01 ± 0.24; human TLG, T0.1 = 0.78 ± 0.16) but exhibited a faster rundown during repetitive GABA applications (Fig. 1A). Interestingly, the same applied to oocytes injected with TL ganglioglioma mRNA (patient 9, Table 1).
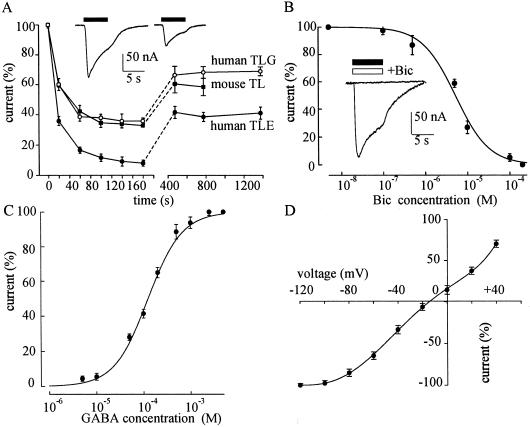
Fig 1.
Properties of GABA currents. (A) Decay of the peak GABA-current amplitude during repetitive applications of GABA (1 mM), in oocytes injected with: TLE mRNA (9 oocytes/2 frogs, 9/2; 2 patients); mouse TL mRNA (6/2; 10 mice); and TLG mRNA (6/2; 2 patients). The amplitudes were normalized to those elicited by the first applications of GABA (current range: 40–300 nA). In this and subsequent figures, the points show means ± SEM. (Inset) Sample traces of GABA currents elicited by the first (Left) and second (Right) GABA applications (filled bars) in an oocyte injected with TLE mRNA. (B) Inhibition of GABA (100 μM) currents by Bic in TLE mRNA-injected oocytes (6/2; 2 patients). The GABA currents were normalized to the currents evoked by GABA alone (Imax = −60 nA; range: 16–102 nA). (Inset) Current evoked by 1 mM GABA (filled bar), and blocked by 0.2 mM Bic (open bar). (C) GABA dose/current response relationship from TLE mRNA-injected oocytes (8/3; 3 patients). Imax = −179 nA; range: 40–330 nA. (D) GABA current/voltage relationship in TLE-oocytes (6/2; 2 patients). The GABA (100 μM) currents were normalized to those evoked at −120 mV (−115 nA; range: 70–210 nA). Reversal potential (Vrev) = −14.5 mV.
The amplitude of the membrane current elicited by GABA increased with the GABA concentration, and the GABA current was inhibited by the classical competitive blocker Bic with IC50 and nH values of 5.2 μM and 1.1, respectively (Fig. 1B). These values are similar to those reported for oocytes injected with mRNA from postnatal day 10 and adult rat cortices (10, 11) and for cultured embryonic mouse neurons (12). GABA dose/current–response curves, fitted to Hill equation, gave nH and EC50 values of 1.2 and 125 μM, respectively (Fig. 1C). The latter value was considerably larger than many EC50 values reported in the literature (11–17), but similar to the EC50 found with TL ganglioglioma mRNA (patient 9, Table 1; not shown).
