Abstract
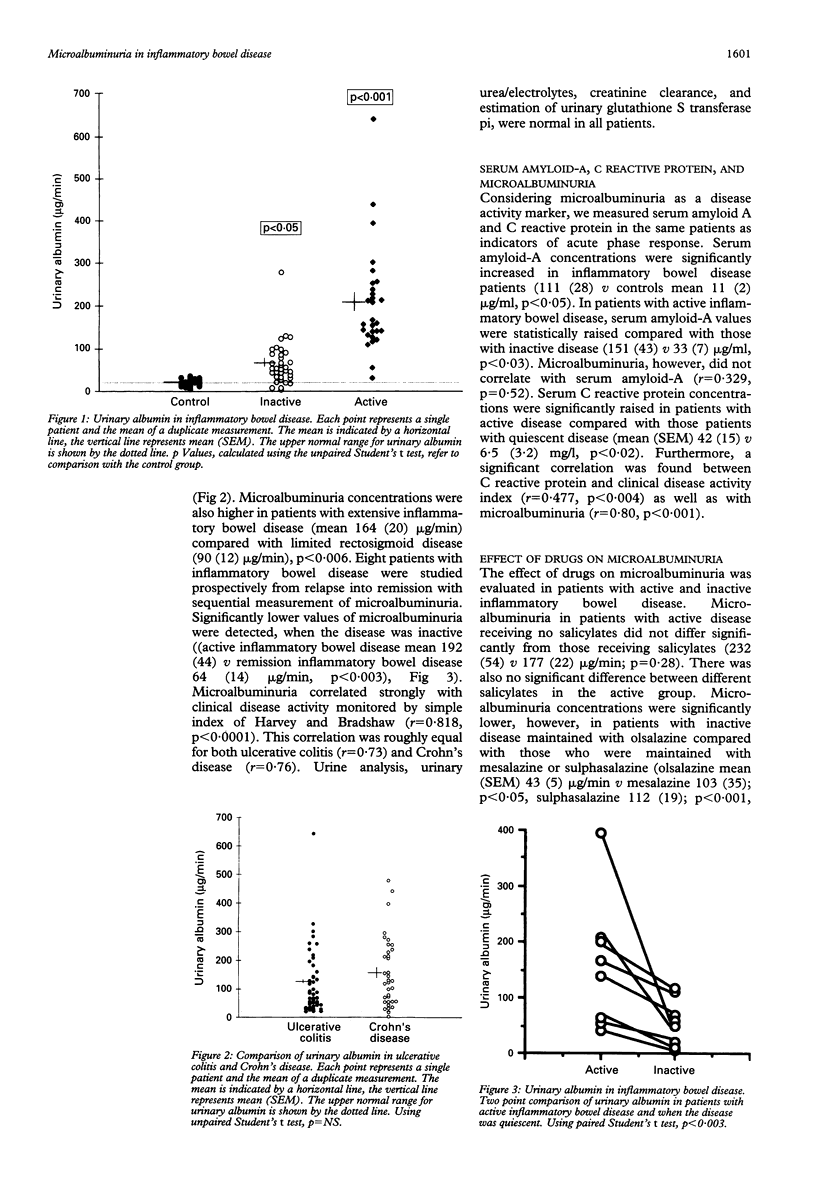
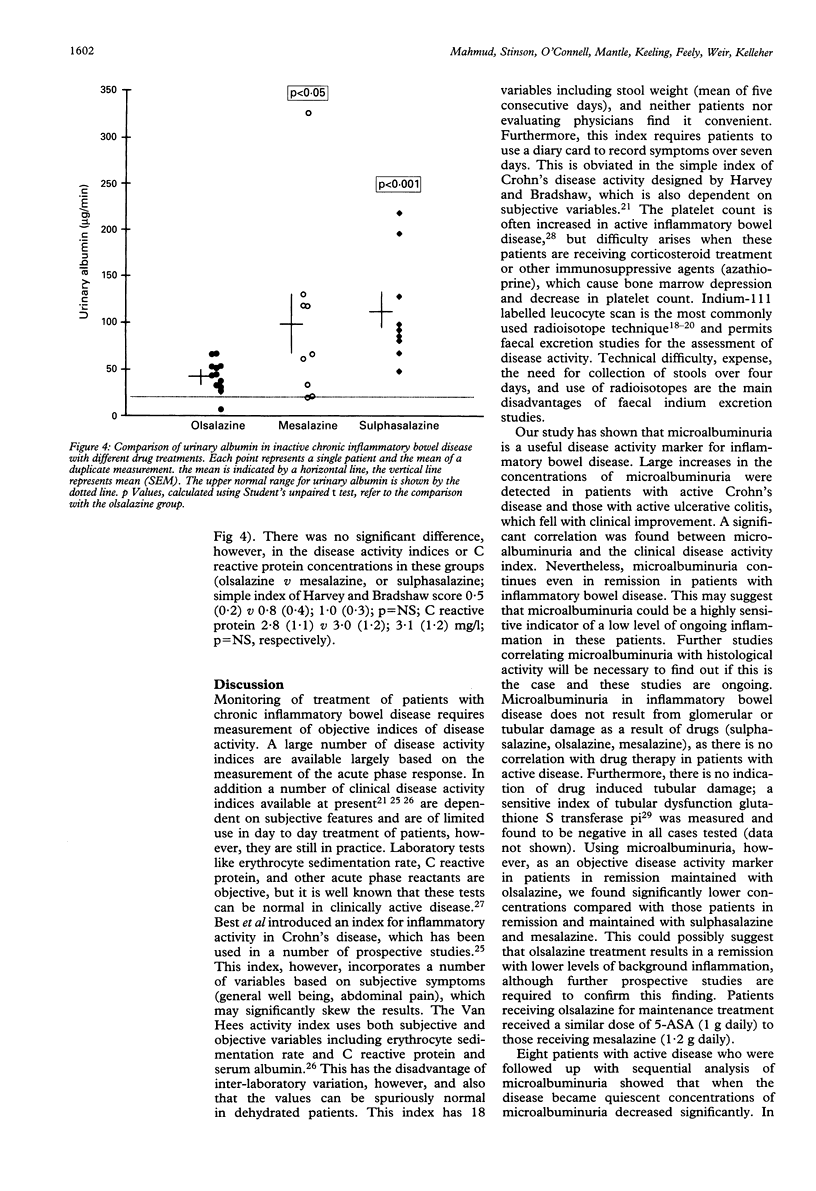
Microalbuminuria independently predicts the development of nephropathy and increased cardiovascular morbidity and mortality in diabetic patients, but it may be an indicator of the acute phase response. This study examined microalbuminuria as a marker of the acute phase response in patients with inflammatory bowel disease and correlated it with the disease activity in 95 patients with inflammatory bowel disease (ulcerative colitis (n = 52), Crohn's disease (n = 43)) determined by the simple index of Harvey and Bradshaw. Fifty patients were in complete clinical remission and 45 patients had active disease. Microalbuminuria was detected in all patients with inflammatory bowel disease (147 (17) v 18 (2) microgram/min, inflammatory bowel disease v controls mean (SEM), p < 0.007). Patients with active inflammatory bowel disease had higher concentrations of microalbuminuria compared with patients in remission (206 (19) v 65 (8) microgram/min, mean (SEM), p < 0.0001). Eight patients with active inflammatory bowel disease who were sequentially followed up with measurements of microalbuminuria had significantly lower values, when the disease was inactive (active inflammatory bowel disease 192 (44) v inactive inflammatory bowel disease 64 (14) microgram/min, p < 0.03). There was a significant correlation with the simple index of Harvey and Bradshaw (r = 0.818, p < 0.0001). Microalbuminuria values were significantly lower in inflammatory bowel disease patients in remission, maintained with olsalazine compared with those patients maintained with mesalazine and salazopyrine, but no significant difference was seen in values of microalbuminuria in active inflammatory bowel disease patients receiving different salicylates. This study also measured serum amyloid-A as an indicator of the acute phase response in the same patients. Serum amyloid-A was significantly increased in active disease compared with inactive disease (151 (43) v 33 (7) or controls 11 (2) micrograms/ml, p < 0.05). In conclusion microalbuminuria is present in abnormal amounts in all patients with active inflammatory bowel disease, and values fall when the disease is quiescent. Microalbuminuria is probably a consequence of an acute phase response and provides a simple, rapid, and inexpensive test, which has the potential to monitor inflammatory bowel disease activity and response to treatment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck I. T. Laboratory assessment of inflammatory bowel disease. Dig Dis Sci. 1987 Dec;32(12 Suppl):26S–41S. doi: 10.1007/BF01312461. [DOI] [PubMed] [Google Scholar]
