Abstract
Over the past 20 years, protein glycation has been implicated in a variety of pathological states. Although smoking also can contribute to many of these diseases, the precise mechanism by which this occurs is not known. Previously, we have demonstrated that nornicotine, a constituent of tobacco and metabolite of nicotine, can catalyze aldol reactions under aqueous conditions. This finding has caused us to question whether this reaction has physiological consequences. We now report that nornicotine causes aberrant protein glycation and catalyzes the covalent modification of certain prescription drugs such as the commonly used steroid, prednisone. Furthermore, we show that the plasma of smokers as compared with nonsmokers contains higher concentrations of nornicotine-modified proteins, suggesting an unrecognized pathway for the development of the pathology of tobacco abuse.
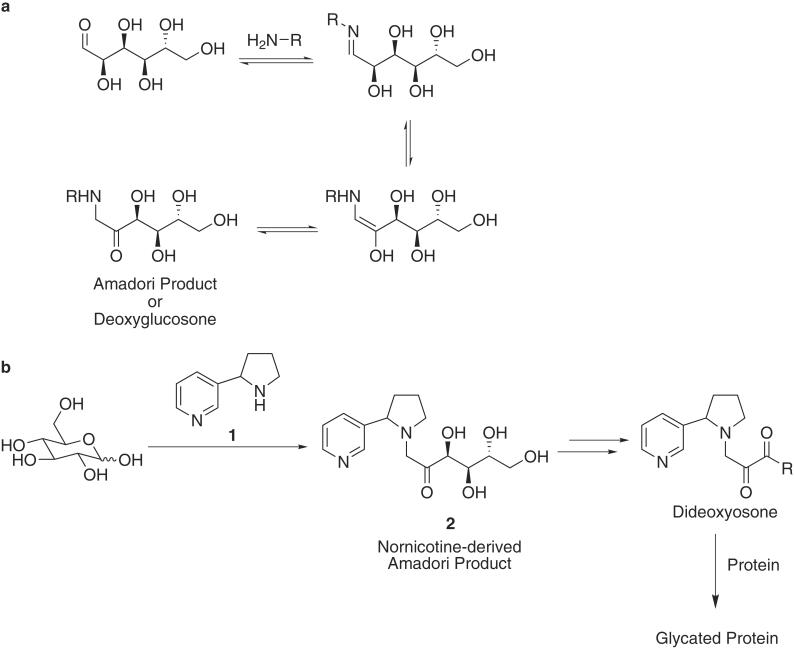
The reaction between reducing sugars and amines, known as the Maillard reaction, was first described 90 years ago (1). Also termed nonenzymatic browning, the Maillard reaction has been extensively reviewed by food chemists for its role in the development and deterioration of flavor and the effects on the nutritional value of foods during processing and storage (2, 3). In the past 20 years, the Maillard reaction has attained special prominence biologically because of its role in certain disease states including diabetes (4), cancer (5), atherosclerosis (6), and Alzheimer's disease (7, 8), as well as normal aging (9). The initial step in this process involves the reversible formation of a Schiff base between an amine and the ring-opened form of a reducing sugar, followed by Amadori rearrangement to give deoxyglucosones (Fig. 1A). Further oxidation of these intermediates produces dideoxyosones, reactive electrophiles capable of reacting with available amino acid side chain nucleophiles to give a variety of characterized and uncharacterized products termed advanced glycation endproducts (AGEs) (10, 11).
Fig 1.
(a) Amadori product derived from the reaction of glucose and a primary amine. (b) Formation of nornicotine-based Amadori product 2 from glucose and nornicotine 1. This intermediate can undergo further chemical reaction including oxidation to a 1,2-dicarbonyl-containing compound, a critical intermediate found in protein glycation.
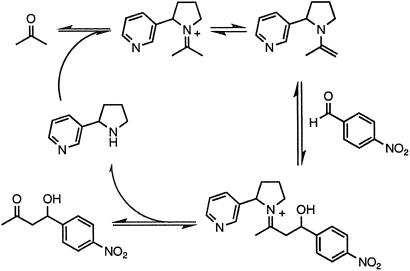
Recently, we have shown that nornicotine 1, a minor tobacco alkaloid (12) and psychoactive (13) nicotine metabolite (14, 15) with an extended half-life (13), is a viable aqueous aldol catalyst (16) (Fig. 2). Furthermore, our exploration into the mechanism of this process revealed that the reaction proceeded through an enamine intermediate. The unique and heretofore undetected ability of a metabolite to form enamine structures under aqueous conditions led us to consider other physiologically relevant chemical reactions that make use of similar intermediates. The Amadori rearrangement fit these requirements, and we hypothesized that nornicotine should form the corresponding Amadori product 2 on incubation with glucose under physiologically relevant conditions (Fig. 1B). Furthermore, this reaction scenario implied that 2 could be an early intermediate in the glycation of proteins by nornicotine.
