Abstract
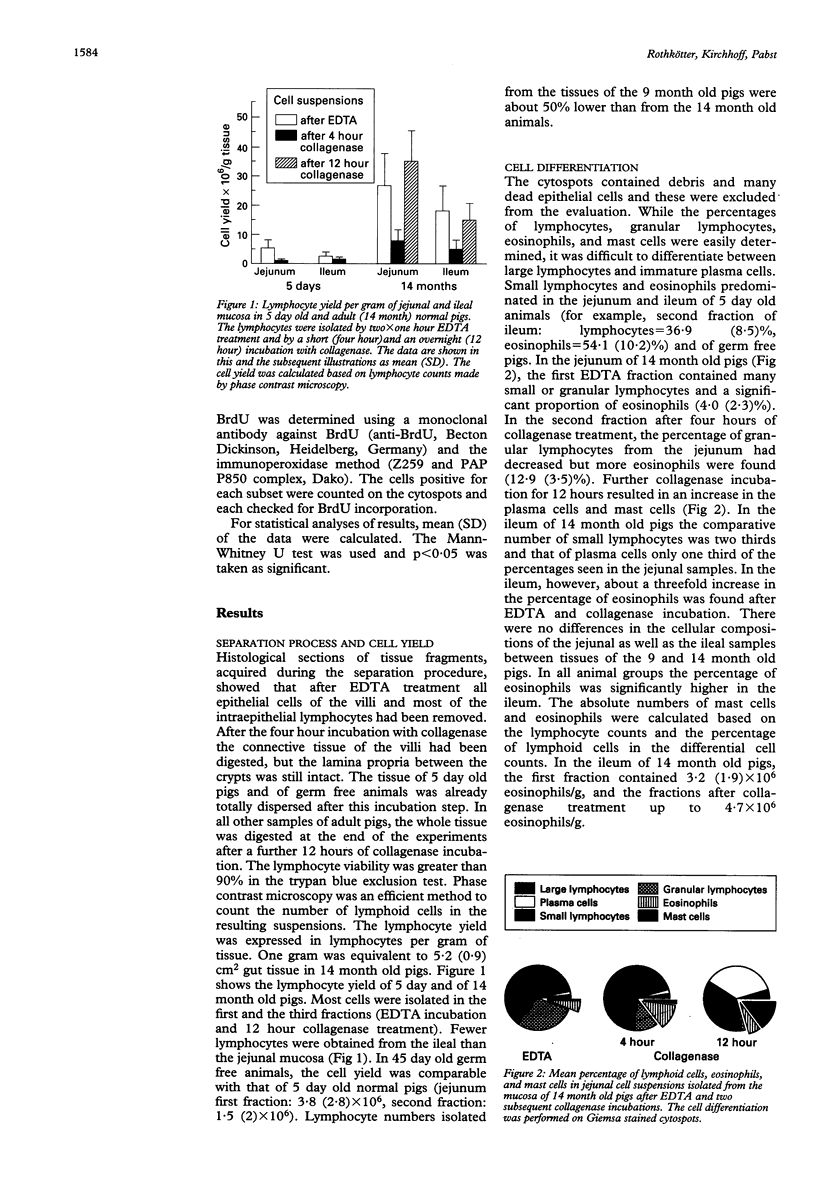
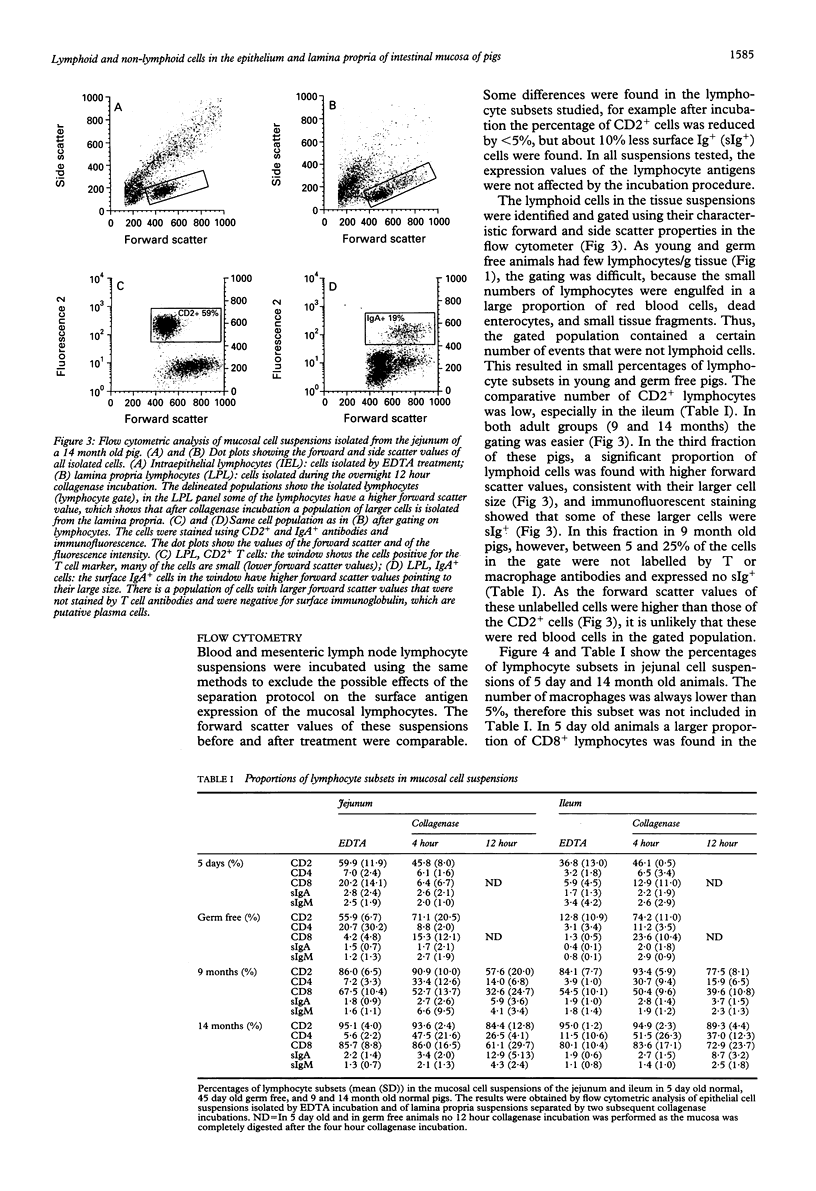
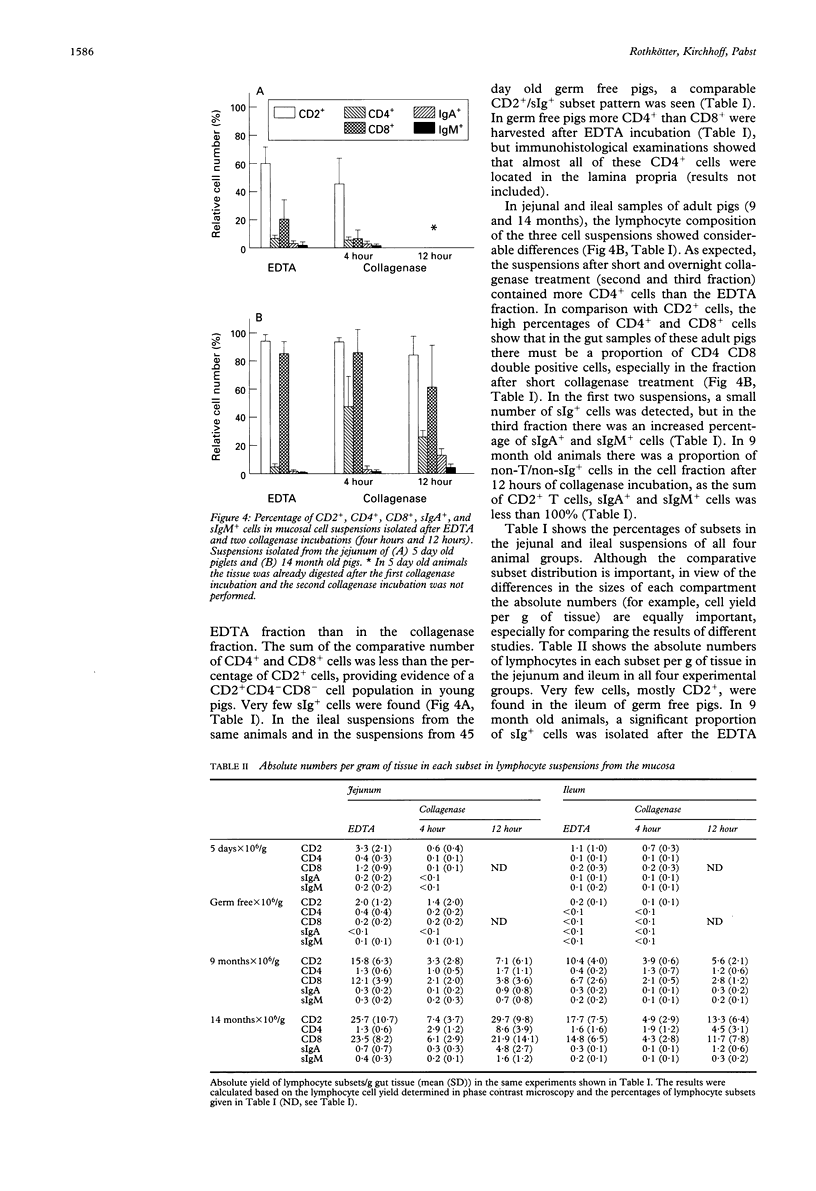
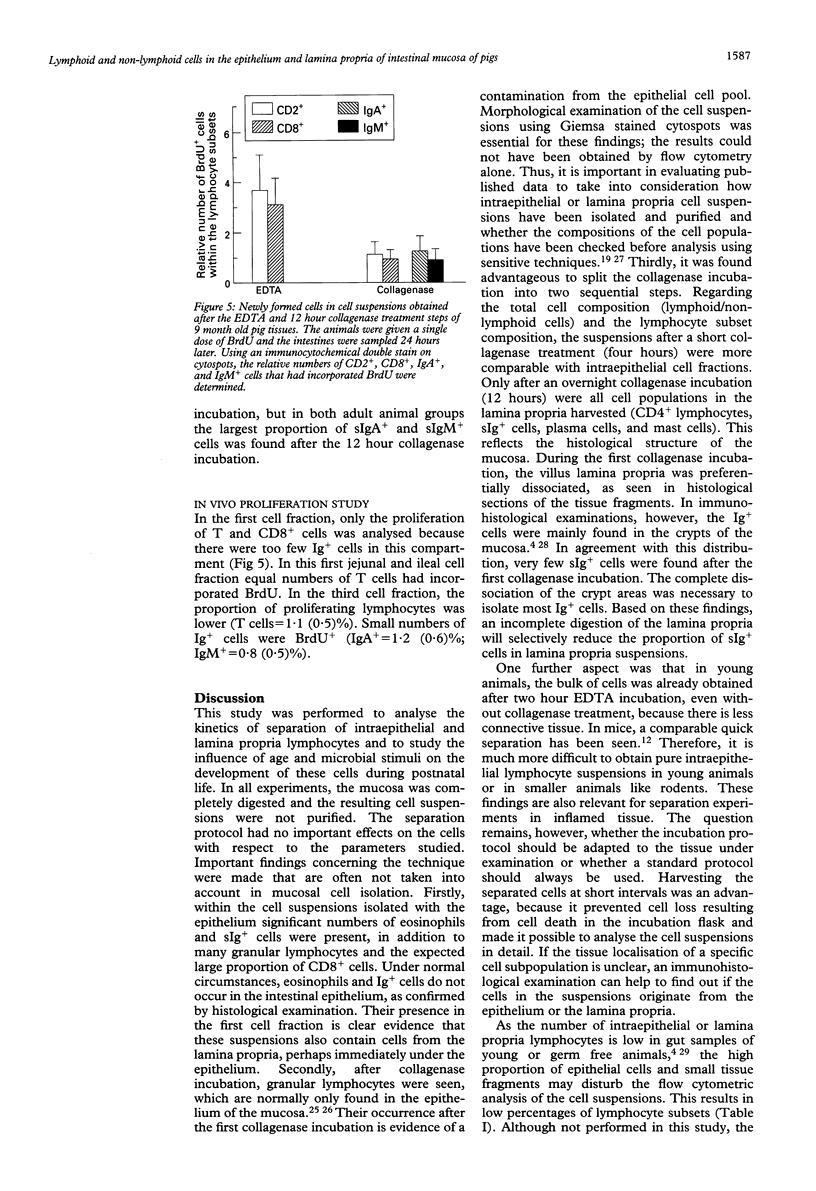
The jejunum and ileum of 5 day old and adult normal pigs and of 45 day old germ free pigs were used to study the lymphocyte pools in the epithelium and lamina propria by sequential treatments with EDTA, four hours, and 12 hours of collagenase treatment. In adult animals the incubation of the jejunal wall with EDTA resulted in mean (SD) 26.8 (10.9) x 10(6) intraepithelial lymphocytes per g of tissue. The ileal wall gave lower cell yields. After complete digestion of the lamina propria by collagenase a further yield of 35.2 (10.2) x 10(6)/g lymphocytes was achieved. The separation of the gut wall from 5 day old pigs resulted in a 10-fold lower total lymphocyte yield, and the tissue was totally digested after four hours of collagenase treatment. Many eosinophils and mast cells were found in the suspensions from adult animal tissues after the collagenase treatment; 4.7 x 10(6)/g and 4.8 x 10(6)/g, respectively. The suspensions after 12 hour collagenase incubation contained up to 30% plasma cells. Almost all cells isolated by EDTA incubation were CD8+ T cells. After collagenase incubation CD4+ and CD8+ T lymphocytes were found in all animal groups, and in adult animals up to 20% surface Ig+ cells were harvested. When the incorporation of the thymidine analogue bromodesoxyuridine was used to study the lymphocyte production in vivo 3 to 7% lymphocytes in the epithelium were labelled 24 hours later (lamina propria T lymphocytes about 1%). In this study lymphoid as well as non-lymphoid cells have been analysed in mucosal cell suspensions. The absolute cell