Abstract
Systems neuroscience addresses the complex circuits made by populations of neurons in the CNS and the cooperative function of these neurons. Improved approaches to the neuroanatomical analysis of CNS circuits are thus of great interest. In fact, significant advances in tract-tracing methods have recently been made by using transgenic mice that express transneuronal lectin tracers under the control of neuron-specific promoters. The utility of those animals, however, is limited to the CNS circuit influenced by the particular promoter. Here, we describe a new transgenic mouse that can be used for transneuronal tracing analysis of circuits in any region of the brain or spinal cord. The transgene in these mice results in expression of LacZ in neurons throughout the CNS. Excision of the LacZ gene by Cre-mediated recombination initiates expression of the lectin, wheat germ agglutinin (WGA). To illustrate the diverse uses of these ZW (LacZ-WGA) mice, we triggered WGA expression either by crossing the mice with two Cre-expressing transgenic mouse lines or by microinjecting a Cre-expressing adeno-associated virus into the cerebellum or cerebral cortex. Both approaches resulted in extensive WGA expression in the cell bodies and dendrites of neurons in which the recombination event occurred, as well as anterograde and transneuronal transport of the lectin to second and third order neurons. Because the lectin can be induced in developing and adult animals, and in all regions of the brain and spinal cord, these ZW may prove extremely valuable for numerous studies of CNS circuit analysis.
The ability to visualize complex and extended neural networks in the CNS is critical to understanding the functional organization of complex sensory and motor systems and is particularly valuable when the objective is to address the dynamic responses of these systems in injury and disease. Classically, circuit organization has been established with tracers that define the projections of subsets of neurons. Because few tracers cross synapses, however, it is more difficult to identify chains of neuronal connections (1). Although transneuronal transport of viruses has been used in several anterograde and retrograde tracing studies, the lytic nature of these viruses has limited their use after CNS injection (2–5).
Building on the ability of plant lectins to transport transneuronally (6, 7), Yoshihara et al. (8) developed a transgenic mouse in which the lectin wheat germ agglutinin (WGA) is driven by a Purkinje cell-specific promoter, L7. In these mice, the lectin was not only strongly expressed in Purkinje cells, but was also detectable in 2nd and 3rd order neurons downstream of the Purkinje cells, i.e., after its transneuronal anterograde transport. In a similar approach, Zou et al. (9) used transneuronal tracing with selective expression of barley lectin to study the central projection of subsets of primary olfactory neurons. Because lectin expression in these mice is under the control of neuron-specific promoters, the circuits that are revealed are system specific. In the present report, we describe the development of a transgenic mouse in which transneuronal labeling of circuits originating from any region of the brain or spinal cord can be induced. To this end, we combined the Cre/loxP site-specific recombination system with the transgenic approach of Yoshihara et al. (8) to produce transgenic mice in which induction of the WGA expression can be controlled. To induce the WGA transgene, either we crossed these mice with others that express Cre recombinase under the control of tissue-specific promoters, or we microinjected an adeno-associated virus (AAV) that expresses Cre. We demonstrate not only that Cre-mediated induction of the lectin reveals exquisite morphology of the neurons that express the WGA transgene, but also that the lectin is efficiently transported in the anterograde direction and transneuronally to higher order neurons in a variety of CNS circuits.
Materials and Methods
Construction of the Transgene and Generation of Transgenic Mice.
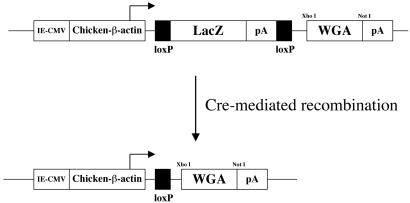
To construct the pCZW expression vector, we inserted a truncated WGA coding sequence (kindly provided by Y. Yoshihara, Brain Science Institute, Wako, Japan and N. Ryba, National Institutes of Health, Bethesda, MD) into the intact XhoI-NotI sites of the pCALL expression vector (10), replacing the alkaline phosphatase gene. In pCZW, the WGA cDNA is located downstream of the following transcription unit: chicken β-actin promoter (CβA)-loxP-flanked βgeo-(LacZneoR)3xpA (ref. 11; Fig. 1). Under normal conditions, only the LacZ gene is expressed. After Cre-mediated recombination, the βgeo/3xpA fragment is excised, and the WGA is expressed. The approach is comparable to that used in the development of ZAP mice (10).
