Abstract
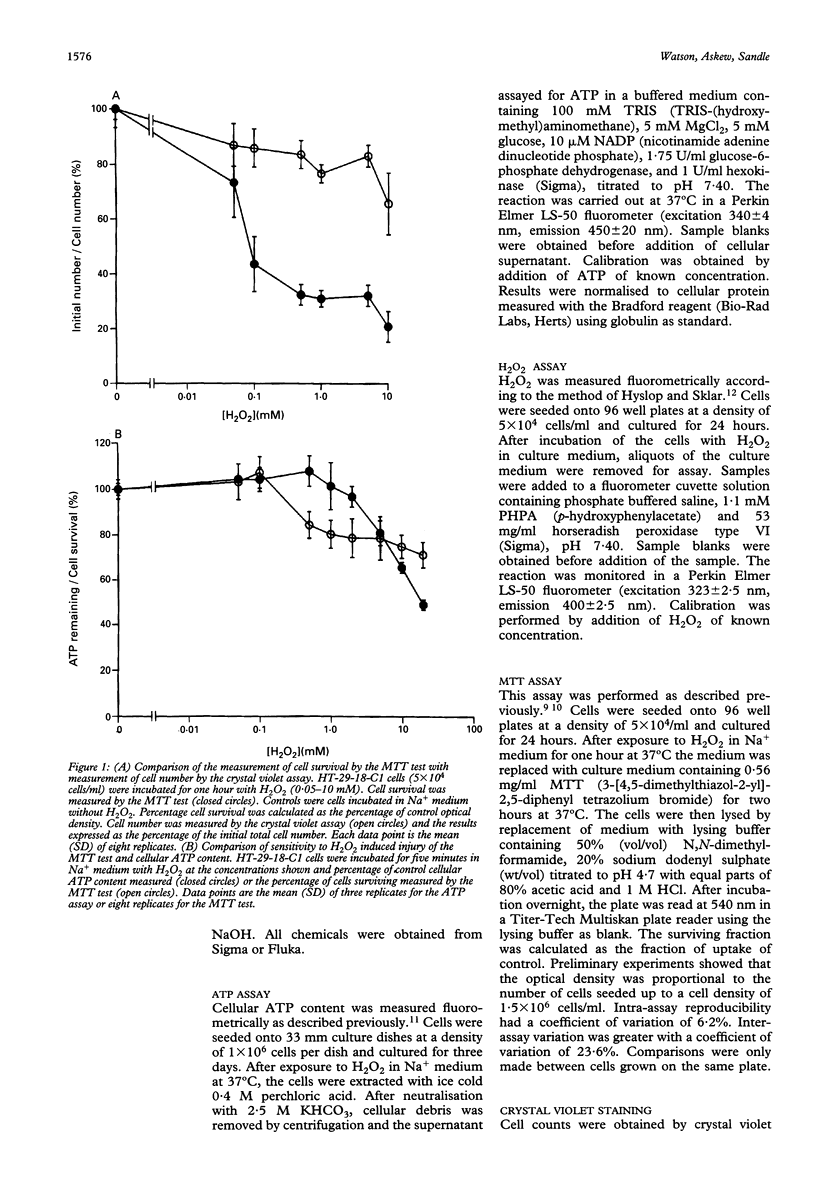
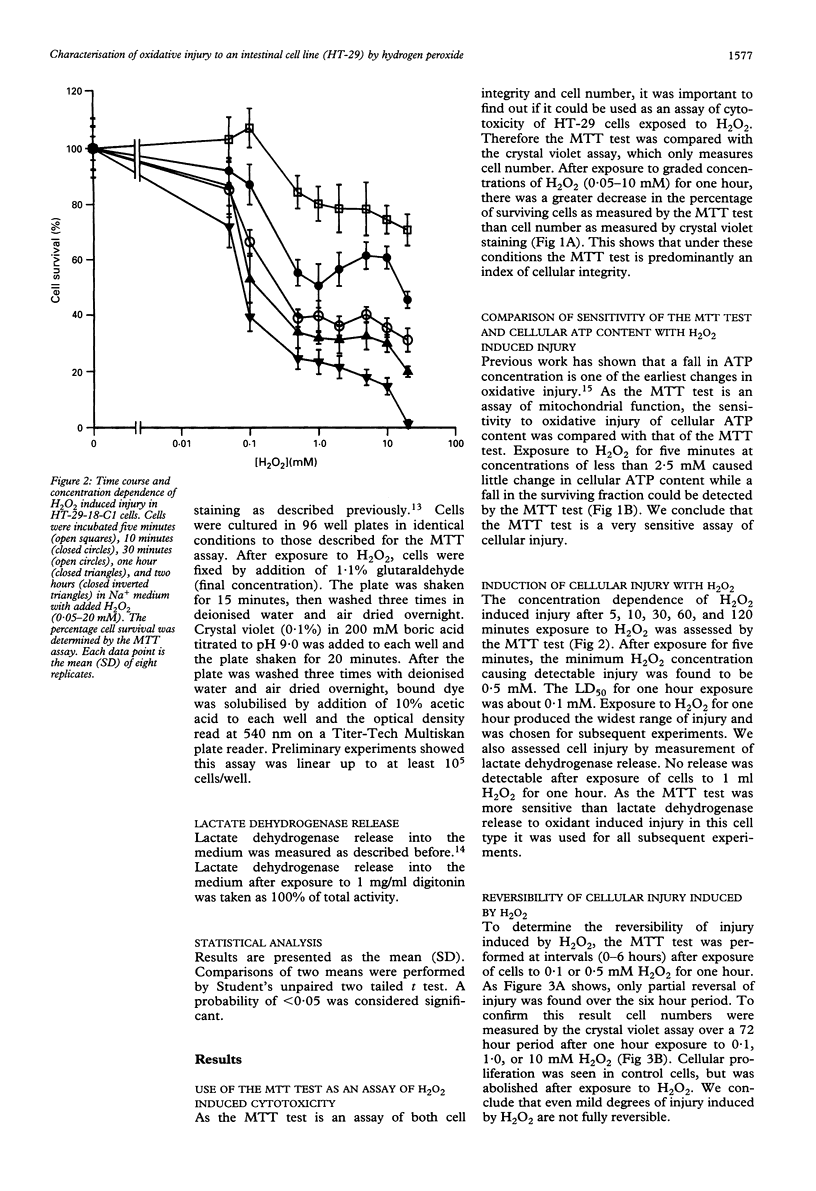
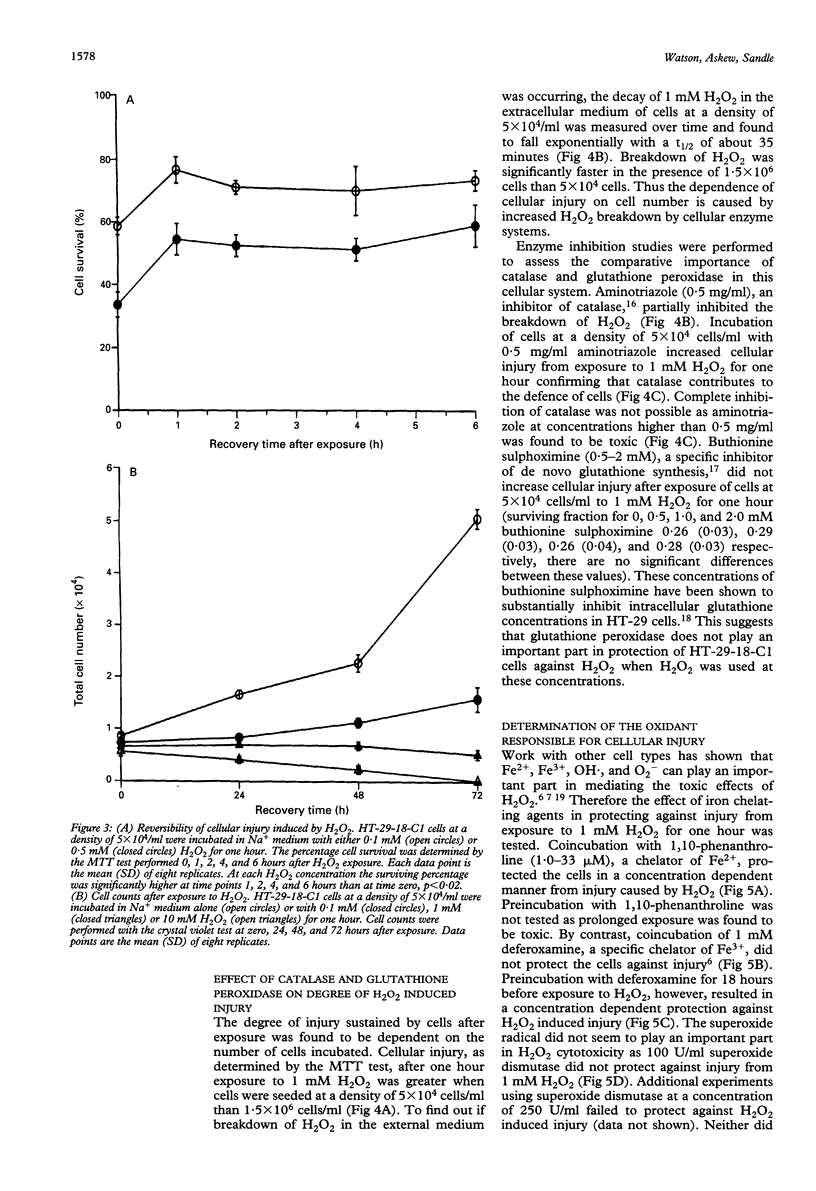
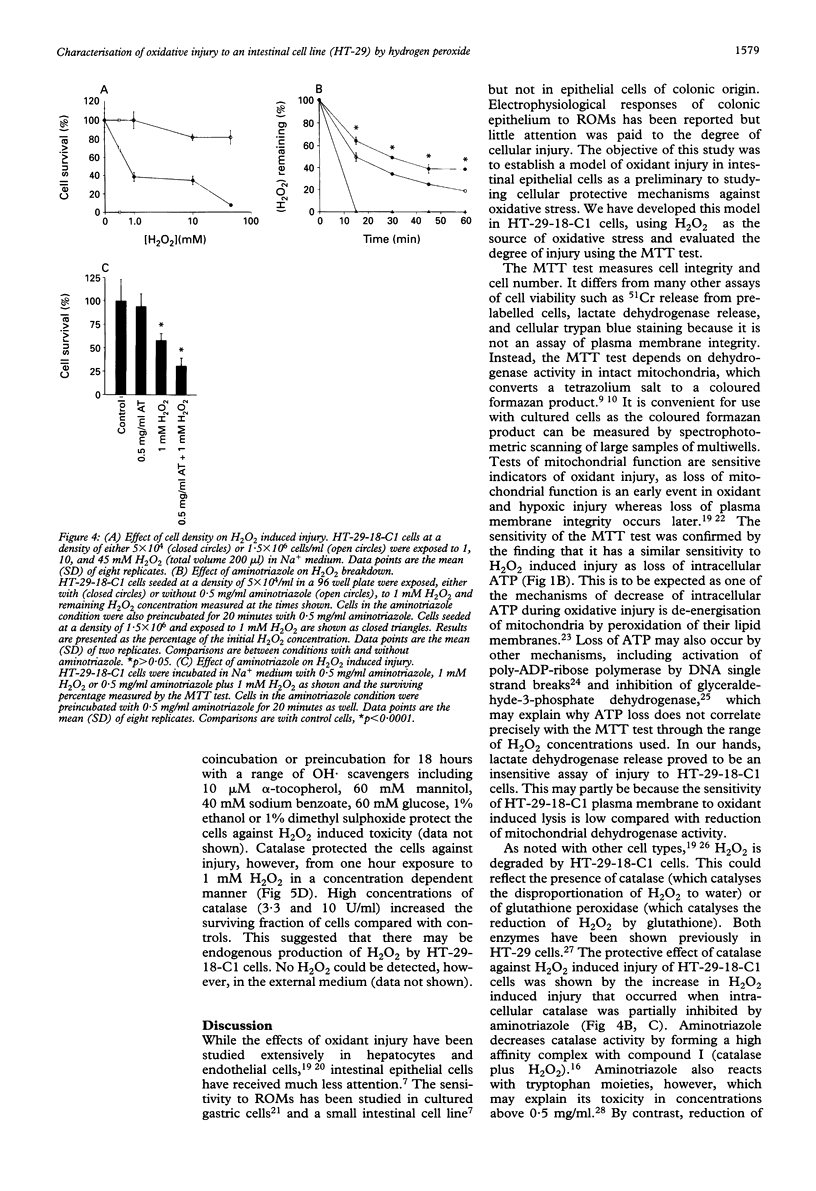
Reactive oxygen metabolites have been implicated in causing epithelial cell injury in colonic inflammation. A model of oxidant injury in intestinal epithelial cells has been developed in which HT-29-18-C1 cells are injured with graded concentrations of hydrogen peroxide and characterised by the MTT test. The MTT test was validated as a cytotoxicity assay and has a similar sensitivity to hydrogen peroxide induced injury as the assay of intracellular adenosine triphosphate. Exposure to a range of hydrogen peroxide concentrations (0.05-20 mM) for varying duration (5-120 min) showed that injury was dependent on time and concentration. The median lethal dose (LD50) for one hour exposure to hydrogen peroxide was approximately 0.1 mM. Injury from hydrogen peroxide was only partially reversible as determined by the MTT test and assay of cellular proliferation by crystal violet staining. There was an exponential loss of hydrogen peroxide when incubated with HT-29-18-C1 cells (t1/2 35 min). Experiments with 0.5 mg/ml aminotriazole and 0.5-2 mM buthionine sulphoximine suggested hydrogen peroxide breakdown was predominantly caused by catalase rather than glutathione peroxidase. Injury resulting from 1 mM hydrogen peroxide could be reduced by either coincubation of cells with 1,10-phenanthroline, an Fe2+ chelator, or preincubation with deferoxamine, and Fe3+ chelator, suggesting the participation of Fe2+ and Fe3+ in hydrogen peroxide induced injury. In conclusion, hydrogen peroxide induces injury in HT-29-18-C1 cells both directly and by generation of the hydroxyl radical.