Abstract
The γ-aminobutyric acid type A (GABAA) receptor mediates fast inhibitory synaptic transmission in the CNS. Dysfunction of the GABAA receptor would be expected to cause neuronal hyperexcitability, a phenomenon linked with epileptogenesis. We have investigated the functional consequences of an arginine-to-glutamine mutation at position 43 within the GABAA γ2-subunit found in a family with childhood absence epilepsy and febrile seizures. Rapid-application experiments performed on receptors expressed in HEK-293 cells demonstrated that the mutation slows GABAA receptor deactivation and increases the rate of desensitization, resulting in an accumulation of desensitized receptors during repeated, short applications. In Xenopus laevis oocytes, two-electrode voltage-clamp analysis of steady-state currents obtained from α1β2γ2 or α1β2γ2(R43Q) receptors did not reveal any differences in GABA sensitivity. However, differences in the benzodiazepine pharmacology of mutant receptors were apparent. Mutant receptors expressed in oocytes displayed reduced sensitivity to diazepam and flunitrazepam but not the imidazopyridine zolpidem. These results provide evidence of impaired GABAA receptor function that could decrease the efficacy of transmission at inhibitory synapses, possibly generating a hyperexcitable neuronal state in thalamocortical networks of epileptic patients possessing the mutant subunit.
Keywords: rapid agonist application, two-electrode voltage clamp, Xenopus oocytes
Epilepsy, one of the most common neurological disorders, is characterized by episodic seizures that are caused by paroxysmal, synchronized discharges of hyperexcitable neuronal populations. Many human epileptic syndromes have a genetic component, and the molecular basis of a few inherited epilepsies is now known (1). Most genes implicated in epilepsy to date code for ion channel subunits, both ligand-gated and voltage-gated (2). For example, mutations within the α- and β-subunits of the voltage-gated sodium channel family have been found in families with generalized epilepsy with febrile seizures plus (GEFS+; refs. 3 and 4). Mutations within the voltage-gated potassium channel subunits KCNQ2 and KCNQ3 cause the benign familial neonatal convulsions phenotype (5–9), whereas mutations in the acetylcholine ligand-gated receptor family are responsible for familial nocturnal frontal lobe epilepsy (10–14). In vitro investigations with the oocyte expression system and two-electrode voltage-clamp assay generally suggest that these mutations would result in neuronal hyperexcitability.
The γ-aminobutyric acid type A (GABAA) receptor is the predominant ligand-gated Cl− ion channel conferring fast inhibitory synaptic transmission in the CNS and, therefore, is a prime candidate for involvement in epileptogenesis. The GABAA receptor is a heterooligomeric complex of five subunits. Each subunit has a large, N-terminal extracellular domain, four transmembrane domains, and a small, extracellular C-terminal tail. Multiple subunits have been cloned and divided into families based on their sequence homology. The subunits also are differentially expressed in various brain regions and confer distinct pharmacology to the expressed GABAA receptor (15, 16). Most native receptors contain α-, β-, and γ-subunits, with α1β2γ2 being the most common combination (17). The receptor has two GABA-binding sites formed by the extracellular domains at the interface of αβ-subunits. The receptor also is modulated allosterically by benzodiazepines, believed to bind at the interface of αγ-subunits. Binding of benzodiazepines potentiates GABA-activated Cl− currents but, alone, is unable to activate the receptor. The exact mechanism of receptor modulation by benzodiazepines remains unclear.
Mutations in GABAA receptor subunit genes recently have been described in human epilepsy (18–20). Two families with the GEFS+ phenotype had different mutations in the γ2-subunit gene, severely reducing or abolishing Cl− currents in response to GABA in Xenopus laevis oocytes. A French family (20) had a K289M mutation that resulted in an amino acid change in the extracellular loop between the second and third transmembrane domains. We described an Australian family with a nonsense mutation that caused truncation of the γ2-subunit at position 351 between the third and forth transmembrane domains (19). Heteromeric GABA receptor complexes containing GFP-tagged truncated γ2-subunits were found to be trapped within the endoplasmic reticulum, thereby inhibiting expression at the cell membrane. Different phenotypes of simple febrile seizures, childhood absence epilepsy and GEFS+, were observed in a large Australian family with a missense mutation in the extracellular domain of the γ2-subunit (18). The mutation (R43Q) resulted in the replacement of a highly conserved arginine, found in most ligand-gated receptor subunits, for a glutamine at position 43. Although expression of this mutant subunit in X. laevis oocytes did not alter GABA-induced Cl− currents, lack of potentiation of such currents with low benzodiazepine concentrations was observed.
Impaired sensitivity to benzodiazepines may explain some aspects of the epilepsy phenotypes observed. Here, we postulate that the γ2(R43Q) mutation may have additional and significant pathophysiological effects. This is due, in part, to the observation that R43 is so highly conserved amongst other ligand-gated channels that do not interact with benzodiazepines that it may play a more fundamental role in receptor function. We now have examined both GABA sensitivity and responses to benzodiazepines by using two-electrode voltage-clamp recordings of Xenopus oocytes heterologously expressing WT and mutation GABA receptors. The oocyte system, however, has relatively slow temporal resolution, whereas during synaptic transmission, receptors are activated by pulses of neurotransmitter that reach millimolar concentrations and decay within a millisecond (21). To obtain sufficient temporal resolution, we used rapid-solution switches on outside-out patches from human embryonic kidney (HEK-293) cells to approximate the conditions at synapses. We found that the γ2(R43Q) mutation slows receptor deactivation and increases fast desensitization. These kinetic differences could lead to reduced inhibition during periods of high activity and, thus, contribute to the epileptic phenotypes.