Fig 1.
pCZW construct used to generate ZW transgenic mice. Both the LacZ (β-galactosidase-neomycin resistance fusion protein) and WGA expression are under the control of the ubiquitous chicken β-actin promoter-cytomegalovirus enhancer. Under normal conditions, only the LacZ is expressed. After Cre recombination, the loxP-flanked LacZ is excised, and the WGA is transcribed.
We linearized the pCZW vector with XmnI and purified it according to the recommendations of the Stanford transgenic facility (www-med.stanford.edu/transgenic). One microgram of linearized pCZW was microinjected into the pronuclei of one-cell embryos of pseudopregnant B6CBAF1/J (C57BL/6 × CBA) mice to produce transgenic mice. Pups were derived from B6CBAF1/J × B6CBAF1/J crosses and were housed in the University of California, San Francisco animal facility. Founder transgenic mice were identified after a standard 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) reaction of the tail and then crossed to C57BL/6 mice. We used F1 progeny in this study.
Recombination After Cre Induction.
To generate transgenic mice that express Cre recombinase under the control of the L7 promoter, we inserted a BglI/BglII fragment containing a nuclear localization signal-Kozac sequence-Cre-Protamine I into the fourth exon of the L7 minigene (kindly provided by J. Oberdick, Department of Neuroscience, Ohio State University, Columbus, OH; ref. 12). Consistent with the fact that L7-Cre transgenic mice, which are on a DBA/C57BL6 background, express Cre exclusively in Purkinje cells, we found that crosses of L7-Cre mice with the ROSA26 reporter mice (13) resulted in recombination in 99% of Purkinje cells (unpublished data).
Pax 6α-Cre transgenic mice were kindly provided by P. Gruss (Max Planck Institute, Göttingen, Germany) and were generated as described elsewhere (14). In the α-Cre mice, the Cre recombinase is expressed under the control of a retina-specific regulatory element, the murine Pax6. Expression occurs only in retinal progenitor cells, and these cells give rise to all cell types of the mature neuroretina, but predominantly in the periphery.
Cre-Mediated Recombination After Virus Injection.
Concerning AAV-Cre-GFP, to trigger Cre recombination postnatally, we injected an AAV that expresses a Cre-GFP (green fluorescent protein). The fusion protein is under the control of the human immediate early cytomegalovirus (CMV) promoter (15, kindly provided by B. Kaspar and F. Gage, Salk Institute). For cerebellar injections of the virus, we anesthetized 6-wk-old ZW transgenic mice with ketamine (60 mg/kg; xylazine 8 mg/kg) and placed them in a stereotaxic instrument. After incision of the skin overlying the occiput, a small burr hole was made directly over the right hemisphere of the cerebellum. A micropipette attached to a manual microinjector (Sutter Instruments, Novato, CA) was inserted to a depth of 1.5 mm below the skull. We made a single injection of 1.0–2.0 μl of the concentrated AAV suspension [≈106 plaque-forming units (pfu)/ml]. The scalp was then sutured, and, after the mouse recovered from anesthesia, it was returned to standard housing. In other studies, we microinjected the AAV in the visual cortex. As described above, we inserted a micropipette containing the viral vector to a depth of 0.5 mm below the skull and then made a single injection of the AAV-Cre-GFP. Both the cerebellar and cortical injected mice were analyzed at 1, 2, and 4 wk post-AAV injection.
Immunohistochemistry.
To localize the transgene and transported WGA, we anesthetized mice with Nembutal (100 mg/kg) and then perfused them transcardially with 10 ml of saline (0.9% NaCl), followed by 30 ml of 10% formalin in 0.1 M phosphate buffer (PB), at room temperature. Tissues were removed, postfixed in the same solution for 1 h, and cryoprotected in 30% sucrose-PB saline (PBS). Thirty-micrometer cryostat sections were preincubated for 30 min at room temperature, in PBS containing 0.5% Triton X-100 and 10% normal goat serum (NPBST) and then immunostained overnight at 4°C in the same buffer containing a polyclonal anti-WGA antibody (1:50,000, Sigma) or a monoclonal anti-NeuN (1:2,000, Chemicon), which marks neurons. After washing in NPBST, sections were incubated for 1 h with rhodamine (Cy3)-conjugated anti-rabbit IgG (1:700, Molecular Probes) or with Oregon green (514)-conjugated anti-mouse IgG (1:700, Molecular Probes), rinsed in NPBST, mounted in fluoromount-G and coverslipped. Sections were viewed with a Nikon Eclipse fluorescence microscope and images were collected with a Spot Camera and processed with Adobe photoshop, version 5.0.
