Abstract
In Drosophila, locomotor activity is sexually dimorphic and the brain area controlling this dimorphism has been mapped. The neurons of the pars intercerebralis (PI) have been suggested to participate in such differences between males and females. However, the precise physical nature of the dimorphism, the identity of the PI neurons involved, and the nature of the neuronal signal coding the dimorphism remain unknown. In this study, we used a video-tracking paradigm to characterize further the pattern of locomotor activity in Drosophila. We show that the number of activity/inactivity periods (start/stop bouts) is also sexually dimorphic, and that it can be genetically feminized in males. Moreover, the transplantation of PI neurons from a female, or of feminized PI neurons from a donor male into a receiver wild-type male is sufficient to induce the feminization of locomotor behavior, confirming that this tiny cluster of ≈10 neurons is directly responsible for the sexual dimorphism in locomotor activity. Finally, feeding males with fluvastatin, a juvenile hormone (JH) inhibitor, also led to start/stop feminization, and this effect is reversible by the simultaneous application of methoprene, a JH analog, suggesting the existence of a neuroendocrine control, by JH, of such behavioral dimorphism.
Invertebrates and vertebrates show sexually dimorphic behaviors, and it has been suggested that males and females differ in the brain structures controlling such behavioral differences (1–4). Such sex differences have often been used as a way of studying how brain structure contributes to brain function. However, although many sexually dimorphic neural structures have been described (2–4), very few studies have yet demonstrated the direct involvement of a brain structure in a sexually dimorphic behavior (5, 6) and still less a behavior expressed in a nonsexual context. We recently showed that Drosophila locomotor activity is sexually dimorphic (7) outside any sexual context. We could abolish this behavioral dimorphism and create males with a female-like activity profile by genetic manipulations (8). Briefly, we used a UAS-transformer transgene (UAS-tra; ref. 9), the feminizing action of which has been shown to be cell-autonomous (10–12), under the control of different brain-specific enhancer trap P[Gal4] lines. This first study suggested strongly that a few neurons located in the mid-anterior part of the pars intercerebralis (PI) might participate in the control of this sexually dimorphic behavior (8). However, because in this previous study the fly was observed in a small tube, the locomotor activity was indirectly quantified when the fly crossed an infra-red light-gate. As a result, the precise locomotor parameters that accounted for the sexual difference could not be elucidated. Furthermore, the involvement of the PI neurons was indirectly deduced on the basis of a genetic ablation, and the molecular mechanisms linking those few PI neurons to the sexually dimorphic behavior remained to be identified. In this study, we developed a new video-tracking paradigm that allowed us to more precisely specify which parameters in locomotor activity differ between males and females. Furthermore, by a transplantation experiment, we provide evidence that PI neurons are directly responsible for the sexual dimorphism. Finally, using a pharmacological approach, we show that this effect could be mediated by juvenile hormone (JH).
Materials and Methods
Drosophila Culture.
Drosophila melanogaster stocks were maintained at 25°C on standard food medium. Genetic feminization was carried out by using the gene transformer, downstream of four UASGAL4 enhancer elements (P[UAS-tra]; ref. 9). All enhancer-trap lines were homozygous viable. Because Gal4-directed transformer expression was tested in flies heterozygous for both the P[Gal4] and P[UAS-tra] constructs, the corresponding heterozygous P[Gal4]/Canton-S (CS) flies were tested as controls.
Quantification of Locomotor Activity by Video-Tracking.
Intact single Drosophila flies (male and female) were allowed to walk freely in 4 × 4-cm-square chambers, 4 mm high (to limit vertical movement), made of two glass plates and illuminated from below. All experiments were performed at 24°C, at 50–60% relative humidity, with 4-day-old males and mated females. Six flies (three controls and three feminized flies) were simultaneously videotaped at a capture rate of 5 images per second for 7 h. Traces were generated by using EthoVision (Noldus Information Technology, Wageningen, The Netherlands). Paths joining contiguous traces were graphically represented as dots (the center of gravity of the fly) connected by lines (path traveled between frames). From these paths, two parameters were calculated: the total distance moved (mm), and the number of start/stop bouts (i.e., the frequency of successive periods of activity and inactivity). For statistical comparisons, ANOVAs were performed by using statistica (StatSoft, Tulsa, OK). All data were normally distributed.
Transplantation of PI Neurons.