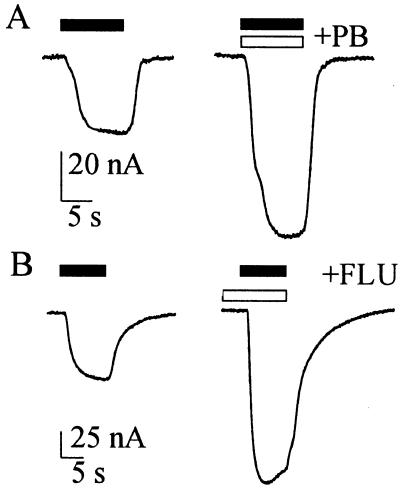
The GABA current decreased in size as the oocyte was depolarized and inverted direction at about −15 mV (Fig. 1D), which is close to the Cl− equilibrium potential in Xenopus oocytes. As the potential was made more negative the GABA-current amplitude increased to a maximum at about −100 mV, but did not increase more with further hyperpolarization. Moreover, the GABA-current amplitude was greatly potentiated when GABA was applied together with pentobarbital (PB) or with flunitrazepam (FLU); and the potentiation was greater if the oocytes were pretreated with FLU (Fig. 2). Specifically, both PB (100 μM) and FLU (0.3 μM, pretreated for 5 s) increased by 2.3- or 2.1-fold, respectively, the currents evoked by 50 μM GABA (8 oocytes, 3 donors; patients 1–6, Table 1). All this is in agreement with previous observations in oocytes expressing rat brain GABAA receptors (16, 17).
Fig 2.
Modulation of GABA currents in TLE mRNA-injected oocytes. (A) Current responses to GABA (50 μM; Left), and to GABA coapplied with PB (100 μM; open bar; Right). (B) Current responses to GABA (50 μM; Left), and to GABA after preincubation with FLU (0.3 μM; open bar; Right). Holding potential: −60 mV.
Interestingly, these GABA receptors functional properties, GABA affinity and current rundown, and those reported below for the glutamate receptors were similar to those of the same receptors expressed by the TL mRNA from patient 9 (Table 1), suggesting that the epileptic seizures parallel altered receptor properties.
AMPA Currents.
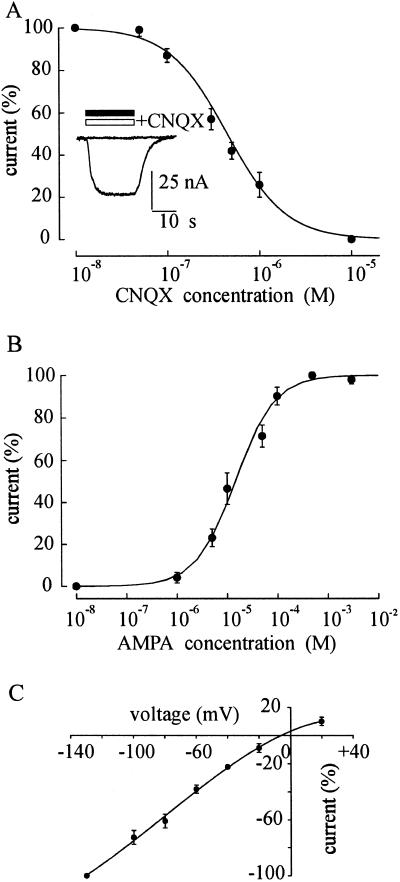
Oocytes injected with human TLE mRNA responded to AMPA (50–100 μM) with very small currents (<10 nA) or not at all. However, if these oocytes were pretreated for 30 s with the allosteric potentiator CTZ (20 μM) (18), they consistently showed relatively large inward AMPA currents. These currents were blocked by CNQX, the selective antagonist of non-NMDA (N-methyl d aspartate) type GluRs, with IC50 and nH values of 0.45 μM and 1.37, respectively (Fig. 3A). The mean concentration of AMPA required to elicit the half maximal response was 14.5 μM, and the nH was 1.1 (Fig. 3B), both values being similar to those reported for rat neurons (19, 20). The reversal potential of the AMPA current was about −7 mV and the I–V relation was fairly linear being slightly bent at positive potentials (Fig. 3C). Thus, these findings show that the mRNA from human TLE tissue induced the oocytes to acquire functional AMPA receptors whose desensitization is potently modulated by CTZ, a drug known to decrease the rate of AMPA receptor desensitization (18, 21).
Fig 3.