Results
Fig. 1 illustrates the pCZW construct that we used to generate the ZW transgenic mouse line. This construct directs expression of WGA after Cre-mediated excision of the loxP-flanked LacZ gene. We obtained three transgenic mouse lines (ZW5, ZW16, and ZW19) that carry the ZW transgene. Because of the widespread expression of the transgene, we identified founder transgenic mice by using a standard X-Gal reaction of the tail. We propagated only the line with the highest rate of transgene transmission (ZW19). In this line, 75% of the offspring were positive for LacZ. As expected, all offspring were negative for WGA (data not shown). This result indicates that WGA expression requires Cre recombination and that there was no evidence of leaky WGA expression from the ZW transgene.
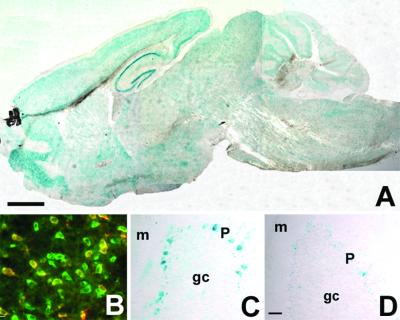
Because the chicken β-actin promoter is very active in diverse cell types, in both adult animals and in embryos (10), we anticipated that the transgenic mice would express WGA in all tissues where Cre-mediated recombination occurred. In fact, we detected β-galactosidase activity throughout the CNS and peripheral nervous system, albeit in a mosaic fashion (Fig. 2A). Double immunostaining experiments using an antibody against a neuronal marker (NeuN) showed that ≈50% of the total neuron population express the ZW transgene (Fig. 2B). Whole-mount staining of ZW transgenic newborns showed very intense and extensive transgene expression in diverse tissues (data not shown). There was no X-Gal staining in nontransgenic mice.
Fig 2.
Expression of the ZW transgene in adult transgenic mice. (A) This photomontage of a sagittal section of the brain of a 2-wk-old ZW mouse shows β-galactosidase activity throughout the CNS, indicating that there is extensive and widespread expression of the transgene. (B) Based on the double labeling (yellow) of NeuN-positive (green) and β-galactosidase-positive neurons (red), we estimate that about half of the total neuron population expresses the ZW transgene. This particular image was taken from a section through the striatum. (C) The ZW transgene is expressed in a mosaic fashion. Although the labeling in Purkinje cells was the most prominent, we observed extensive, albeit light labeling of all cell layers of the cerebellum. (D) After Cre recombination in an ZW/L7-Cre double transgenic mouse, Purkinje cells no longer express the LacZ transgene and thus the Purkinje cell layer appears white. Shown are granule cell (gc), molecular (m), and Purkinje cell layers (P). (Bar = 1.0 mm in A and 75 μm in B–D.)
L7 Cre-Mediated WGA Expression in Cerebellar Circuits.
To trigger WGA synthesis by Cre-mediated recombination, we first mated the ZW transgenic line with a heterozygous L7-Cre transgenic mouse line that expresses Cre exclusively in cerebellar Purkinje cells. F1 double transgenic animals that carried both the ZW and the L7-CRE transgenes were mosaic for β-galactosidase activity, throughout the brain (data not shown), but in Purkinje cells we found an almost complete loss of LacZ expression. Compare the Purkinje cell lacZ expression in a ZW mouse (Fig. 2C) with that in a ZW/L7-Cre double transgenic (Fig. 2D). These results establish that a Cre recombination event occurred.