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Craven P. A., Pfanstiel J., Saito R., DeRubertis F. R. Actions of sulfasalazine and 5-aminosalicylic acid as reactive oxygen scavengers in the suppression of bile acid-induced increases in colonic epithelial cell loss and proliferative activity. Gastroenterology. 1987 Jun;92(6):1998–2008. doi: 10.1016/0016-5085(87)90635-4. [DOI] [PubMed] [Google Scholar]
- DeTraglia M. C., Brand J. S., Tometsko A. M. The reaction of 3-diazonium-1,2,4-triazole with tryptophan at acid pH: a scintillation method for tryptophan determination in proteins. Anal Biochem. 1979 Nov 1;99(2):464–473. doi: 10.1016/s0003-2697(79)80034-2. [DOI] [PubMed] [Google Scholar]
- Griffith O. W. Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J Biol Chem. 1982 Nov 25;257(22):13704–13712. [PubMed] [Google Scholar]
- Grisham M. B., Gaginella T. S., von Ritter C., Tamai H., Be R. M., Granger D. N. Effects of neutrophil-derived oxidants on intestinal permeability, electrolyte transport, and epithelial cell viability. Inflammation. 1990 Oct;14(5):531–542. doi: 10.1007/BF00914274. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Protection against tissue damage in vivo by desferrioxamine: what is its mechanism of action? Free Radic Biol Med. 1989;7(6):645–651. doi: 10.1016/0891-5849(89)90145-7. [DOI] [PubMed] [Google Scholar]
- Hansen M. B., Nielsen S. E., Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989 May 12;119(2):203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- Hiraishi H., Terano A., Razandi M., Sugimoto T., Harada T., Ivey K. J. Role of iron and superoxide in mediating hydrogen peroxide injury to cultured rat gastric cells. Gastroenterology. 1993 Mar;104(3):780–788. doi: 10.1016/0016-5085(93)91013-8. [DOI] [PubMed] [Google Scholar]