Properties of AMPA currents in TLE mRNA-injected oocytes. (A) Inhibition of AMPA (10 μM) currents by CNQX. AMPA currents in the presence of CNQX normalized to those without CNQX (Imax = −70 nA; 6/2; 2 patients). (Inset) Superimposed traces of the current evoked by AMPA (50 μM; filled bar) and then blocked by CNQX (20 μM; open bar). (B) AMPA dose/current response relationship from 8 oocytes (3 donors; 2 patients). Imax = −268 nA. (C) AMPA current/voltage relationship from 4 oocytes (2 donors; 2 patients) with AMPA at 10 μM. AMPA currents were normalized to those obtained at −130 mV (−165 nA). Vrev = −7 mV.
KAI Currents.
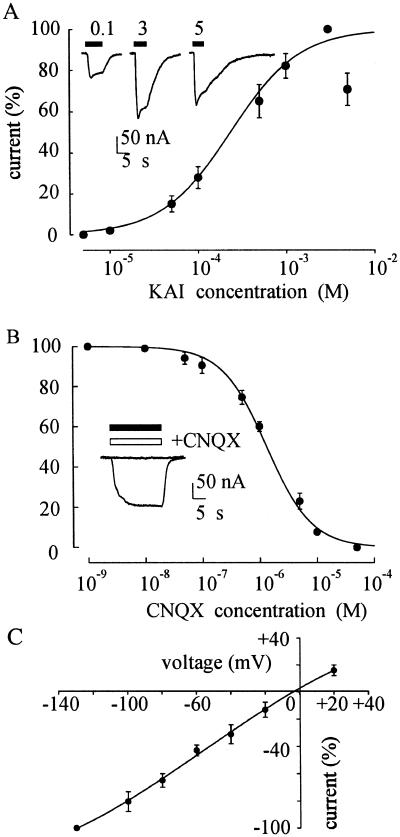
The TLE-oocytes also acquired kainate receptors; and KAI currents became detectable at concentrations of about 10 μM, peaked at about 3 mM and became smaller with larger doses (Fig. 4A), presumably because of the receptor desenzitization. The dose/response relation gave EC50 and nH values of 235 μM and 1.1, respectively (Fig. 4A), which are in agreement with those reported previously for KAI receptors induced by rat (22, 23) and human brain mRNAs (24, 25).
Fig 4.
Properties of KAI currents in TLE mRNA-injected oocytes. KAI dose/current response relationship from 6 oocytes (3 donors; 3 patients). KAI currents were normalized to those evoked by 3 mM KAI (Imax = −239 nA). (Inset) Sample current at indicated KAI concentrations (mM). (B) Inhibition of KAI currents by CNQX. Currents evoked by KAI (200 μM) coapplied with CNQX were normalized to those evoked by KAI without CNQX (Imax = −62 nA). Same oocytes and patients as for Fig. 3A. (Inset) Superimposed currents evoked by KAI (50 μM; filled bar) and blocked by CNQX coapplication (50 μM; open bar). (C) KAI current/voltage relation for the same oocytes as in Fig. 3C. The amplitude of the currents evoked by KAI (200 μM) were normalized to the mean value obtained at −130 mV. Vrev = −4 mV.
The KAI currents were potently blocked by CNQX. As illustrated in Fig. 4B, CNQX blocked the response to KAI (200 μM) with IC50 and nH values of 1.32 and 1.1 respectively, in agreement with values found in oocytes injected with human brain mRNA (25). As was the case for AMPA currents, the I–V relation for the KAI-currents was linear; and the reversal potential was at about −4 mV (Fig. 4C). This value is similar to the reversal potential for the KAI current in oocytes injected with mRNA from human brain (25, 26).