yield per gram of mucosal tissue is a basis to estimate the pool sizes of intraepithelial and lamina propria lymphocytes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baklien K., Brandtzaeg P. Immunohistochemical characterization of local immunoglobulin formation in Crohn's disease of the ileum. Scand J Gastroenterol. 1976;11(5):447–457. [PubMed] [Google Scholar]
- Bookman M. A., Bull D. M. Characteristics of isolated intestinal mucosal lymphoid cells in inflammatory bowel disease. Gastroenterology. 1979 Sep;77(3):503–510. [PubMed] [Google Scholar]
- Brandtzaeg P., Halstensen T. S., Kett K., Krajci P., Kvale D., Rognum T. O., Scott H., Sollid L. M. Immunobiology and immunopathology of human gut mucosa: humoral immunity and intraepithelial lymphocytes. Gastroenterology. 1989 Dec;97(6):1562–1584. doi: 10.1016/0016-5085(89)90406-x. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Overview of the mucosal immune system. Curr Top Microbiol Immunol. 1989;146:13–25. doi: 10.1007/978-3-642-74529-4_2. [DOI] [PubMed] [Google Scholar]
- Brown S. L., Morrison S. L. Regulation of the production of secretory and membrane immunoglobulin during lymphocyte development. Clin Immunol Immunopathol. 1989 Feb;50(2):155–170. doi: 10.1016/0090-1229(89)90125-6. [DOI] [PubMed] [Google Scholar]
- Bull D. M., Bookman M. A. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977 May;59(5):966–974. doi: 10.1172/JCI108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Guy-Grand D., Griscelli C. Intraepithelial lymphocytes of human gut: isolation, characterisation and study of natural killer activity. Gut. 1985 Jan;26(1):81–88. doi: 10.1136/gut.26.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Guy-Grand D. Intestinal intraepithelial lymphocytes. Gastroenterol Clin North Am. 1991 Sep;20(3):549–576. [PubMed] [Google Scholar]