Fig 2.
Covalent catalysis of the aldol reaction by nornicotine via an enamine intermediate.
Materials and Methods
General Synthetic Methods.
Unless otherwise stated, all reactions were performed under an inert atmosphere with dry reagents and solvents and flame-dried glassware. Analytical TLC was performed by using 0.25 mm precoated silica gel Kieselgel 60 F254 plates. Visualization of the chromatogram was by UV absorbance, iodine, dinitrophenylhydrazine, ceric ammonium molybdate, ninhydrin, or potassium permanganate as appropriate. 1H NMR spectra were recorded on either a Bruker AMX-500 or Bruker DRX-600 spectrometer at 500 and 600 MHz, respectively. 13C NMR spectra were recorded on a Bruker AMX-500 spectrometer at 125 MHz. Matrix-assisted laser desorption/ionization Fourier transform MS experiments were performed on an IonSpec FTMS mass spectrometer. Electrospray ionization MS experiments were performed on an API 100 Perkin–Elmer SCIEX single quadrupole mass spectrometer. Analytical reverse phase HPLC (RP-HPLC) was performed on a Hitachi L-5000 series instrument equipped with a Vydac-C18 analytical column, a UV detector at 254 nm, and mobile phases comprised of mixtures of acetonitrile/water (0.1% trifluoroacetic acid).
Synthesis of Nornicotine-Derived Amadori Product 2.
The Amadori product 2 was prepared as reported (17). Nornicotine (250 mg, 1.69 mmol) and glucose (258 mg, 1.43 mmol) were dissolved in dry methanol (3.25 ml) and heated to reflux for 1 h. Malic acid (19 mg, 0.14 mmol) was then added, and the reaction was refluxed for an additional 2 h. The mixture was then concentrated under reduced pressure, and water (3 ml) was added to the residue. This solution was passed through a column of Amberlyte IR-120(plus) strongly acidic ion exchange resin and the column washed with deionized water (65 ml). The desired crude compound was then eluted with 2 M NH4OH (125 ml), and the combined fractions concentrated under reduced pressure to remove any residual NH3. The resulting aqueous solution was lyophilized to give a white powder, which was further purified on silica by using ethanol/water (30:70) as the eluent. (167 mg, 32%).
Formation in Vitro of Nornicotine-Derived Amadori Product 2.
Nornicotine (10 mM) was incubated in a solution of glucose (200 mM) in PBS (200 mM, pH 7.4). The reaction was protected from light and heated to 37°C. At given time intervals, aliquots of the reaction (100 μl) were taken and analyzed by electrospray ionization MS for the presence of the Amadori product (m/z = 310). Additionally, aliquots (20 μl) were removed and diluted to a final volume of 1 ml with phosphate buffer. Aliquots of these diluted solutions (20 μl) were then removed and injected onto the HPLC system described above (HPLC conditions: isocratic mobile phase consisting of water with 0.1% trifluoroacetic acid. Solvent flow rate of 1 ml⋅min−1 and detection at 254 nm). Independent confirmation of the Amadori product in these reactions was accomplished by coinjection with a known synthetically pure standard (retention time = 4.90 min).
Glycation of Proteins by Nornicotine.
Glucose (200 mM) was added to PBS (200 mM; pH 7.4) to obtain a glucose-enriched buffer. The protein in question [RNase A, BSA, or human serum albumin (HSA)] was then dissolved in this buffer in an microcentrifuge tube to attain a final concentration of protein of 10 mg/ml (final reaction volume = 1 ml). Immediately after the addition of protein, nornicotine was added to the reaction (0.77 μM), and the solution was filtered through a 0.2 μM syringe filter. The reactions were incubated at 37°C in the dark, and aliquots were removed at given time intervals, diluted 1:1,000 with PBS, and analyzed by ELISA.
Western Blot Detection of Nornicotine-Modified Proteins in Vitro and in Vivo.