In the first instance, a few neurons (≈10) of the PI were transplanted from a 4-day-old female of the enhancer-trap line P[Gal4]30Y, expressing the labeling gene green fluorescent protein (UAS-GFP), which made it possible to visualize the target neurons. The PI neurons were dissected, and the female neurons were introduced into the abdomen of a recipient 4-day-old wild-type male. Dissections were performed under a binocular microscope (MZFLIII; Leica, Deerfield, IL) equipped with a UV-fluorescent lamp. The donor fly was placed in Drosophila Ringer solution, the head was opened, and the labeled neurons were dissected and extracted with a pulled glass micropipette. The neurons were then immediately introduced into the abdomen of a recipient wild-type male of the same age (4 days old). Next, 4-day-old males of the same enhancer-trap line P[Gal4]30Y, expressing both the feminizing gene (UAS-transformer) and UAS-GFP, were dissected, and PI neurons were transplanted into a wild-type male recipient. Controls were conducted in parallel: males of the same age from the same enhancer-trap line (P[Gal4]30Y), expressing solely the labeling gene green fluorescent protein (UAS-GFP) were dissected, and the nonfeminized neurons were introduced into the abdomen of a recipient 4-day-old wild-type male. All flies were cold-anesthetized (ice) and allowed to recover overnight. Locomotor activity recording was performed the following day (on 5-day-old flies).
Fluvastatin Feeding Protocol and Methoprene Treatment.
Fluvastatin, kindly provided by F. Couillaud (Institut François Magendie, Bordeaux, France) and L. Duportets (Université Paris-Sud, Orsay, France), was dissolved in water (5 μg/μl) and added to glass vials lined with paper 3 mm thick. Three-day-old males were kept overnight (12 h) in a vial without food or water. They were then given access to fluvastatin solution for 10 h. Sibling CS control flies were treated in parallel, receiving water only. The flies were then introduced into the individual arenas, and their locomotor activity was video-recorded for 7 h. To reverse the fluvastatin effect, under the same conditions as described above, the JH analog methoprene (0.5 μg/μl, dissolved in acetone; Sigma) was topically applied (0.2 μl) to the abdomen of male flies fed with fluvastatin 3 h previously. In parallel, sibling CS control male flies received the same amount of the acetone vehicle. The flies were then introduced into the arenas, and their locomotor activity was video-recorded for 7 h.
Results
Number of Start/Stop Bouts Is Sexually Dimorphic.
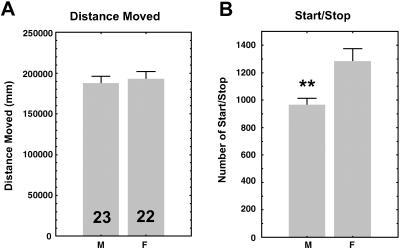
To define precisely the difference between how males and females walk, we developed a video-tracking system that follows the fly in “real time.” Over a 7-h period, a single fly walked spontaneously in a small square arena. Two parameters were analyzed: the total distance moved and the number of successive periods of activity/inactivity (start/stop bouts), a parameter that reflects the overall organization of walking. The total distance covered was similar in males and females, as shown in the previous paradigm (ref. 7; Fig. 1A). However, females showed significantly more start/stop bouts than males (Fig. 1B), showing that the temporal organization of locomotor activity differs between the sexes.
Fig 1.
Sex difference in two parameters of locomotor activity (wild-type CS flies). The sex (M, males; F, females) and number of flies recorded are indicated in the box. (A) Total distance moved (mm) over 7 h. Males and females traveled the same distance. (B) Number of start/stop bouts. Females showed significantly more start/stop bouts than males. Values shown are the mean ± SEM. **, P < 0.01.
Genetic Feminization of PI Neurons.