- Dziaba K. A., Lambrecht G., Petzoldt K. Intestinal and serum antibody response in gnotobiotic piglets to oral immunization with Escherichia coli. Comp Immunol Microbiol Infect Dis. 1985;8(3-4):267–272. doi: 10.1016/0147-9571(85)90005-0. [DOI] [PubMed] [Google Scholar]
- Fritz F. J., Pabst R., Binns R. M. Lymphocyte subsets and their proliferation in a model for a delayed-type hypersensitivity reaction in the skin. Immunology. 1990 Dec;71(4):508–516. [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The mouse gut T lymphocyte, a novel type of T cell. Nature, origin, and traffic in mice in normal and graft-versus-host conditions. J Exp Med. 1978 Dec 1;148(6):1661–1677. doi: 10.1084/jem.148.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D., Vanden Broecke C., Briottet C., Malassis-Seris M., Selz F., Vassalli P. Different expression of the recombination activity gene RAG-1 in various populations of thymocytes, peripheral T cells and gut thymus-independent intraepithelial lymphocytes suggests two pathways of T cell receptor rearrangement. Eur J Immunol. 1992 Feb;22(2):505–510. doi: 10.1002/eji.1830220232. [DOI] [PubMed] [Google Scholar]
- Halstensen T. S., Brandtzaeg P. Activated T lymphocytes in the celiac lesion: non-proliferative activation (CD25) of CD4+ alpha/beta cells in the lamina propria but proliferation (Ki-67) of alpha/beta and gamma/delta cells in the epithelium. Eur J Immunol. 1993 Feb;23(2):505–510. doi: 10.1002/eji.1830230231. [DOI] [PubMed] [Google Scholar]
- Harvey J., Jones D. B. Human mucosal T-lymphocyte and macrophage subpopulations in normal and inflamed intestine. Clin Exp Allergy. 1991 Sep;21(5):549–560. doi: 10.1111/j.1365-2222.1991.tb00846.x. [DOI] [PubMed] [Google Scholar]