Monoclonal antibody NIC6C12 does not react significantly with HSA, BSA, or RNase A at 1:1,000 dilution. Serum samples were obtained from subjects after informed consent was given in accordance with a protocol approved by the Institution Review Board at The Scripps Research Institute. Although some reactivity was observed with certain plasma proteins (see Results and Discussion), this did not interfere with the specific detection of glycated proteins in serum samples. Western blot analysis was performed by using 10% NuPage polyacrylamide gels (Invitrogen) according to published procedures (18). Blots were developed by using either the colorimetric reagent TMB (3,3′5,5′-tetramethylbenzidine; Pierce), or SuperSignal West Pico chemoluminescent horseradish peroxidase substrate (Pierce).
Qualitative NMR Test for Nornicotine-Based Glycation.
1D 1H NMR spectra were acquired by using a standard pulse sequence with water suppression (512 scans) on a Bruker DRX 600 spectrometer at 298 K.
Immunochemical Detection of Nornicotine-Modified Proteins in Vitro and in Vivo.
All ELISA assays were performed by using published procedures (18). Briefly, an ELISA plate (Costar; 96-well) was coated overnight at 4°C with serum diluted to 50 μg/ml total protein (as determined by UV absorbance at 280 nm) and then blocked with 50 μl of Blotto (4% skim milk powder in PBS) for 1 h at 37°C. Typically, 25 μl of a 1:500 dilution of mAb 6C12 in Blotto was then added and incubated 1.5 h at 37°C. After washing, 25 μl of a 1:2,000 dilution of a goat-anti-mouse/horseradish peroxidase conjugate in Blotto was added and incubated for 1 h. The plate was developed with the colorimetric reagent TMB, quenched with an equal volume of 2 M H2SO4, and the absorbance was measured on an ELISA plate reader at 450 nm.
Results and Discussion
Formation of the Nornicotine-Derived Amadori Product in Vitro.
Incubation of nornicotine and glucose in phosphate buffer for 3 days yielded a product with a mass corresponding to the Amadori product 2 and a characteristic peak by HPLC analysis. Independent synthesis and characterization of the Amadori product (17), followed by coinjection of this known material with the presumed Amadori product confirmed the synthesis of this compound in vitro. Although 2 is known to exist to a minor extent in tobacco (i.e., ≈0.01% by weight in nornicotine-rich tobacco) (17, 19), its formation in vivo and its potential to participate in protein glycation has not been described. As a potential glycotoxin (20) present in tobacco, we propose that Amadori product 2 derived from nornicotine and glucose is an early intermediate in the glycation of proteins. Furthermore, conversion of 2 to the corresponding dideoxyosone provides an electrophilic nicotine-derived metabolite, a class of compounds that could play a role in the mechanism of tobacco addiction (21).
Development of an Immunoassay for Nornicotine Protein Glycation.
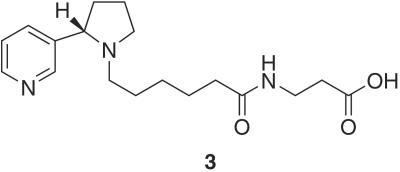
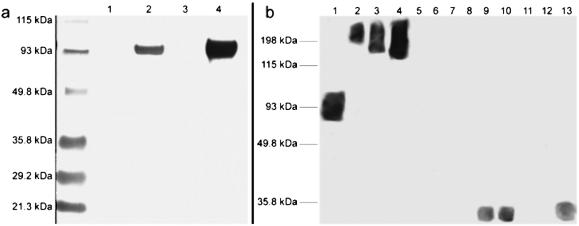
Immunoassays for the detection of glycation products have attracted significant attention (22). Although previous immunoassays have proven effective for the detection of AGE's both in vitro and in vivo, the inherent heterogeneity of both the antigen and polyclonal immune response significantly complicates understanding the chemical nature of in vivo glycation (23). To provide evidence that 2 can glycate proteins, a rapid and sensitive high-throughput immunoassay was developed to facilitate specific detection of nornicotine-derived protein glycation products. With this goal in mind, we chose to use the nornicotine alkaloid nucleus as the primary antigenic determinant. Our reasoning was based on our knowledge that the glucose-derived crosslinking region may undergo a variety of chemical transformations over time, whereas