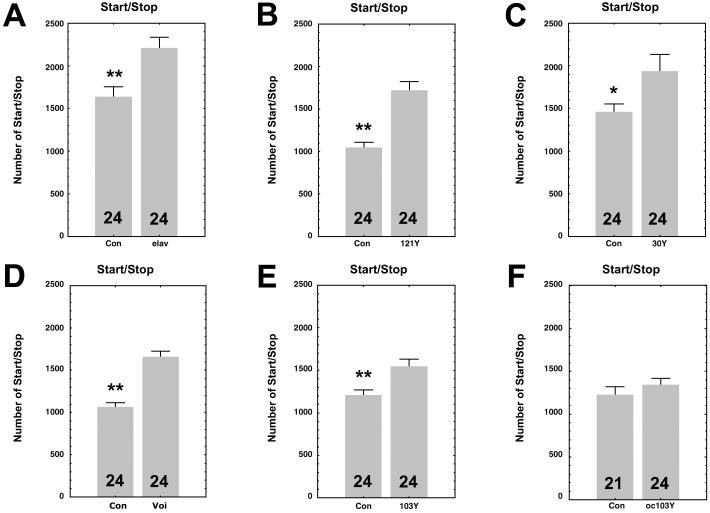
To investigate whether the start/stop parameter is under the control of neurons of the PI, we feminized specific neurons in the brain, using a previously described genetic technique, combining a UAS-transformer transgene and enhancer-trap P[Gal4] lines (8). Fig. 2 shows that, in all five previously identified feminized lines (8), the effect on male locomotor activity is similar, making males walk like females. In transformed males from P[elav-Gal4], P[Gal4]121Y, P[Gal4]30Y, P[Gal4]Voi, and P[Gal4]103Y lines (8), the number of start/stop bouts is significantly greater than in control males (Fig. 2 A–E). P[elav-Gal4] is expressed pan-neurally, whereas the other four lines share a few neurons, localized in the mid-anterior part of the PI, that appear to be responsible for the sexual dimorphism. We also performed the experiment in the ocelliless (oc1) mutant, known to lack PI neurons (13). Against an oc1 genetic background, the expression of transformer under the control of the P[Gal4]103Y fails to induce the feminization of start/stop bouts (Fig. 2F), suggesting that the presence of certain PI neurons is of fundamental importance for this sexually dimorphic behavior. Two P[Gal4] lines, P[Gal4]C35 and P[Gal4]C232, were used as controls. P[Gal4]C35 is expressed in the mushroom-bodies (14) and P[Gal4]C232 in the ring neurons of the ellipsoid-body (15–17), a substructure of the central complex. Expression of transformer in these strains did not lead to the feminization of start/stop bouts (data not shown), suggesting that the mushroom-bodies and the ellipsoid-body are not involved in the control of this sexually dimorphic behavior.
Fig 2.
Histograms of number of start/stop bouts in males of five enhancer-trap P[Gal4]lines driving the UAS-tra gene. For all groups, the controls (Con) correspond to the appropriate heterozygous P[Gal4]/Canton-S (CS) flies. (A) P[elav-Gal4]; (B) P[Gal4]121Y; (C) P[Gal4]30Y; (D) P[Gal4]Voi; (E) P[Gal4]103Y. In the five groups of flies, males expressing transformer in certain parts of the brain showed more start/stop bouts than control males. (F) Control flies correspond to male hemizygous oc1;P[Gal4]103Y/CS flies and feminized males (oc103Y) to oc1;P[Gal4]103Y/UAS-tra. Males in which certain neurons of the PI are missing because of the oc1 mutation do not show an increase in the number of start/stop bouts. Numbers of observed flies are shown in each box. Values given are means ± SEM. *, P < 0.5; **, P < 0.01.
Transplantation of PI Neurons.
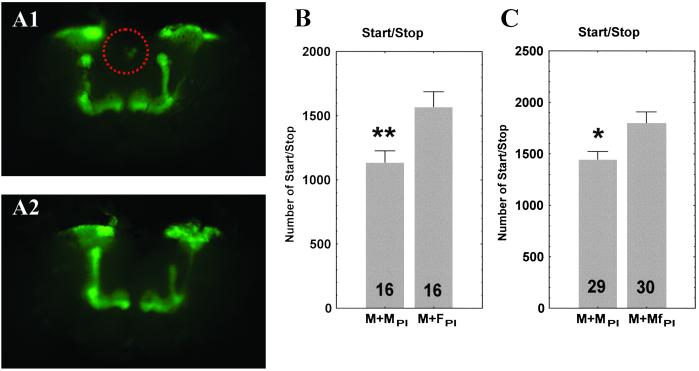
To assess whether PI neurons exercise a direct and autonomous control of sexually dimorphic locomotor behavior, we transplanted the identified tiny cluster of about 10 neurons of the PI (Fig. 3A). In Drosophila, transplantation of brain tissue has already been performed for the circadian pacemaker (18). First, PI neurons from a donor female of the P[Gal4]30Y line expressing the UAS-GFP were dissected, and PI cells were injected into the abdomen of a recipient wild-type male. Second, PI neurons of feminized P[Gal4]30Y males expressing both UAS-tra and UAS-GPF were dissected and injected into the abdomen of a recipient wild-type male. As a control, PI neurons from a P[Gal4]30Y male expressing only the UAS-GFP were dissected and injected into the abdomen of a wild-type male. The transplantation of either female donor neurons (Fig. 3B) or feminized neurons from a donor male (Fig. 3C) led to the feminization of start/stop bouts in the receiver wild-type male, whereas transplantation of control neurons (from a nonfeminized donor male) produced no change in the walking activity of the recipient male. This result shows that the transplanted neurons are sufficient to affect the number of start/stop bouts, providing evidence that PI neurons control this sexually dimorphic locomotor behavior. Because there were no functional neural connections between the abdominally implanted neurons and the locomotor system of the recipient, the action of the transplanted neurons must be mediated by humoral factors, implying that those neurons are neurosecretory.