- James S. P., Graeff A. S. Spontaneous and lymphokine-induced cytotoxic activity of monkey intestinal mucosal lymphocytes. Cell Immunol. 1985 Jul;93(2):387–397. doi: 10.1016/0008-8749(85)90143-1. [DOI] [PubMed] [Google Scholar]
- James S. P. Mucosal T-cell function. Gastroenterol Clin North Am. 1991 Sep;20(3):597–612. [PubMed] [Google Scholar]
- Lunney J. K. Characterization of swine leukocyte differentiation antigens. Immunol Today. 1993 Apr;14(4):147–148. doi: 10.1016/0167-5699(93)90227-C. [DOI] [PubMed] [Google Scholar]
- Marsh M. N., Leigh R. J., Loft D. E., Garner G. V., Gordon D. B. Studies of intestinal lymphoid tissue. X-observations on granular epithelial lymphocytes (gEL) in normal and diseased human jejunum. Virchows Arch A Pathol Anat Histopathol. 1988;412(4):365–370. doi: 10.1007/BF00750263. [DOI] [PubMed] [Google Scholar]
- Mosley R. L., Klein J. R. A rapid method for isolating murine intestine intraepithelial lymphocytes with high yield and purity. J Immunol Methods. 1992 Nov 25;156(1):19–26. doi: 10.1016/0022-1759(92)90006-f. [DOI] [PubMed] [Google Scholar]
- Pabst R., Beil W. Mast cell heterogeneity in the small intestine of normal, gnotobiotic and parasitized pigs. Int Arch Allergy Appl Immunol. 1989;88(3):363–366. doi: 10.1159/000234827. [DOI] [PubMed] [Google Scholar]
- Pabst R., Trepel F. Quantitative evaluation of the total number and distribution of lymphocytes in young pigs. Blut. 1975 Aug;31(2):77–86. doi: 10.1007/BF01633723. [DOI] [PubMed] [Google Scholar]
- Perkkiö M., Savilahti E. Time of appearance of immunoglobulin-containing cells in the mucosa of the neonatal intestine. Pediatr Res. 1980 Aug;14(8):953–955. doi: 10.1203/00006450-198008000-00012. [DOI] [PubMed] [Google Scholar]