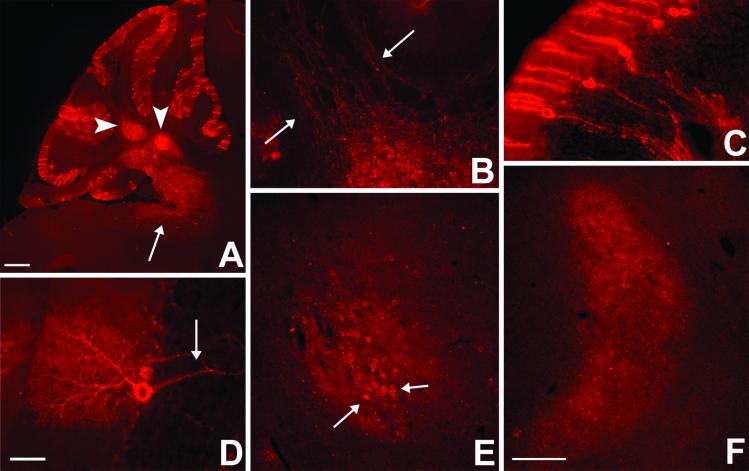
We next asked whether the Cre-mediated lacZ gene excision had indeed triggered WGA expression and transport. As illustrated in sagittal sections taken from a 2-wk-old double transgenic mouse (Fig. 3A), we observed an intense, punctate WGA labeling in all lobules of the cerebellar cortex. The lectin was present in Purkinje cell bodies and dendritic arbors and in bundles of axons (Figs. 3 C and D) that coursed through the underlying granule cell layer and cerebellar white matter, en route to the deep cerebellar nuclei (Figs. 3 A and B). We traced other axons to the vestibular nuclei (Fig. 3A). We only very rarely observed WGA-positive granule cells, which indicates that the WGA synthesized in Purkinje cells was not retrogradely transported to neurons that target Purkinje cells.
Fig 3.
WGA expression and transneuronal transport in cerebellar pathways of ZW/L7-Cre double transgenic mice. After Cre recombination, the WGA immunostaining (red) localizes extensively in Purkinje cells (A and C). The lectin is uniformly distributed in Purkinje cell somata, dendrites, and axons (arrow in D), and axonal arbors are readily detected in the deep cerebellar and vestibular nuclei (arrowheads and arrow in A). The tracer is axonally transported and transneuronally transferred to second order neurons in the deep cerebellar nuclei (B) and to third order neurons (arrows) in the red nucleus (E). (F) Transneuronal label in the ventrolateral nucleus of the thalamus appeared to be restricted to terminals. (Bar = 450 μm in A and 150 μm in B–F.)
Fig. 3A illustrates that there is intense anterograde WGA labeling in neuronal cell bodies and processes of the deep cerebellar and vestibular nuclei. Consistent with the known projection of the deep nuclei to the red nucleus in the midbrain and the ventrolateral nucleus (VL) of the thalamus, we also found significant terminal expression of WGA in these regions (Figs. 3 E and F). We also observed large numbers of WGA-positive cell bodies in the red nucleus, but not in VL, which suggests that the extent of transneuronal transport can vary significantly in different circuits. Finally, we observed a few labeled cell bodies in the dorsal and ventral horns of the spinal cord, as well as in the brainstem reticular formation (data not shown), both of which are targets of axons of deep cerebellar nuclei neurons. The pattern of labeling that we observed did not differ significantly in ZW/L7-Cre double transgenic mice that were studied at 4, 6, or 10 wk of age.
Pax6 Cre-Mediated WGA Expression in Visual Circuits.
In crosses of ZW and (α-Cre) mice, we observed significant transneuronal labeling in the visual system of double transgenic progeny. As expected, F1 double transgenic animals that carried both the ZW and the α-CRE transgenes were mosaic for β-galactosidase activity, throughout the brain, but in retinal cells we found an almost complete loss of LacZ activity (data not shown), indicating that a Cre recombination event had occurred.
Fig. 4 A–C is taken from a 4-wk-old double transgenic mouse and illustrates punctate WGA immunoreactivity in cell bodies and dendrites throughout the depth of the retina, in all cell types (Fig. 4A). As expected, the WGA was concentrated in the peripheral retina, i.e., where the Cre was most abundantly expressed (14). In mice that were studied at 2 wk of age, we found label only in the retina. By contrast, at 4 wk of age, we observed significant transneuronal transport of WGA to brainstem targets of retinal ganglion cells, including the pretectal nucleus (Fig. 4B), the lateral geniculate nucleus (Fig. 4C), and in the superior colliculus (data not shown). We did not observe labeling in visual cortical areas, which suggests that we had reached the limit of the transneuronal transport. Longer survival times, up to 10 wk, did not increase the density of label in the brainstem and did not result in labeling in the visual cortex, at least by using an immunofluorescence detection method.
Fig 4.