- Best W. R., Becktel J. M., Singleton J. W., Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976 Mar;70(3):439–444. [PubMed] [Google Scholar]
- Braegger C. P., Nicholls S., Murch S. H., Stephens S., MacDonald T. T. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet. 1992 Jan 11;339(8785):89–91. doi: 10.1016/0140-6736(92)90999-j. [DOI] [PubMed] [Google Scholar]
- Bäckman L., Appelkvist E. L., Ringdén O., Dallner G. Glutathione transferase in the urine: a marker for post-transplant tubular lesions. Kidney Int. 1988 Feb;33(2):571–577. doi: 10.1038/ki.1988.35. [DOI] [PubMed] [Google Scholar]
- Castell J. V., Gómez-Lechón M. J., David M., Hirano T., Kishimoto T., Heinrich P. C. Recombinant human interleukin-6 (IL-6/BSF-2/HSF) regulates the synthesis of acute phase proteins in human hepatocytes. FEBS Lett. 1988 May 23;232(2):347–350. doi: 10.1016/0014-5793(88)80766-x. [DOI] [PubMed] [Google Scholar]
- Chambers R. E., Stross P., Barry R. E., Whicher J. T. Serum amyloid A protein compared with C-reactive protein, alpha 1-antichymotrypsin and alpha 1-acid glycoprotein as a monitor of inflammatory bowel disease. Eur J Clin Invest. 1987 Oct;17(5):460–467. doi: 10.1111/j.1365-2362.1987.tb01143.x. [DOI] [PubMed] [Google Scholar]
- Choy M. Y., Walker-Smith J. A., Williams C. B., MacDonald T. T. Differential expression of CD25 (interleukin-2 receptor) on lamina propria T cells and macrophages in the intestinal lesions in Crohn's disease and ulcerative colitis. Gut. 1990 Dec;31(12):1365–1370. doi: 10.1136/gut.31.12.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsgaard E. M., Frøland A., Jørgensen O. D., Mogensen C. E. Microalbuminuria as predictor of increased mortality in elderly people. BMJ. 1990 Feb 3;300(6720):297–300. doi: 10.1136/bmj.300.6720.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing D. J., Campbell I. W., Clarke B. F. The natural history of diabetic autonomic neuropathy. Q J Med. 1980 Winter;49(193):95–108. [PubMed] [Google Scholar]