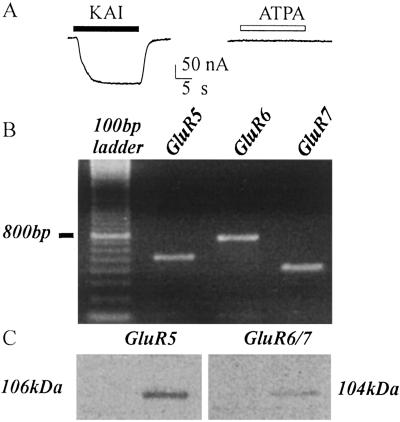
Oocytes injected with TLE mRNA from patients 1–9 (e.g., Fig. 5A) were unresponsive to ATPA (50–500 μM), which is considered to be a potent and specific agonist of human GluR5 receptors (27). Nevertheless, mRNA encoding the GluR5 subunit was detected by RT-PCR analysis of the poly(A+) RNA used for the oocyte injections (Fig. 5B); and Western blot analysis (Fig. 5C) gave evidence of the expression of GluR5–7 receptors in the TL of the same epileptic patients. Therefore, we conclude that GluR5 is expressed in the epileptic TL, but the receptors are unable to generate currents when “transplanted” into oocytes.
Fig 5.
Absence of functional GluR5 in TLE mRNA-injected oocytes. (A) Current response to KAI (50 μM) and lack of response to ATPA (200 μM) in the same oocyte. (B) RT-PCR analysis of GluR5, GluR6, and GluR7 subunits in TLE mRNA. (C) Western blot analysis of the expression of GluR5, GluR6/7 subunits in TLE tissue. (B and C) Same patient as in A. Note the presence of GluR5. Results are representative of all experiments from patients 1–9 of Table 1.
Responses from Oocytes Injected with Mouse TL or Human TLG mRNAs.
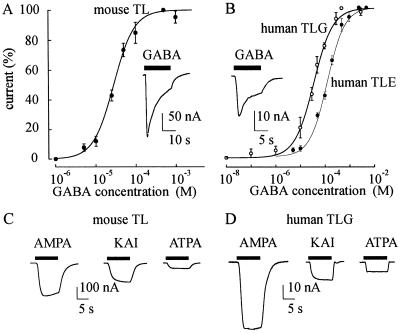
Experiments were done to compare the TLE mRNA-injected oocytes with oocytes injected with mRNA extracted from either mouse TL or human TLG. Oocytes injected with mouse TL exhibited GABA dose–current response relations with an EC50 of 28.5 μM and an nH of 1.6 (Fig. 6A); whereas oocytes injected with TLG mRNA gave an EC50 of 36 μM and an nH of 1.1 (Fig. 6B). Both EC50 values were much smaller than the one obtained in oocytes injected with TLE mRNA (Figs. 1C and 6B). In other experiments, the sensitivity of mouse and TLG oocytes to the glutamate agonists, KAI, AMPA, and ATPA, was examined. All of the oocytes tested (10/3) responded with currents to AMPA > KAI > ATPA (Fig. 6 C and D). This indicates that the oocytes injected with either mouse TL or human TLG mRNA express functional AMPA and KAI receptors, including the GluR5 receptors. Interestingly, height oocytes (two donors) injected with TLE brain membranes (28) from patients 2 and 3 (Table 1) gave similar results as those obtained after mRNA injections (data not shown).
Fig 6.
Current responses in oocytes injected with mRNA extracted from either mouse TL or human TL. (A) GABA dose/current relationship in mouse TL mRNA-injected oocytes (Imax = −274 nA; 0.5 mM GABA; 6/2). (Inset) Sample current evoked by GABA (1 mM). (B) GABA dose/current relationship from TLG mRNA-injected oocytes (○) (Imax = −25 nA; 8/2; 2 patients) compared with the GABA dose/current relationship from TLE mRNA-injected oocytes (•) described in Fig. 1C. (Inset) Sample current evoked by GABA (1 mM) in one oocyte injected with human TLG mRNA. (C) Sample currents evoked by AMPA (50 μM), KAI (200 μM), or ATPA (200 μM) in one oocyte injected with the same mRNA preparation as in A. (D) Currents evoked by the same drugs as in C in another oocyte injected with the same human TLG mRNA as for B. Note the ATPA sensitivity of the oocytes in C and D.