Fig 3.
Transplantation of PI neurons. (A) Microphotography of the same dissected brain showing PI neurons from a feminized male donor (P[Gal4]30Y/tra-GFP) before (A1) and after (A2) harvesting. The PI neurons are circled by a dotted line. Microphotography, using a numerical camera (CoolSNAP, Princeton Instruments, Trenton, NJ), was performed under a binocular microscope (Leica MZFLIII) equipped with a UV-fluorescent lamp. (B) Number of start/stop bouts of wild-type CS recipient males transplanted with female PI neurons. Control (M + MPI) standard male donor (P[Gal4]30Y/GFP), and (M + FPI) female donor (P[Gal4]30Y/GFP) flies. (C) Number of start/stop bouts of wild-type CS recipient males transplanted with feminized male PI neurons. Control (M + MPI) standard male donor (P[Gal4]30Y/GFP), and (M + MfPI) feminized male donor (P[Gal4]30Y/tra-GFP) flies. Transplanted PI neurons from either a female or a feminized male donor led to a significant increase in number of start/stop bouts, when compared with appropriate control males. The number of flies observed is shown in each box. Values shown are means ± SEM. *, P < 0.05; **, P < 0.01. Because the two series of transplantations were not accomplished at the same period of the year, the base level of start/stop bouts is slightly different. This result emphasizes the importance of recording control flies simultaneously with transplanted flies, as was done here.
Ingestion of Fluvastatin, a JH Biosynthesis Inhibitor, Mimics the Feminization Effect.
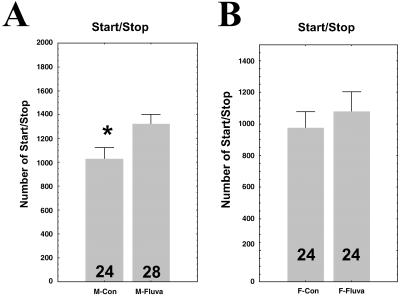
The PI zone of the insect brain contains several neuronal cell bodies, some of which are neurosecretory (19, 20). In Drosophila, their identity and number are still poorly characterized. In other insects, such as the locust, a subgroup of these neurons (A and B) has been characterized, sending projections into the corpora cardiaca (cc) and the corpora allata (ca) to control the secretions of this gland complex (20). In adult insects, the main secretion of the cc-ca is JH, involved in female vitellogenesis (20–22); however, JH also has many other functions in a range of tissues (21, 22). In male Drosophila, although JH has been shown to stimulate protein synthesis in the accessory glands (23, 24), possible additional JH-regulated physiological processes remain to be revealed. To test the hypothesis that JH might be involved in sexual dimorphic locomotor activity and could account for a molecular link between PI neurons, the cc-ca gland, and locomotor activity, we used a pharmacological approach to block JH biosynthesis. Fluvastatin, a hydroxymethylglutaryl-CoA reductase inhibitor, inhibits JH biosynthesis in insects (25, 26). After ingestion of fluvastatin, wild-type male Drosophila showed an increase in start/stop frequency (Fig. 4A), mimicking the feminization effect, whereas in females, fluvastatin produced no effect (Fig. 4B). This result strongly suggests that PI neurons control the dimorphism in locomotor activity through JH-based neuroendocrine transmission.
Fig 4.