- Fagan E. A., Dyck R. F., Maton P. N., Hodgson H. J., Chadwick V. S., Petrie A., Pepys M. B. Serum levels of C-reactive protein in Crohn's disease and ulcerative colitis. Eur J Clin Invest. 1982 Aug;12(4):351–359. doi: 10.1111/j.1365-2362.1982.tb02244.x. [DOI] [PubMed] [Google Scholar]
- Gosling P., Sutcliffe A. J., Cooper M. A., Jones A. F. Burn and trauma associated proteinuria: the role of lipid peroxidation, renin and myoglobin. Ann Clin Biochem. 1988 Jan;25(Pt 1):53–59. doi: 10.1177/000456328802500107. [DOI] [PubMed] [Google Scholar]
- Gross V., Andus T., Caesar I., Roth M., Schölmerich J. Evidence for continuous stimulation of interleukin-6 production in Crohn's disease. Gastroenterology. 1992 Feb;102(2):514–519. doi: 10.1016/0016-5085(92)90098-j. [DOI] [PubMed] [Google Scholar]
- Haffner S. M., Stern M. P., Gruber M. K., Hazuda H. P., Mitchell B. D., Patterson J. K. Microalbuminuria. Potential marker for increased cardiovascular risk factors in nondiabetic subjects? Arteriosclerosis. 1990 Sep-Oct;10(5):727–731. doi: 10.1161/01.atv.10.5.727. [DOI] [PubMed] [Google Scholar]
- Harries A. D., Fitzsimons E., Fifield R., Dew M. J., Rhoades J. Platelet count: a simple measure of activity in Crohn's disease. Br Med J (Clin Res Ed) 1983 May 7;286(6376):1476–1476. doi: 10.1136/bmj.286.6376.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. F., Bradshaw J. M. A simple index of Crohn's-disease activity. Lancet. 1980 Mar 8;1(8167):514–514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- Jarrett R. J., Viberti G. C., Argyropoulos A., Hill R. D., Mahmud U., Murrells T. J. Microalbuminuria predicts mortality in non-insulin-dependent diabetics. Diabet Med. 1984 May;1(1):17–19. doi: 10.1111/j.1464-5491.1984.tb01915.x. [DOI] [PubMed] [Google Scholar]
- Klein N. J., Shennan G. I., Heyderman R. S., Levin M. Alteration in glycosaminoglycan metabolism and surface charge on human umbilical vein endothelial cells induced by cytokines, endotoxin and neutrophils. J Cell Sci. 1992 Aug;102(Pt 4):821–832. doi: 10.1242/jcs.102.4.821. [DOI] [PubMed] [Google Scholar]
- Krentz A. J., Hale P. J., Albutt E. C., Nattrass M. HbA1 in the diagnosis of factitious remission of diabetes. Ann Clin Biochem. 1988 Mar;25(Pt 2):150–154. doi: 10.1177/000456328802500204. [DOI] [PubMed] [Google Scholar]