the 3-pyrrolidine-2-yl-pyridine nucleus is expected to be conserved. Thus, antibodies were raised against hapten 3 (Fig. 3), which prominently features the 3-pyrrolidine-2-yl-pyridine nucleus in enantiopure form (24). Our initial efforts focused on the detection of glycation of the model protein, RNase A (25). Nornicotine, glucose, and RNase A were incubated in buffer and aliquots of the mixture screened at given time points for binding to our panel of antibodies. Under our reaction conditions (see Materials and Methods), detectable glycation was first observed by ELISA after 7 days. Specific glycation resulting from nornicotine was detectable by using mAb NIC6C12, causing us to postulate that the recognized epitope of this antibody resides with the nornicotine alkaloid nucleus. As confirmation of this hypothesis, molecules that contain this nucleus such as nicotine and nornicotine, as well as 2 and nornicotine glycated RNase A, were found to compete for binding by ELISA (Table 1). To extend our studies to other proteins, we examined the glycation of HSA and BSA. Nornicotine-based glycation of these proteins occurred readily and again, only glycation in the presence of nornicotine and glucose could be detected by using mAb NIC6C12 (Fig. 4A). This assay is very sensitive, as glycation can be detected with as little as one covalently attached nornicotine molecule per protein. Even after extended incubations (>7 months), the nornicotine moiety remains covalently bound to the protein, implying that, similar to other reported AGE structures, the nornicotine-derived AGE also forms irreversibly. This result strongly suggests that the nornicotine AGE could exist for extended periods of time when attached to a suitable protein leading to increased antigenicity (25) and/or irreversible loss of protein function (26).
Fig 3.
Hapten 3 used to elicit anti-nicotine mAbs.
Table 1.
Apparent dissociation constants obtained using mAb NIC6C12
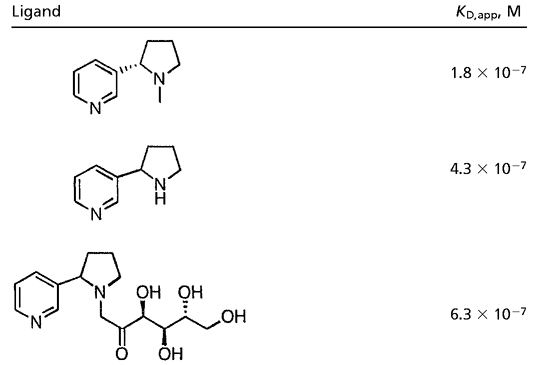 |
Determined by competition ELISA using nornicotine-glycated RNase A as the competing antigen.
Fig 4.
Chemical modification of proteins with nornicotine. SDS/PAGE and Western blot with mAb NIC6C12 of samples are shown. (a) BSA (lane 1), BSA + nornicotine and glucose (lane 2), HSA (lane 3), HSA + nornicotine and glucose (lane 4). (b) Chemoluminescent development of Western blot from human plasma; HSA + nornicotine and glucose (lane 1), nonsmoker sample A total protein concentrations of 2.5 mg/ml (lane 2), 5.0 mg/ml (lane 3), 10 mg/ml (lane 4); nonsmoker sample B total protein concentrations of 2.5 mg/ml (lane 5), 5.0 mg/ml (lane 6), 10 mg/ml (lane 7); smoker sample C total protein concentrations of 2.5 mg/ml (lane 8), 5.0 mg/ml (lane 9), 10 mg/ml (lane 10); smoker sample D total protein concentrations of 2.5 mg/ml (lane 11), 5.0 mg/ml (lane 12), 10 mg/ml (lane 13). Reactions were performed by incubating sterile solutions (0.2-μm filter) of glucose (200 mM), nornicotine (0.8 mM), and protein (0.8 mM) in phosphate buffer (200 mM, pH 7.4) in the dark at 37°C.
Detection of Nornicotine-Based Protein Glycation by NMR.
Previously, 1H NMR has been exploited to directly observe conformationally flexible regions on protein surfaces as increased flexibility leads to less line broadening because of longer T2 relaxation times (27). Although we could not directly detect covalently bound nornicotine moieties on the surface of BSA by NMR, we were able to develop a qualitative test for nornicotine-based glycation using 1D 1H NMR. Proteins with glucose-based glycation displayed sharply defined peaks different from the native protein, whereas proteins that had been incubated with nornicotine and glucose displayed no analogous sharp peaks, possibly indicating a loss of conformational flexibility on nornicotine-based glycation (data not shown).