Discussion
Medically intractable TLE is a relatively common adult seizure disorder of uncertain ethiology. Evidence from animal models, and from human studies, suggests that altered expression of ionotropic GABAA and GluRs may contribute to the pathophysiology of TLE (2, 29). To study in more detail the neurotransmitter receptors of the epileptic brain, we decided to see whether it was possible to transfer the receptors from human brain tissue, surgically resected from intractable TLE patients, into the membrane of Xenopus oocytes.
We found that oocytes injected with human TLE mRNA consistently express GABAA, and both AMPA-type and KAI-type glutamate receptors. Some functional properties of the GABAA receptors appear to be the normal ones; namely, the GABA current reversal at a potential close to the Cl− equilibrium potential; the competitive bicuculline block and the barbiturate and benzodiazepine potentiation of the GABA currents. However, some other properties of the GABA receptors expressed by the TLE mRNA are special. For example, in oocytes injected with TLE mRNA the GABA currents exhibited a faster and more pronounced rundown than the GABA currents elicited in oocytes injected with human TLG or mouse TL mRNA. In addition, the affinity for GABA is much smaller than that found in native nerve cells or in heterologous cell expression systems (11–17). Interestingly, it has recently been reported that a mutation of the α1 subunit of GABAA receptors in an autosomal dominant form of human juvenile myoclonic epilepsy impairs considerably the receptor affinity (30). The unusual GABAA receptor behavior reported here could be due to GABA receptor mutations (3, 17, 30) or to altered dynamic regulation (31). etc. Whatever turns out to be the basis of the abnormal GABA-receptor function, the present findings may provide an avenue for understanding better the role of the GABAergic system in TLE.
It is interesting to note that GluR5 receptors are expressed in the TLE brain; but the GluR5 transcripts of the same epileptic brain fail to express functional receptors when injected into the oocytes. In contrast, mouse TL and human TLG mRNAs do express functional GluR5 receptors in the oocytes. The reasons for this difference could be many, including receptor mutations or posttranscriptional modifications. However, it should be noted that in oocytes injected with membranes (28) prepared from the temporal neocortex of two TLE patients (patients 2 and 3 of Table 1), we obtained GABA and ATPA sensitivities similar to those found in oocytes injected with TLE mRNA. These findings indicate that both the unusual properties of GABAA receptors and the expression of “silent” GluR5 receptors are unlikely to be related to altered posttranslational processes or to receptor modulation by host membrane proteins and lipids. Interestingly, changes in receptor editing during seizures may be a reason for altered receptor activity (32).
The mechanism of drug resistance in TLE is still an open question. Our findings indicate that the benzodiazepine resistance in TLE cannot be strictly attributed to the GABA receptor dysfunction, because we found that the “epileptic” GABA receptor is positively modulated by both pentobarbital and flunitrazepam; findings that are in agreement with the reported benzodiazepine sensitivity of TLE neurons (33). Therefore, it is likely that TLE drug resistance may depend on a variety of factors, including modulation of the blood–brain barrier permeability (34).
In conclusion, our work strongly suggests that the unusual properties of the epileptic GABAA receptors, together with the disfunction of KAI receptors, reduce the inhibition of GABAergic neurons, thus contributing to the development of TLE and points to both GABAA and GluR5 receptors as important targets to improve the treatment of TLE (see refs. 35 and 36).
Acknowledgments
We are especially grateful to patients 1–11 of Table 1, R.G., V.E., L.A., S.M.T., R.F., L.E., F.G., B.A., M.V., S.A., and C.M., who so generously contributed to this work. We thank Prof. Fabio Ruzzier and Dr. Francesca Grassi for critical reading of the manuscript. This work was supported in part by Ministero Università Ricerca Scientifica (to F.E.), by Ministero della Salute (to F.E.), and by Consiglio Nazionale delle Ricerche (to R.M.).