- Mahida Y. R., Wu K., Jewell D. P. Enhanced production of interleukin 1-beta by mononuclear cells isolated from mucosa with active ulcerative colitis of Crohn's disease. Gut. 1989 Jun;30(6):835–838. doi: 10.1136/gut.30.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modigliani R., Mary J. Y., Simon J. F., Cortot A., Soule J. C., Gendre J. P., Rene E. Clinical, biological, and endoscopic picture of attacks of Crohn's disease. Evolution on prednisolone. Groupe d'Etude Thérapeutique des Affections Inflammatoires Digestives. Gastroenterology. 1990 Apr;98(4):811–818. doi: 10.1016/0016-5085(90)90002-i. [DOI] [PubMed] [Google Scholar]
- Murch S. H., MacDonald T. T., Walker-Smith J. A., Levin M., Lionetti P., Klein N. J. Disruption of sulphated glycosaminoglycans in intestinal inflammation. Lancet. 1993 Mar 20;341(8847):711–714. doi: 10.1016/0140-6736(93)90485-y. [DOI] [PubMed] [Google Scholar]
- Ramadori G., Sipe J. D., Dinarello C. A., Mizel S. B., Colten H. R. Pretranslational modulation of acute phase hepatic protein synthesis by murine recombinant interleukin 1 (IL-1) and purified human IL-1. J Exp Med. 1985 Sep 1;162(3):930–942. doi: 10.1084/jem.162.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven G. M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988 Dec;37(12):1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Rosenzweig L. J., Kanwar Y. S. Removal of sulfated (heparan sulfate) or nonsulfated (hyaluronic acid) glycosaminoglycans results in increased permeability of the glomerular basement membrane to 125I-bovine serum albumin. Lab Invest. 1982 Aug;47(2):177–184. [PubMed] [Google Scholar]
- Saverymuttu S. H., Camilleri M., Rees H., Lavender J. P., Hodgson H. J., Chadwick V. S. Indium 111-granulocyte scanning in the assessment of disease extent and disease activity in inflammatory bowel disease. A comparison with colonoscopy, histology, and fecal indium 111-granulocyte excretion. Gastroenterology. 1986 May;90(5 Pt 1):1121–1128. doi: 10.1016/0016-5085(86)90376-8. [DOI] [PubMed] [Google Scholar]
- Saverymuttu S. H., Hodgson H. J., Chadwick V. S., Pepys M. B. Differing acute phase responses in Crohn's disease and ulcerative colitis. Gut. 1986 Jul;27(7):809–813. doi: 10.1136/gut.27.7.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saverymuttu S. H., Peters A. M., Lavender J. P., Pepys M. B., Hodgson H. J., Chadwick V. S. Quantitative fecal indium 111-labeled leukocyte excretion in the assessment of disease in Crohn's disease. Gastroenterology. 1983 Dec;85(6):1333–1339. [PubMed] [Google Scholar]