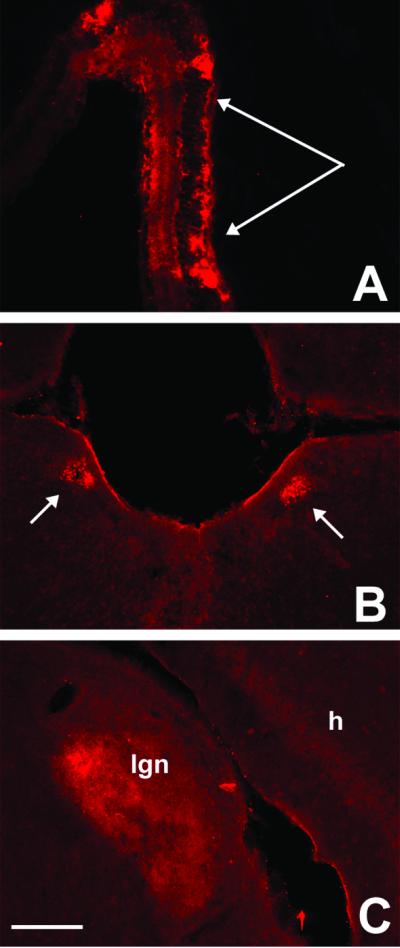
WGA expression in vision circuits of α-Cre/ZW double transgenic mice. α-Cre mice express the Cre recombinase only in retinal progenitor cells that give rise to all cell types of the mature neuroretina. (A) After crossing with ZW mice, we found WGA immunoreactivity in cell bodies and dendrites through all retinal layers, with a concentration in the peripheral retina. Arrows in A point to photoreceptor layer. The WGA protein was anterogradely transferred from retinal ganglion cells to the pretectal nuclei (B) and to neurons in the dorsal lateral geniculate nucleus (C). We did not find labeled cortical neurons in these animals. (Bar = 200 μm in A and 100 μm in B and C.)
Cre-Mediated WGA Expression After Virus Injection in Adult ZW Mice.
To determine whether WGA expression can also be induced in adult mice, we injected a Cre/GFP-expressing AAV vector into the cerebellar cortex or into the visual cortex of adult ZW mice. Most animals were injected at 6 wk of age; injections made into older mice resulted in similar patterns of labeling. Because the AAV vector expresses a fusion protein (Cre/GFP), we could readily define the precise pattern of viral infection by monitoring for neuronal expression of the GFP. Fig. 5A illustrates an injection site that was confined to one folium of the cerebellum; this mouse was studied 7 days after AAV injection.
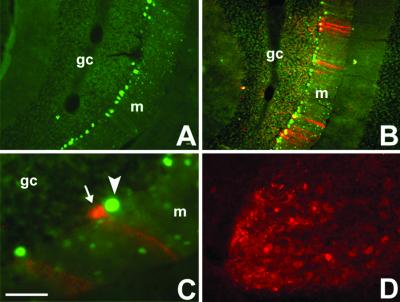
Fig 5.
WGA expression and transneuronal transport after AAV-mediated Cre-GFP delivery to the cerebellar cortex of an adult ZW mouse. (A) GFP fluorescence (resulting from expression of the Cre-GFP fusion protein) identifies the location of the AAV injection, which was made 7 days before, in this 6-wk-old mouse. The Cre recombinase was mainly found in the nuclei of infected cells. (B) An adjacent section reveals that Cre recombination triggered WGA expression (red) in several cerebellar cells. (C) The WGA (red) was transneuronally transferred to noninfected Purkinje cells (arrow), i.e., which contained the βgeo transgene. Some Cre-expressing Purkinje cells (green, arrowhead) are not WGA positive, due to the mosaicism of the transgene expression. (D) As observed in the ZW/L7-Cre double transgenic mice, AAV induced-Cre recombination in Purkinje cells was followed by transneuronal transport of the WGA to neurons in the deep cerebellar nuclei. (Bar = 200 μm in A–D.)
There was no spread of the AAV to the deep cerebellar nuclei or to the contralateral cerebellar cortex. Serial section analysis revealed that the area of infection extended up to 1.2 mm along the rostral-caudal axis of the cerebellum and up to 0.5 mm dorsoventrally. Consistent with the translocation of Cre to the nucleus, we found that the Cre/GFP signal was largely confined to the nucleus, of both Purkinje and granule cells; however, we did observe some cytoplasmic labeling. Fig. 5B shows that AAV-induced Cre expression triggered significant WGA expression. The labeling was mainly associated with Cre/GFP-positive Purkinje cells, and there was intense WGA labeling throughout the dendritic arbor of these neurons. We also observed WGA immunoreactivity in some GFP-negative, i.e., nonrecombined cells (arrow, Fig. 5C), at the injection site, and in Purkinje cells remote from the injection site (data not shown). This result suggests that transneuronal transport of the lectin can occur between synaptically connected Purkinje cells, presumably after anterograde transport by their recurrent collaterals. We did not systemically evaluate the molecular layer of the cerebellar cortex for transneuronal transport to basket and stellate cells.