- Holmsen H., Robkin L. Hydrogen peroxide lowers ATP levels in platelets without altering adenyalte energy charge and platelet function. J Biol Chem. 1977 Mar 10;252(5):1752–1757. [PubMed] [Google Scholar]
- Huet C., Sahuquillo-Merino C., Coudrier E., Louvard D. Absorptive and mucus-secreting subclones isolated from a multipotent intestinal cell line (HT-29) provide new models for cell polarity and terminal differentiation. J Cell Biol. 1987 Jul;105(1):345–357. doi: 10.1083/jcb.105.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyslop P. A., Hinshaw D. B., Halsey W. A., Jr, Schraufstätter I. U., Sauerheber R. D., Spragg R. G., Jackson J. H., Cochrane C. G. Mechanisms of oxidant-mediated cell injury. The glycolytic and mitochondrial pathways of ADP phosphorylation are major intracellular targets inactivated by hydrogen peroxide. J Biol Chem. 1988 Feb 5;263(4):1665–1675. [PubMed] [Google Scholar]
- Hyslop P. A., Sklar L. A. A quantitative fluorimetric assay for the determination of oxidant production by polymorphonuclear leukocytes: its use in the simultaneous fluorimetric assay of cellular activation processes. Anal Biochem. 1984 Aug 15;141(1):280–286. doi: 10.1016/0003-2697(84)90457-3. [DOI] [PubMed] [Google Scholar]
- Kueng W., Silber E., Eppenberger U. Quantification of cells cultured on 96-well plates. Anal Biochem. 1989 Oct;182(1):16–19. doi: 10.1016/0003-2697(89)90710-0. [DOI] [PubMed] [Google Scholar]
- Kvietys P. R., Inauen W., Bacon B. R., Grisham M. B. Xanthine oxidase-induced injury to endothelium: role of intracellular iron and hydroxyl radical. Am J Physiol. 1989 Nov;257(5 Pt 2):H1640–H1646. doi: 10.1152/ajpheart.1989.257.5.H1640. [DOI] [PubMed] [Google Scholar]