- Sawyer N., Wadsworth J., Wijnen M., Gabriel R. Prevalence, concentration, and prognostic importance of proteinuria in patients with malignancies. Br Med J (Clin Res Ed) 1988 May 7;296(6632):1295–1298. doi: 10.1136/bmj.296.6632.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D. T., Gray G. M., Gregory P. B., Anderson M., Goodwin D. A., McDougall I. R. Location and activity of ulcerative and Crohn's colitis by indium 111 leukocyte scan. A prospective comparison study. Gastroenterology. 1983 Feb;84(2):388–393. [PubMed] [Google Scholar]
- Wakefield A. J., Sankey E. A., Dhillon A. P., Sawyerr A. M., More L., Sim R., Pittilo R. M., Rowles P. M., Hudson M., Lewis A. A. Granulomatous vasculitis in Crohn's disease. Gastroenterology. 1991 May;100(5 Pt 1):1279–1287. [PubMed] [Google Scholar]
- Wakefield A. J., Sawyerr A. M., Dhillon A. P., Pittilo R. M., Rowles P. M., Lewis A. A., Pounder R. E. Pathogenesis of Crohn's disease: multifocal gastrointestinal infarction. Lancet. 1989 Nov 4;2(8671):1057–1062. doi: 10.1016/s0140-6736(89)91078-7. [DOI] [PubMed] [Google Scholar]
- Winocour P. H., Dhar H., Anderson D. C. The relationship between autonomic neuropathy and urinary sodium and albumin excretion in insulin-treated diabetics. Diabet Med. 1986 Sep-Oct;3(5):436–440. doi: 10.1111/j.1464-5491.1986.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Winocour P. H., Durrington P. N., Bhatnagar D., Ishola M., Mackness M., Arrol S. Influence of early diabetic nephropathy on very low density lipoprotein (VLDL), intermediate density lipoprotein (IDL), and low density lipoprotein (LDL) composition. Atherosclerosis. 1991 Jul;89(1):49–57. doi: 10.1016/0021-9150(91)90006-o. [DOI] [PubMed] [Google Scholar]
- Winocour P. H., Harland J. O., Millar J. P., Laker M. F., Alberti K. G. Microalbuminuria and associated cardiovascular risk factors in the community. Atherosclerosis. 1992 Mar;93(1-2):71–81. doi: 10.1016/0021-9150(92)90201-q. [DOI] [PubMed] [Google Scholar]
- Yi E. S., Ulich T. R. Endotoxin, interleukin-1, and tumor necrosis factor cause neutrophil-dependent microvascular leakage in postcapillary venules. Am J Pathol. 1992 Mar;140(3):659–663. [PMC free article] [PubMed] [Google Scholar]
- van Hees P. A., van Elteren P. H., van Lier H. J., van Tongeren J. H. An index of inflammatory activity in patients with Crohn's disease. Gut. 1980 Apr;21(4):279–286. doi: 10.1136/gut.21.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]