In contrast to the exclusive expression of WGA in Purkinje cells after L7-Cre-ZW crosses, the AAV injection occasionally resulted in labeling of a cluster of granule cells in a halo just ventral to the Purkinje cell layer (Fig. 5B). This labeling was manifest as an intense, punctate WGA staining in all layers of the cerebellar cortex and in the white matter immediately underlying the center of the injection site. The high correspondence of GFP and WGA labeling indicated that the WGA is induced in all infected transgenic neurons. The labeling in the granule cell layer was found both in cells and in bundles of Purkinje cell axons destined for the deep cerebellar nuclei. As shown in Fig. 5D, the pattern of deep cerebellar labeling induced by the AAV-Cre-GFP was comparable to that seen in the L7Cre/ZW mice, although not as extensive.
Cre-Mediated WGA Expression After AAV Injection in Adult Visual Cortex.
A great advantage of the ZW mice is that circuit analysis from subsets of neurons can be studied in diverse regions of the CNS. To illustrate this property, we evaluated the labeling produced by a localized injection of the AAV-Cre-GFP into the visual cortex. Fig. 6A illustrates an injection site in a mouse that was studied 2 wk after injection. There are several interesting features of the WGA labeling that was induced. The pattern of GFP labeling reveals that the injection was concentrated in the middle laminae of the cortex, with a few isolated neurons located in more superficial and deep laminae. As expected, the pattern of WGA-immunoreactive neurons overlapped that of the GFP-positive distribution, but the numbers of labeled neurons were much larger, and included many neurons located throughout the dorsal-ventral extent of the cortex, creating a columnar pattern. The WGA-positive, GFP-negative neurons were presumably labeled after transneuronal transport of the lectin within a cortical column. Fig. 6 further illustrates transneuronally labeled cells in a cluster located lateral to the heart of the AAV injection, perhaps in a functionally related cortical column.
Fig 6.
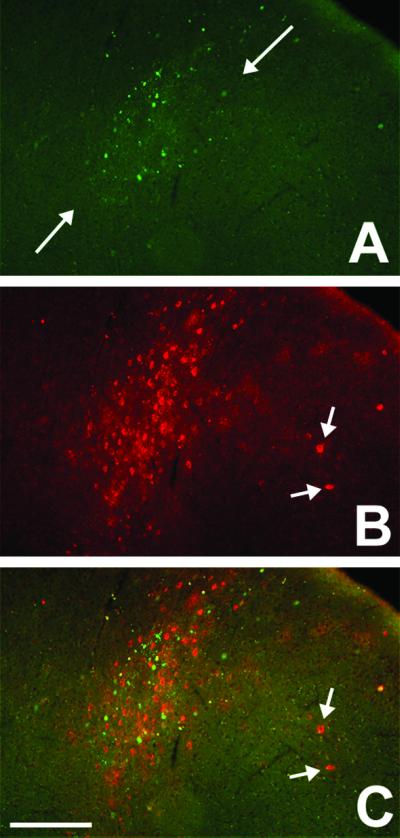
WGA expression and transneuronal transport after AAV-mediated Cre-GFP delivery to the visual cortex of an adult ZW mouse. (A) GFP fluorescence shows that AAV-infected neurons were concentrated in the middle laminae of the cortex. (B) The WGA immunoreactivity (red) overlaps extensively with the GFP labeling but also extends to more superficial and deep cortical layers, in a columnar pattern. (C) The merged image shows that there is also transneuronal transport of the lectin into cortical neurons that are likely located in an adjacent column (arrow). (Bar = 150 μm in A–C.)
Discussion
To understand how extended neural circuits develop and are altered (in adaptive and maladaptive ways, as can occur after injury), it would be helpful to be able to trace the multisynaptic connections made by subpopulations of neurons in diverse regions of the brain and spinal cord. We believe that the ZW transgenic mouse described in this report will prove extremely valuable in such circuit analyses. Not only can we induce long-term expression of the WGA transneuronal tracer after Cre-mediated recombination, but we also find extensive dendritic and axonal labeling of the primary neurons (in which the Cre recombination occurs). This Golgi-like labeling permits a detailed morphological analysis of the cells of origin of the pathway that is being analyzed. Most importantly, the WGA expression can be induced in all part of the CNS and in embryonic, postnatal, or adult animals. The breadth of studies in which these mice will prove useful is thus tremendous.