Number of start/stop bouts in controls (Con) and fluvastatin-treated (Fluva) wild-type CS flies. (A) males; (B) females. The number of flies observed is shown in each box. Values given are means ± SEM. *, P < 0.05. As in Fig. 3, because the two series of fluvastatin-treated flies (males and females) were not accomplished at the same period of the year, the base level of start/stop bouts is slightly different. This result emphasizes the importance of recording control flies simultaneously with transplanted flies, as was done here.
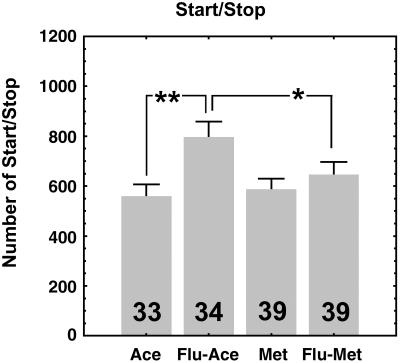
The Fluvastatin Effect Is Reversed by Methoprene, a JH Analog.
In other insects, such as cockroaches, the hydroxymethylglutaryl-CoA reductase enzyme has been shown to be present in other tissues (27), and, consequently, other putative side effects on tissues besides the corpora allata could not be excluded. To confirm that the observed fluvastatin effect on locomotor activity is conveyed by JH, we used methoprene, a JH analog, to reverse the fluvastatin effect. Methoprene is a well known JH analog that has already been successfully used to reverse JH deficiency effects (21, 28, 29). After starting the fluvastatin treatment, we topically applied the methoprene solution to the abdomen of the flies. Seven hours later, the locomotor activity of the flies was recorded. Fig. 5 shows that males that received both products, fluvastatin followed by methoprene, exhibit the same number of start/stop bouts as the appropriate controls, indicating that methoprene is sufficient to reverse the JH deficiency-induced effect caused by fluvastatin ingestion. This reversal of the effect, by methoprene, confirms that this sexual dimorphic effect depends on JH.
Fig 5.
Number of start/stop bouts of the two sets of appropriate Control flies (Ace, males treated with the acetone vehicle only; Flu-Ace, males treated with fluvastatin + acetone) compared with males who received either only methoprene (Met) or both fluvastatin and methoprene (Flu-Met). The number of flies observed is shown in each box. Values given are means ± SEM. *, P < 0.05; **, P < 0.01. The statistical difference between the fluvastatin-treated males that received methoprene compared with the males that received only fluvastatin indicates that methoprene is sufficient to reverse the effect (JH deficiency) caused by fluvastatin. The relatively lower number of start/stop bouts observed in this experiment, compared with Fig. 4, could be attributable to deleterious secondary side effects caused by the acetone vehicle. Nevertheless, the reversal effect is clearly apparent.
Finally, one of the main advantages of these last three experimental approaches (transplantation, fluvastatin, and methoprene) is that the difference in locomotor activity cannot be due to some uncontrolled genetic background side effect because the recipient males, both those treated with fluvastatin alone and those treated with fluvastatin and methoprene, share the same wild-type genotype as their respective controls.
Discussion
Although a sexual dimorphism in locomotor activity has been previously reported (8), because of the design of the apparatus used in that study, the fine difference between male and female locomotor activity remained unknown. In this study, using a video-tracking paradigm, we were able to describe a more precise parameter, the number of episodes of activity/inactivity (start/stop bouts), that accounts for the sexual dimorphism. This increased precision in the characterization of the subtle male/female difference in locomotor activity allowed us to identify with more acuity the origin of the dimorphism. Moreover, we demonstrated that this parameter (start/stop bouts) could be feminized.
Our transplantation experiments provided clear evidence that the sexually dimorphic organization of walking pattern in Drosophila is under the control of a few neurons of the PI, and consequently imply that those neurons act via a humoral factor to transmit their effect. Additionally, as suggested by the feminization induced by the apparent depletion of JH after the ingestion of fluvastatin, which can be reversed by the application of a JH analog, methoprene, this effect is hormone dependent. This pharmacological approach strongly suggests that JH could act as a molecular link between the PI neurons and the locomotor center. Interestingly, it has been recently proposed that the Drosophila takeout gene, which encodes for a putative juvenile hormone-binding protein, may participate in conveying information between locomotor activity, survival, and food status (30). Therefore, a male/female difference in JH regulation, either quantitative and/or affecting the timing of release, might possibly underlie the sexual dimorphism in locomotor activity. Indeed, it is known that, in most insects, variation in the rate of JH biosynthesis is the main factor influencing JH titer and, consequently, the physiological response to this hormone (31).