Abbreviations
GABA, γ-aminobutiric acid
AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
KAI, kainic acid
ATPA, 2-amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl)propanoic acid
TL, temporal lobe
TLE, TL focal epilepsy
TLG, human TL glioma
CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione
Bic, bicuculline
CTZ, cyclothiazide
GluR5, glutamate receptor 5
EEG, electroencephalogram
References
- 1.Olsen R. W., DeLorey, T. M., Gordey, M. & Kang, M. H. (1999) in Jasper's Basic Mechanisms of the Epilepsies, eds. Delgado-Escueta, A. V., Wilson, W., Olsen, R. W. & Porter, R. J. (Lippincott Williams and Wilkins, New York), Vol. III, pp. 499–510. [Google Scholar]
- 2.Loup F., Weiser, H.-G., Yonekawa, Y., Aguzzi, A. & Fritschy, J.-M. (2000) J. Neurosci. 20, 5401-5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baulac S., Huberfeld, G., Gourfinkel-An, I., Mitropoulou, G., Beranger, A., Proud'homme, J.-F., Baulac, A., Bruzzone, R. & LeGuern, E. (2001) Nat. Genet. 28, 46-48. [DOI] [PubMed] [Google Scholar]
- 4.Wallace R. H., Marini, C., Petrou, S., Harkin, L. A., Bowser, D. N., Panchal, R. G., Williams, D. A., Sutherland, G. R., Mulley, J. C., Cheffer, I. E. & Berkovic, S. F. (2001) Nat. Genet. 28, 49-52. [DOI] [PubMed] [Google Scholar]
- 5.Avanzini G. & Ptacek, L. J. (2002) in Epilepsy and Movement Disorders, eds. Guerrini, R., Aicardi, J., Andermann, F. & Hallet, M. (Cambridge Univ. Press, Cambridge, U. K.), pp. 1–14.
- 6.Miledi R., Parker, I. & Sumikawa, K., (1989) Fidia Research Foundation Neuroscience Lectures (Raven, New York), Vol. 3, pp. 57–90. [Google Scholar]
- 7.Matute C., Arellano, R., Conde-Guerri, B. & Miledi, R. (1992) Proc. Natl. Acad. Sci. USA 89, 3399-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palma E., Mileo, A. M., Eusebi, F. & Miledi, R. (1996) Proc. Natl. Acad. Sci. USA 93, 11231-11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miledi R. (1982) Proc. R. Soc. London B 215, 491-497. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter M. K., Parker, I. & Miledi, R. (1988) Proc. R. Soc. London B 225, 299-313. [DOI] [PubMed] [Google Scholar]
- 11.Polenzani L., Woodward, R. M. & Miledi, R. (1991) Proc. Natl. Acad. Sci. USA 88, 4318-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wahl P., Ragsdale, D., Schousboe, A. & Miledi, R. (1993) J. Neurochem. 60, 57-65. [DOI] [PubMed] [Google Scholar]
- 13.Chang Y., Wang, R., Barot, S. & Weiss, D. S. (1996) J. Neurosci. 16, 5415-5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demuro A., Martinez-Torres, A., Francesconi, W. & Miledi, R. (1999) Br. J. Pharmacol. 127, 57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagaya N. & Macdonald, R. L. (2001) J. Physiol. 532, 17-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker I., Gundersen, C. B. & Miledi, R. (1986) J. Neurosci. 6, 2290-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walters R. J., Hadley, S. H., Morris, K. D. W. & Amin, J. (2000) Nat. Neurosci. 3, 1274-1281. [DOI] [PubMed] [Google Scholar]