- Reddehase M. J., Saalmüller A., Hirt W. Gamma/delta T-lymphocyte subsets in swine. Curr Top Microbiol Immunol. 1991;173:113–117. doi: 10.1007/978-3-642-76492-9_16. [DOI] [PubMed] [Google Scholar]
- Röpke C., Everett N. B. Kinetics of intraepithelial lymphocytes in the small intestine of thymus-deprived mice and antigen-deprived mice. Anat Rec. 1976 May;185(1):101–108. doi: 10.1002/ar.1091850110. [DOI] [PubMed] [Google Scholar]
- Rüthlein J., Heinze G., Auer I. O. Anti-CD2 and anti-CD3 induced T cell cytotoxicity of human intraepithelial and lamina propria lymphocytes. Gut. 1992 Dec;33(12):1626–1632. doi: 10.1136/gut.33.12.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmüller A., Reddehase M. J., Bühring H. J., Jonjić S., Koszinowski U. H. Simultaneous expression of CD4 and CD8 antigens by a substantial proportion of resting porcine T lymphocytes. Eur J Immunol. 1987 Sep;17(9):1297–1301. doi: 10.1002/eji.1830170912. [DOI] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Bofill M., Jewell D. P. Intestinal lymphocyte subpopulations in inflammatory bowel disease: an analysis by immunohistological and cell isolation techniques. Gut. 1984 Jan;25(1):32–40. doi: 10.1136/gut.25.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman R. J., Henning S. J., Nichols B. L. The miniature pig as an animal model for the study of intestinal enzyme development. Pediatr Res. 1988 Mar;23(3):311–315. doi: 10.1203/00006450-198803000-00016. [DOI] [PubMed] [Google Scholar]
- Spencer J., Isaacson P. G., Walker-Smith J. A., MacDonald T. T. Heterogeneity in intraepithelial lymphocyte subpopulations in fetal and postnatal human small intestine. J Pediatr Gastroenterol Nutr. 1989 Aug;9(2):173–177. doi: 10.1097/00005176-198908000-00007. [DOI] [PubMed] [Google Scholar]