Moreover, the molecular nature of the neuroendocrine products expressed by the PI neurons controlling JH synthesis/release by the cc-ca gland complex remains to be determined. It is possible that allatotropins and allatostatins are involved: these neuropeptides are secreted by certain brain cells and up- or down-regulate JH synthesis, respectively (22). The recent identification of Drosophila allatostatin genes (32–34) will make it possible to study this hypothesis. Recent studies have also reported that a mutation in the Drosophila insulin receptor (InR) gene extends lifespan and is due to a JH deficiency (29). A mutation in the chico gene, which codes for an insulin receptor substrate protein, also extends lifespan (35). Interestingly, in situ hybridization has shown that the InR is expressed in the cerebral cortex (36), whereas immunocytochemical studies have revealed that an insulin-like peptide is expressed in medial neurosecretory cells of the adult brain (37). These neurosecretory cells may correspond to the PI neurons controlling sexually dimorphic locomotor behavior. Although JH was discovered >60 years ago, its molecular action remains poorly understood, partly because its receptor has yet to be unambiguously identified (21, 22). JH plays essential roles in insects, including metamorphosis, caste differentiation, diapause, regulation of reproduction, and behavior (21, 22). It also stimulates neuroblast proliferation in the mushroom-bodies of the adult cricket (38). The small cluster of PI neurons controlling the sexually dimorphic locomotor behavior in Drosophila could therefore represent a new model of neural sexual dimorphism, like, for instance, the higher vocal center in songbirds or the preoptic area in rats (2).
Acknowledgments
We thank L. Duportets and F. Couillaud for kindly providing us with fluvastatin, and M. Cobb, J.-F. Ferveur, and H. Courvoisier for critical reading of the manuscript. J.-R.M. was supported by the Centre National de la Recherche Scientifique.
Abbreviations
UAS-tra, UAS-transformer transgene
PI, pars intercerebralis
JH, juvenile hormone
CS, Canton-S
oc1, ocelliless
cc, corpora cardiaca
ca, corpora allata
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Short R. V. & Balaban, E., (1994) The Differences Between the Sexes (Cambridge Univ. Press, Cambridge, U.K.).
- 2.Breedlove S. M. (1992) J. Neurosci. 12, 4133-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelley D. B. (1988) Annu. Rev. Neurosci. 11, 225-251. [DOI] [PubMed] [Google Scholar]
- 4.De Vries G. J. & Boyle, P. A. (1998) Behav. Brain Res. 92, 205-213. [DOI] [PubMed] [Google Scholar]
- 5.Cooke B. M., Tabibnia, G. & Breedlove, S. M. (1999) Proc. Natl. Acad. Sci. USA 96, 7538-7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodson J. L. & Baas, A. H. (2000) Nature 403, 769-772. [DOI] [PubMed] [Google Scholar]
- 7.Martin J.-R., Ernst, R. & Heisenberg, M. (1999) J. Comp. Physiol. A 184, 73-84. [DOI] [PubMed] [Google Scholar]
- 8.Gatti S., Ferveur, J.-F. & Martin, J.-R. (2000) Curr. Biol. 10, 667-670. [DOI] [PubMed] [Google Scholar]
- 9.Ferveur J.-F., Störtkuhl, K. F., Stocker, R. F. & Greenspan, R. J. (1995) Science 267, 902-905. [DOI] [PubMed] [Google Scholar]
- 10.Baker B. S. & Ridge, K. A. (1980) Genetics 94, 383-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKeown M., Belote, J. M. & Baker, B. S. (1987) Cell 48, 489-499. [DOI] [PubMed] [Google Scholar]