Detection of Nornicotine-Modified Proteins in Vivo.
To determine whether nornicotine-derived glycation occurred preferentially in smokers, a set of plasma samples from both smoker and nonsmokers was analyzed by ELISA. The plasma of smokers was found to contain statistically higher amounts of nornicotine-modified proteins than the plasma of nonsmokers (P = 0.015, n = 10, unpaired Student's t test). Because mAb NIC6C12 displays cross-reactivity with certain high molecular weight species in plasma in some, but not all, nonsmokers (Fig. 4B, lanes 2–7), these proteins cannot be considered markers of smoking-related protein modifications. Our assumption was that this observed reactivity was caused by either recognition of NIC6C12 by serum proteins that bind antibody constant regions (e.g., C1q) or other immunoglobulins present in the serum. However, heat treatment of the serum before ELISA or treatment with immobilized protein G had little effect on the strength of the observed signal. Furthermore, assays using either the F(ab′)2, Fab, or scFv fragments of NIC6C12 showed no significant reduction of this polyreactivity. Despite this complication, the lower molecular weight nornicotine-glycated proteins occur only in the serum of smokers (Fig. 4B, lanes 9, 10, and 13). As noted previously, nornicotine has been found in minor amounts both in tobacco and as a nicotine metabolite (see above). In light of these findings, it is evident that its concentration and extended half-life in vivo are sufficient to cause aberrant protein modification.
Covalent Modification of Steroids by Nornicotine.
It has been demonstrated that reactions similar to the Amadori rearrangement can also occur between lysine side chains and steroids that contain an α-hydroxyketone moiety (28). This reaction, the Heyns rearrangement, covalently modifies steroids and provides α-aminoketones analogous to the α-aminoaldehydes produced by the Amadori rearrangement. Based on the observed protein glycation results (see above), we surmised that nornicotine could also covalently modify steroids. Interestingly, two products were formed on incubation of prednisone, a common steroidal pharmaceutical, with nornicotine. Characterization of the observed products by standard synthetic techniques revealed the expected Heyns rearrangement product as well as a previously undescribed 22-hydroxy-prednisone adduct (Fig. 5). This adduct is not stable over time and is observed to decrease in favor of the Heyns rearrangement product. Both of these compounds have the potential to not only have altered efficacy in their therapeutic use, but more importantly, altered toxicity as well.
Fig 5.
Two products formed on reaction of nornicotine with prednisone, the prednisone Heyns rearrangement product, and 22-hydroxy-prednisone.
Our results provide a direct chemical link between tobacco use and the development of AGEs, a class of compounds previously implicated in various disease states. Although nornicotine is both a minor tobacco alkaloid and minor nicotine metabolite, serum concentrations are enough to accumulate measurable concentrations of abnormal glycated proteins in vivo. Furthermore, we present evidence that nornicotine can modify commonly used steroids and thus demonstrate a previously unrecognized pathway for drug interactions. The compilation of our data suggests a number of detrimental scenarios; for example, nornicotine-based glycation products could serve as long-lived sources of “nicotine-like” moieties, contributing to the mechanism of nicotine addiction. Nornicotine-based glycation could also provide a pathway for protein inactivation resulting from covalent modification of critical residues (26). Finally, as protein immunogenicity is increased on glycation (25), nornicotine-based glycation could lead to an immune response against nicotine in the serum of smokers. Our work highlights the need for increased knowledge into the consequences of exposure to secondary nicotine metabolites and their potential pathology.
Acknowledgments
We gratefully acknowledge Dr. Paul Wentworth, Jr., Dr. Louis Hom, Jonathan McDunn, and Diane Kubitz for helpful discussions, Dr. Richard Lerner for a critical reading of the manuscript, and The Skaggs Institute for Chemical Biology for financial support.
Abbreviations
HSA, human serum albumin
References
- 1.Maillard L. C. (1912) C. R. Acad. Sci. Ser. II 154, 66-68. [Google Scholar]
- 2.Yaylayan V. A. & Huyghues-Despointes, A. (1994) Crit. Rev. Food Sci. Nutr. 34, 321-369. [DOI] [PubMed] [Google Scholar]
- 3.Ledl F. & Schleicher, E. (1990) Angew. Chem. Int. Ed. Engl. 29, 565-594. [Google Scholar]
- 4.Monnier V. M., Kohn, R. R. & Cerami, A. (1984) Proc. Natl. Acad. Sci. USA 81, 583-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bucala R. & Cerami, A. (1996) in The Maillard Reaction: Consequences for the Chemical and Life Sciences, ed. Ikan, R. (Wiley, New York), pp. 161–181.