- Takimoto H., Nakamura T., Takeuchi M., Sumi Y., Tanaka T., Nomoto K., Yoshikai Y. Age-associated increase in number of CD4+CD8+ intestinal intraepithelial lymphocytes in rats. Eur J Immunol. 1992 Jan;22(1):159–164. doi: 10.1002/eji.1830220124. [DOI] [PubMed] [Google Scholar]
- Van der Heijden P. J., Stok W. Improved procedure for the isolation of functionally active lymphoid cells from the murine intestine. J Immunol Methods. 1987 Nov 5;103(2):161–167. doi: 10.1016/0022-1759(87)90285-7. [DOI] [PubMed] [Google Scholar]
- Westermann J., Ronneberg S., Fritz F. J., Pabst R. Proliferation of lymphocyte subsets in the adult rat: a comparison of different lymphoid organs. Eur J Immunol. 1989 Jun;19(6):1087–1093. doi: 10.1002/eji.1830190619. [DOI] [PubMed] [Google Scholar]
- Wilson A. D., Stokes C. R., Bourne F. J. Morphology and functional characteristics of isolated porcine intraepithelial lymphocytes. Immunology. 1986 Sep;59(1):109–113. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Fujihashi K., Beagley K. W., McGhee J. R., Kiyono H. Cytokine synthesis by intestinal intraepithelial lymphocytes. Both gamma/delta T cell receptor-positive and alpha/beta T cell receptor-positive T cells in the G1 phase of cell cycle produce IFN-gamma and IL-5. J Immunol. 1993 Jan 1;150(1):106–114. [PubMed] [Google Scholar]
- Zeitz M., Greene W. C., Peffer N. J., James S. P. Lymphocytes isolated from the intestinal lamina propria of normal nonhuman primates have increased expression of genes associated with T-cell activation. Gastroenterology. 1988 Mar;94(3):647–655. doi: 10.1016/0016-5085(88)90235-1. [DOI] [PubMed] [Google Scholar]