- Kyle M. E., Nakae D., Sakaida I., Miccadei S., Farber J. L. Endocytosis of superoxide dismutase is required in order for the enzyme to protect hepatocytes from the cytotoxicity of hydrogen peroxide. J Biol Chem. 1988 Mar 15;263(8):3784–3789. [PubMed] [Google Scholar]
- Lemasters J. J., DiGuiseppi J., Nieminen A. L., Herman B. Blebbing, free Ca2+ and mitochondrial membrane potential preceding cell death in hepatocytes. Nature. 1987 Jan 1;325(6099):78–81. doi: 10.1038/325078a0. [DOI] [PubMed] [Google Scholar]
- Ma T. Y., Hollander D., Freeman D., Nguyen T., Krugliak P. Oxygen free radical injury of IEC-18 small intestinal epithelial cell monolayers. Gastroenterology. 1991 Jun;100(6):1533–1543. doi: 10.1016/0016-5085(91)90650-a. [DOI] [PubMed] [Google Scholar]
- Masaki N., Kyle M. E., Serroni A., Farber J. L. Mitochondrial damage as a mechanism of cell injury in the killing of cultured hepatocytes by tert-butyl hydroperoxide. Arch Biochem Biophys. 1989 May 1;270(2):672–680. doi: 10.1016/0003-9861(89)90550-x. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nunez M. T., Cole E. S., Glass J. The reticulocyte plasma membrane pathway of iron uptake as determined by the mechanism of alpha, alpha'-dipyridyl inhibition. J Biol Chem. 1983 Jan 25;258(2):1146–1151. [PubMed] [Google Scholar]
- Repine J. E., Fox R. B., Berger E. M. Hydrogen peroxide kills Staphylococcus aureus by reacting with staphylococcal iron to form hydroxyl radical. J Biol Chem. 1981 Jul 25;256(14):7094–7096. [PubMed] [Google Scholar]
- Sandström B. E., Marklund S. L. Effects of variation in glutathione peroxidase activity on DNA damage and cell survival in human cells exposed to hydrogen peroxide and t-butyl hydroperoxide. Biochem J. 1990 Oct 1;271(1):17–23. doi: 10.1042/bj2710017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstatter I. U., Hinshaw D. B., Hyslop P. A., Spragg R. G., Cochrane C. G. Oxidant injury of cells. DNA strand-breaks activate polyadenosine diphosphate-ribose polymerase and lead to depletion of nicotinamide adenine dinucleotide. J Clin Invest. 