The results from the ZW/L7-Cre double transgenic animals are comparable, but not identical to those reported by Yoshihara et al. (8), who studied mice in which WGA was constitutively driven off of the L7 promoter. We found extensive labeling of Purkinje cells and their axonal projections, as well as 2nd order neurons in the deep cerebellar and vestibular nuclei. We also found extensive terminal labeling in the red nucleus and thalamus and in some 3rd order neurons of the former region. On the other hand, we did not find labeling in the inferior olive, a region heavily labeled in the Yoshihara et al. (8) study. Although the labeling in the latter study could have derived from a retrograde transport of the lectin by olivocerebellar afferents, it is also possible that the strength of the L7 promoter permitted sufficient lectin expression to reveal rubroolivary projections, i.e., via a distant anterograde connection. Consistent with this interpretation we never observed labeling of cerebellar granule cells, which would be labeled if retrograde transport of the expressed lectin were common. The absence of retrograde transport of the lectin in the ZW mice is, of course, critical to the utility of these animals for tracing circuits. Concurrent retrograde transport would make interpretation of the labeling patterns, including the direction of circuit flow, extremely difficult.
The great utility of these new mice will derive from studies in which Cre recombinase is induced in specific brain regions. Because only a few promoters are available to induce regional Cre-mediated recombination, we used a viral approach to administer the Cre transgene. Our initial studies with adenoviral vectors were somewhat disappointing. For example, we found that injection of a Cre-expressing adenovirus into the cerebellar cortex of ZW mice (data not shown) produced only labeled Bergmann glial cells. This result indicates that adenoviral vectors are not adequately incorporated into neurons via the cell body (16), or that rapid cell death had occurred. Furthermore, in the adenovirus-injected mice, we found extensive label in precerebellar neurons of the inferior olive and pons, which suggests that there was retrograde transport of the adenoviral vector. Consistent with other reports (15, 17, 18), we found that the most efficient Cre expression was produced after AAV injections, which labeled large numbers of neurons, in both the cerebellum and the visual cortex. We found no evidence that the GFP component of the Cre-GFP fusion protein reduced the ability of the Cre protein to target the nucleus, where the recombination event occurs. In other studies, we found that AAV injection was also effective after injection into the spinal cord and thalamus (data not shown), establishing the more general value of these animals for studies in diverse CNS regions. Because there is transport in postnatal and adult animals, it will be of great interest to use this transneuronal label approach to study the development of different CNS circuits.
Despite using a purportedly ubiquitous promoter, the ZW mice showed mosaic expression of the transgene. We estimate that ≈50% of the CNS and peripheral nervous system neurons have the potential to synthesize tracer after Cre-mediated recombination. Clearly one useful modification of this approach will be to use a truly “ubiquitous” promoter to drive the expression of the construct. To this end, we are generating mice in which the LacZ/WGA transgene is inserted into the ROSA-26 locus (13). There are, however, some distinct advantages that are possible because of the mosaicism. For example, short distance transneuronal transfer between neurons or reciprocal connections between two regions will be apparent only if neurons that express the transgene can be clearly distinguished from neurons that do not express it. The mosaic expression of the transgene also permitted a very detailed morphological analysis of neurons that express the WGA, in much the same way that Golgi staining provides useful information because it labels only isolated neurons. If every neuron were labeled, then the dendritic arbor of individual neurons would be obscured. Because of this property of ZW mice, we can concurrently study the dendritic arbors of, and the circuits engaged by, subsets of developing neurons.
In summary, we have generated a mouse strain (ZW) that efficiently expresses a neuronal tracer (WGA) after Cre-mediated recombination. Because the expression of the transgene is driven by a relatively “ubiquitous” promoter, tracing of diverse neuronal circuits is possible, in developing and adult animals. We anticipate use of these mice in a variety of neurobiological studies of the development, anatomy, and functions of the CNS. Because the WGA expression can be turned on at any time and in almost any region of the brain, developmental analysis of the projection and central connections of subsets of neurons is possible. These mice should also be of particular value in studies of the reorganization of CNS that occurs in the setting of tissue or nerve injury. For example, it will be of particular interest to study second and third order connections made by axons that undergo either collateral sprouting or regeneration after injury.