- 18.Patneau D. K., Vicklicky, L. & Mayer, M. L. (1993) J. Neurosci. 13, 3496-3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Traynelis S. & Cull-Candy, S. G. (1991) J. Physiol. (London) 433, 727-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lax P., Limatola, C., Fucile, S., Trettel, F., Di Bartolomeo, S., Renzi, M., Ragozzino, D. & Eusebi, F. (2002) J. Neuroimmunol. 129, 66-67. [DOI] [PubMed] [Google Scholar]
- 21.Seifert G., Schoroeder, W., Hinterkeuser, S., Schumacher, T., Schramm, J. & Steinhauser, C. (2002) Epilepsia 43, 162-167. [DOI] [PubMed] [Google Scholar]
- 22.Verdoon T. A. & Dingledine, R. (1988) Mol. Pharmacol. 34, 298-307. [PubMed] [Google Scholar]
- 23.Ragsdale D., Gant, D. B., Anis, N. A., Eldefrawi, A. T., Eldefrawi, M. E., Konno, K. & Miledi, R. (1989) J. Pharmacol. Exp. Ther. 251, 156-163. [PubMed] [Google Scholar]
- 24.Gundersen C. B., Miledi, R. & Parker, I. (1984) Nature 308, 421-424. [DOI] [PubMed] [Google Scholar]
- 25.Matute C. & Miledi, R. (1993) Proc. Natl. Acad. Sci. USA 90, 3270-3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Umbach J. A. & Gundersen, C. B. (1989) Mol. Pharmacol. 36, 582-588. [PubMed] [Google Scholar]
- 27.Clarke V. R. J., Ballyk, B. A., Hoo, K. H., Mandelzys, A., Pellizzari, A., Bath, C. P., Thomas, J., Sharpe, E. F., Davies, C. H., Ornstein, P. L., et al. (1997) Nature 389, 599-603. [DOI] [PubMed] [Google Scholar]
- 28.Miledi R., Eusebi, F., Martinez-Torres, A., Palma, E. & Trettel, F. (2002) Proc. Natl. Acad. Sci USA 99, 13238-13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonald J. W., Garofalo, E. A., Hood, T., Sackellares, J. C., Gilman, S., McKeever, P. E., Troncoso, J. C. & Johnston, M. V. (1991) Ann. Neurol. 29, 529-541. [DOI] [PubMed] [Google Scholar]
- 30.Cossette P., Liu, L., Brisebois, K., Dong, H., Lortie, A., Vanasse, M., Saint-Hilaire, J. M., Verner, A., Lu, W. Y., Wang, Y. T. & Rouleau, G. A. (2002) Nat. Genet. 31, 184-189. [DOI] [PubMed] [Google Scholar]
- 31.Moss S. J. & Smart, T. G. (2001) Nat. Neurosci. 2, 240-250. [DOI] [PubMed] [Google Scholar]
- 32.Bernard A., Ferhat, L., Dessi, F., Charton, G., Represa, A., Ben Ari, Y. & Khrestchatisky, M. (1999) Eur. J. Neurosci. 11, 604-616. [DOI] [PubMed] [Google Scholar]
- 33.Gibbs J. W., Zhang, Y. F., Kao, C. Q., Holloway, K. L., Oh, K. S. & Coulter, D. A. (1996) J. Neurophysiol. 75, 1458-1471. [DOI] [PubMed] [Google Scholar]
- 34.Abbott N. J, Khan, E. U., Rollinson, C. M., Reichel, A., Janigro, D., Dombrowski, S. M., Dobbie, M. S. & Begley, D. J. (2002) Novartis Found. Symp. 243, 38-53. [PubMed] [Google Scholar]
- 35.Ben-Ari Y. & Cossart, R. (2000) Trends Neurosci. 23, 580-587. [DOI] [PubMed] [Google Scholar]
- 36.Cossart R., Tyzio, R., Dinocourt, C., Esclapez, M., Hirsch, J. C., Ben-Ari, Y. & Bernard, C. (2001) Neuron 29, 497-508. [DOI] [PubMed] [Google Scholar]