- 6.Bucala R. & Cerami, A. (1992) Adv. Pharmacol. 23, 1-34. [DOI] [PubMed] [Google Scholar]
- 7.Vitek M. P., Bhattacharya, K., Glendening, J. M., Stopa, E., Vlassara, H., Bucala, R., Manogue, K. & Cerami, A. (1994) Proc. Natl. Acad. Sci. USA 91, 4766-4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith M. A., Taneda, S., Richey, P. L., Miyata, S., Yan, S.-D., Stern, D., Sayre, L. M., Monnier, V. M. & Perry, G. (1994) Proc. Natl. Acad. Sci. USA 91, 5710-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monnier V. M. (1990) J. Gerontol. 45, B105-B111. [DOI] [PubMed] [Google Scholar]
- 10.Ho C.-T. (1996) in The Maillard Reaction: Consequences for the Chemical and Life Sciences, ed. Ikan, R. (Wiley, New York), pp. 27–53.
- 11.Biemel K. M., Condra, J. & Lederer, M. O. (2002) Angew. Chem. Int. Ed. Engl. 41, 801-804. [DOI] [PubMed] [Google Scholar]
- 12.Kisaki T. & Tamaki, E. (1961) Arch. Biochem. Biophys. 92, 351-355. [DOI] [PubMed] [Google Scholar]
- 13.Bardo M. T., Green, T. A., Crooks, P. A. & Dwoskin, L. P. (1999) Psychopharmacology 146, 290-296. [DOI] [PubMed] [Google Scholar]
- 14.Curvall M. & Kazeni, V. E. (1993) in Nicotine and Related Alkaloids: Absorption, Distribution, Metabolism and Excretion, eds. Gorrod, J. W. & Wahren, J. (Chapman & Hall, Stockholm), pp. 147–179.
- 15.Crooks P. A., Li, M. & Dwoskin, L. P. (1997) Drug Metab. Dispos. 25, 47-54. [PubMed] [Google Scholar]
- 16.Dickerson T. J. & Janda, K. D. (2002) J. Am. Chem. Soc. 124, 3220-3221. [DOI] [PubMed] [Google Scholar]
- 17.Siddiqui I. R., Rosa, N. & Benzing, L. (1981) Carbohydr. Res. 98, 57-63. [Google Scholar]
- 18.Harlow E. & Lane, D., (1988) Antibodies: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 19.Koiwai A., Mikami, Y., Matsushita, H. & Kisaki, T. (1979) Agric. Biol. Chem. 43, 1421-1426. [Google Scholar]
- 20.Cerami C., Founds, H., Nicholl, I., Mitsuhashi, T., Giordano, D., Vanpatten, S., Lee, A., Al-Abed, Y., Vlassara, H., Bucala, R. & Cerami, A. (1997) Proc. Natl. Acad. Sci. USA 94, 13915-13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cashman J. R. (1997) NIDA Res. Monogr. 173, 225-258. [PubMed] [Google Scholar]
- 22.Al-Abed Y., Kapurniotu, A. & Bucala, R. (1999) Methods Enzymol. 309, 152-172. [DOI] [PubMed] [Google Scholar]
- 23.Makita Z., Vlassara, H., Cerami, A. & Bucala, R. (1992) J. Biol. Chem. 267, 5133-5138. [PubMed] [Google Scholar]
- 24.Isomura S., Wirsching, P. & Janda, K. D. (2001) J. Org. Chem. 66, 4115-4121. [DOI] [PubMed] [Google Scholar]
- 25.Brownlee M., Vlassara, H. & Cerami, A. (1984) Ann. Intern. Med. 101, 527-537. [DOI] [PubMed] [Google Scholar]
- 26.Vlassara H., Brownlee, M., Manogue, K. R., Dinarello, C. A. & Pasagian, A. (1988) Science 240, 1546-1548. [DOI] [PubMed] [Google Scholar]
- 27.Wüthrich K., (1986) NMR of Proteins and Nucleic Acids (Wiley, New York).
- 28.Yu S. C. & Fishman, J. (1985) Biochemistry 24, 8017-8021. [DOI] [PubMed] [Google Scholar]