Acknowledgments
We thank Drs. Cori Bargmann, David Julius, and Gail Martin for suggestions during the development of the WGA construct and Dr. Simona Neumann for helpful comments with the manuscript. This work was supported by National Institutes of Health Grants NS 14627, 21445, and DA 08377 (to A.I.B.), and P01NS 16033 (to B.R.), Association Française pour la Recherche Therapeutique (J.M.B.), Ministerio de Educacion y Ciencia, Spain (B.R.), and an unrestricted gift from Bristol-Myers Squibb (A.I.B.).
Abbreviations
WGA, wheat germ agglutinin
AAV, adeno-associated virus
X-Gal, 5-bromo-4-chloro-3-indolyl β-d-galactoside
References
- 1.Kobbert C., Apps, R., Bechmann, I., Lanciego, J. L., Mey, J. & Thanos, S. (2000) Prog. Neurobiol. 62, 327-351. [DOI] [PubMed] [Google Scholar]
- 2.Card J. P., Rinaman, L., Schwaber, J. S., Miselis, R. R., Whealy, M. E., Robbins, A. K. & Enquist, L. W. (1990) J. Neurosci. 10, 1974-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Card J. P. & Enquist, L. W. (1995) Crit. Rev. Neurobiol. 9, 137-162. [PubMed] [Google Scholar]
- 4.Jasmin L., Burkey, A. R., Card, J. P. & Basbaum, A. I. (1997) J. Neurosci. 17, 3751-3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loewy A. D. (1998) Neurosci. Biobehav. Rev. 22, 679-684. [DOI] [PubMed] [Google Scholar]
- 6.Ruda M. & Coulter, J. D. (1982) Brain Res. 249, 237-246. [DOI] [PubMed] [Google Scholar]
- 7.Shipley M. T. (1985) Brain Res. Bull. 15, 129-142. [DOI] [PubMed] [Google Scholar]
- 8.Yoshihara Y., Mizuno, T., Nakahira, M., Kawasaki, M., Watanabe, Y., Kagamiyama, H., Jishage, K., Ueda, O., Suzuki, H., Tabuchi, K., et al. (1999) Neuron 22, 33-41. [DOI] [PubMed] [Google Scholar]
- 9.Zou Z., Horowitz, L. F., Montmayeur, J. P., Snapper, S. & Buck, L. B. (2001) Nature 414, 173-179. [DOI] [PubMed] [Google Scholar]
- 10.Lobe C. G., Koop, K. E., Kreppner, W., Lomeli, H., Gertsenstein, M. & Nagy, A. (1999) Dev. Biol. 208, 281-292. [DOI] [PubMed] [Google Scholar]
- 11.Friedrich G. & Soriano, P. (1991) Genes Dev. 5, 1513-1523. [DOI] [PubMed] [Google Scholar]
- 12.Smeyne R. J., Chu, T., Lewin, A., Bian, F., S.-, Crisman, S., Kunsch, C., Lira, S. A. & Oberdick, J. (1995) Mol. Cell. Neurosci. 6, 230-251. [DOI] [PubMed] [Google Scholar]
- 13.Soriano P. (1999) Nat. Genet. 21, 70-71. [DOI] [PubMed] [Google Scholar]
- 14.Marquardt T., Ashery-Padan, R., Andrejewski, N., Scardigli, R., Guillemot, F. & Gruss, P. (2001) Cell 105, 43-55. [DOI] [PubMed] [Google Scholar]
- 15.Kaspar B. K., Vissel, B., Bengoechea, T., Crone, S., Randolph-Moore, L., Muller, R., Brandon, E. P., Schaffer, D., Verma, I. M., Lee, K., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 2320-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terashima T., Miwa, A., Kanegae, Y., Saito, I. & Okado, H. (1997) Anat. Embryol. 196, 363-382. [DOI] [PubMed] [Google Scholar]
- 17.Kaplitt M. G., Leone, P., Samulski, R. J., Xiao, X., Pfaff, D. W., O'Malley, K. L. & During, M. J. (1994) Nat. Genet. 8, 148-154. [DOI] [PubMed] [Google Scholar]
- 18.Kaemmerer W. F., Reddy, R. G., Warlick, C. A., Hartung, S. D., McIvor, R. S. & Low, W. C. (2000) Mol. Ther. 2, 446-457. [DOI] [PubMed] [Google Scholar]