1986 Apr;77(4):1312–1320. doi: 10.1172/JCI112436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds N. J., Allen R. E., Stevens T. R., Van Someren R. N., Blake D. R., Rampton D. S. Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology. 1992 Jul;103(1):186–196. doi: 10.1016/0016-5085(92)91112-h. [DOI] [PubMed] [Google Scholar]
- Spragg R. G., Hinshaw D. B., Hyslop P. A., Schraufstätter I. U., Cochrane C. G. Alterations in adenosine triphosphate and energy charge in cultured endothelial and P388D1 cells after oxidant injury. J Clin Invest. 1985 Oct;76(4):1471–1476. doi: 10.1172/JCI112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke P. E., Farber J. L. Ferric iron and superoxide ions are required for the killing of cultured hepatocytes by hydrogen peroxide. Evidence for the participation of hydroxyl radicals formed by an iron-catalyzed Haber-Weiss reaction. J Biol Chem. 1985 Aug 25;260(18):10099–10104. [PubMed] [Google Scholar]
- Sutton H. C., Winterbourn C. C. On the participation of higher oxidation states of iron and copper in Fenton reactions. Free Radic Biol Med. 1989;6(1):53–60. doi: 10.1016/0891-5849(89)90160-3. [DOI] [PubMed] [Google Scholar]
- Tamai H., Kachur J. F., Grisham M. B., Gaginella T. S. Scavenging effect of 5-aminosalicylic acid on neutrophil-derived oxidants. Possible contribution to the mechanism of action in inflammatory bowel disease. 1991 Mar 15-Apr 1Biochem Pharmacol. 41(6-7):1001–1006. doi: 10.1016/0006-2952(91)90207-l. [DOI] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]