- 12.McKeown M., Belote, J. M. & Boggs, R. T. (1988) Cell 53, 887-895. [DOI] [PubMed] [Google Scholar]
- 13.Hirth F., Therianos, S., Loop, T., Gehring, W. J., Reichert, H. & Furukubo-Tokunaga, K. (1995) Neuron 15, 769-778. [DOI] [PubMed] [Google Scholar]
- 14.Yang M. Y., Armstrong, J. D., Vilinsky, I., Strausfeld, N. J. & Kaiser, K. (1995) Neuron 15, 45-54. [DOI] [PubMed] [Google Scholar]
- 15.Connolly J. B., Roberts, I. J. H., Armstrong, J. D., Kaiser, K., Forte, M., Tully, T. & O'Kane, C. J. (1996) Science 274, 2104-2107. [DOI] [PubMed] [Google Scholar]
- 16.Renn S. C., Armstrong, J. D., Yang, M., Wang, Z., An, X., Kaiser, K. & Taghert, P. H. (1999) J. Neurobiol. 41, 189-207. [PubMed] [Google Scholar]
- 17.Martin J.-R., Faure, F. & Ernst, R. (2001) J. Neurogenet. 15, 205-219. [DOI] [PubMed] [Google Scholar]
- 18.Handler A. M. & Konopka, R. J. (1979) Nature 279, 236-238. [DOI] [PubMed] [Google Scholar]
- 19.Köpf H. (1957) Biol. Zentralbl. 76, 28-42. [Google Scholar]
- 20.Orchard I. & Loughton, B. G. (1984) in Comprehensive Insect Physiology, Biochemistry and Pharmacology, eds. Kerkut, G. A. & Gilbert, L. I. (Pergamon, Oxford), Vol. 7, pp. 61–107. [Google Scholar]
- 21.Wyatt G. R. & Davey, K. G. (1996) Adv. Insect Physiol. 26, 1-155. [Google Scholar]
- 22.Gäde G., Hoffmann, K.-H. & Spring, J. H. (1997) Physiol. Rev. 77, 963-1032. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto K., Chadarevian, A. & Pellegrini, M. (1988) Science 239, 916-919. [DOI] [PubMed] [Google Scholar]
- 24.Shemshedini L., Lanoue, M. & Wilson, T. G. (1990) J. Biol. Chem. 265, 1913-1918. [PubMed] [Google Scholar]
- 25.Debernard S., Rossignol, F. & Couillaud, F. (1994) Gen. Comp. Endocrinol. 95, 92-98. [DOI] [PubMed] [Google Scholar]
- 26.Duportets L., Dufour, M.-C., Bécard, J.-M., Gadenne, C. & Couillaud, F. (1996) Arch. Insect Biochem. Physiol. 32, 601-611. [Google Scholar]
- 27.Martinez-Gonzalez J., Buesa, C., Piulachs, M. D., Belles, X. & Hegardt, F. G. (1993) Eur. J. Biochem. 213, 233-241. [DOI] [PubMed] [Google Scholar]
- 28.Handler A. & Postlethwait, J. H. (1977) J. Exp. Zool. 202, 389-402. [DOI] [PubMed] [Google Scholar]
- 29.Tatar M., Kopelman, A., Epstein, D., Tu, M.-P., Yin, C.-M. & Garofalo, R. S. (2001) Science 292, 107-110. [DOI] [PubMed] [Google Scholar]
- 30.Sarov-Blat L., So, W. V., Liu, L. & Rosbash, M. (2000) Cell 101, 647-656. [DOI] [PubMed] [Google Scholar]
- 31.Tobe S. S. & Feyereisen, R. (1983) in Endocrinology of Insects, eds. Downer, R. & Laufer, H. (Liss, New York), pp. 161–178.
- 32.Lenz C., Williamson, M. & Grimmelikhuijzen, C. J. P. (2000) Biochem. Biophys. Res. Commun. 273, 1126-1131. [DOI] [PubMed] [Google Scholar]
- 33.Williamson M., Lenz, C., Winther, M. E., Nässel, D. R. & Grimmelikhuijzen, C. J. P. (2001) Biochem. Biophys. Res. Commun. 281, 544-550. [DOI] [PubMed] [Google Scholar]
- 34.Williamson M., Lenz, C., Winther, A. M. E., Nässel, D. R. & Grimmelikhuijzen, C. J. P. (2001) Biochem. Biophys. Res. Commun. 282, 124-130. [DOI] [PubMed] [Google Scholar]
- 35.Clancy D. J., Gems, D., Harshmann, L. G., Oldham, S., Stocker, H., Hafen, E., Leevers, S. J. & Partridge, L. (2001) Science 292, 104-106. [DOI] [PubMed] [Google Scholar]
- 36.Garofalo R. S. & Rosen, O. M. (1988) Mol. Cell. Biol. 8, 1638-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao C. & Brown, M. R. (2001) Cell Tissue Res. 304, 317-321. [DOI] [PubMed] [Google Scholar]
- 38.Cayre M., Strambi, C. & Strambi, A. (1994) Nature 368, 57-59. [Google Scholar]