Materials and Methods
Construction of Receptor Subunits and Site-Directed Mutagenesis.
Human GABAA receptor α1-, β2-, and γ-short subunit cDNA were subcloned into pcDNA3.1(+). A point mutation, corresponding to the G-to-A nucleotide substitution at position 471 found in affected individuals, was introduced into γ2 of the human GABAA receptor gene by using the QuickChange site-directed mutagenesis kit (Stratagene). The following column-purified oligonucleotide was used to incorporate the mutation into γ2: 5′-GGA TAT GAC AAT AAA CTT CAG CCT GAT ATA GGA GTG AAG CC-3′. Successful mutagenesis was verified by double-stranded DNA sequencing.
Expression in Oocytes and Two-Electrode Voltage-Clamp Analysis.
WT and mutant cDNAs were linearized with appropriate restriction enzymes, and cRNA was made by using Ambion's T7 RNA polymerase mMessage mMachine in vitro RNA synthesis kit (Austin, TX). At the completion of the reaction, cRNA was purified on a Chromaspin-100 diethyl pyrocarbonate H2O equilibrated column. cRNAs were run on denaturing agarose RNA gels to check for correct size, quantity, and quality. Xenopus oocytes (stage V or VI) were microinjected with 25 nl (≈25 ng) of cRNA containing GABAA subunits in specific ratios. Because GABAA receptors can assemble as a binary complex of α1 and β2 alone, γ2 was used in a 10-fold molar excess to promote assembly of α1β2γ2 receptors (22). Oocytes then were stored in OR2 buffer (82.5 mM NaCl/2 mM KCl/1 mM MgCl2/5 mM Hepes, pH 7.5, supplemented with 2% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin; Life Technologies, Gaithersburg, MD) and incubated at 18°C for 3–5 days to allow for adequate receptor expression.
Current recordings from oocytes were made by using a two-electrode voltage clamp (AxoClamp 2B; Axon Instruments), with recordings made at a holding potential of −70 mV. Electrodes were filled with 3 M KCl and were of 1–2 MΩ resistance. Data were low-pass-filtered (300-Hz Bessel) and digitized at 1 kHz by using the FETCHEX module of the pclamp 6 software suite (Axon Instruments). Data for display was low-pass-filtered (10-Hz Gaussian) by using clampfit 8 software (Axon Instruments). Oocytes were perfused continuously with ND96 solution (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM BaCl2/5 mM Hepes, pH 7.5). GABA (Sigma) was applied in the presence or absence of the benzodiazepines diazepam (Sigma), flunitrazepam (School of Pharmacy, Monash University, Australia), or zolpidem (Tocris Neuramin, Bristol, U.K.) at concentrations and times indicated.
Expression in HEK-293 Cells and Outside-Out Patch-Clamp Assay.
HEK-293 cells were cultured in minimum essential medium with Earle's salts (Life Technologies) containing 10% FBS (Sigma) in a 37°C incubator under a 5% CO2 atmosphere. When the cells reached 60–90% confluence in 60-mm culture dishes, they were transfected with 1 μg each of α1, β2, and γ2 (WT or R43Q) cDNAs subcloned into the pcDNA3.1(+) vector and 200 ng of a vector containing the T cell membrane antigen CD8 cDNA (gift from Robert Coronado, Univ. of Wisconsin, Madison, WI) by using the Lipofectamine 2000 reagent (GIBCO) according to the supplied protocol. Twenty to 28 h posttransfection, the cells were replated on 12-mm sterile glass coverslips, transferred to a 30°C incubator, and recorded at 24–96 h postreplating. Transfected cells were recognized by incubation with anti-CD8 antibody beads (Dynal, Oslo).
Recordings from outside-out patches excised from HEK cells were made by using borosilicate glass pipettes filled with 140 mM KCl/10 mM EGTA/2 mM MgATP/20 mM phosphocreatine/10 mM Hepes, pH 7.3. Patches were voltage-clamped at −60 mV and placed in the stream of a multibarreled flowpipe array (Vitro Dynamics, Rockaway, NY) mounted on a piezoelectric bimorph (Vernitron, Bedford, OH). A computer-controlled constant current source drove the bimorph to move solution interfaces over the patch with 10–90% exchange times of ≈200 μs, as measured by the liquid-junction current at the open pipette tip after each experiment. GABA and Zn2+ were dissolved in the perfusion solution, which contained 145 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM Hepes, and 4 mM glucose, pH 7.4. Currents were low-pass-filtered at 2–5 kHz with a four-pole Bessel filter and digitized at a rate no less than twice the filter frequency. Data were collected by using an Axopatch 200B amplifier and Digidata 1320A digitizer (Axon Instruments) controlled by axograph 4 software running on a Macintosh G4. Analysis was performed with Axograph and homewritten routines under matlab (Mathworks, Natick, MA). Analysis of dose-response curves used a bootstrap curve-fitting process in which 100 runs were executed, randomly drawing half the data points at each concentration on each run. Differences between WT and mutant were judged to be statistically significant when the 95% confidence limits of two fitted curves or the underlying equation parameters did not overlap.
Results and Discussion
The γ2(R43Q) Subunit Mutation Alters Deactivation and Desensitization Kinetics of GABAA Receptors Expressed in HEK Cells.
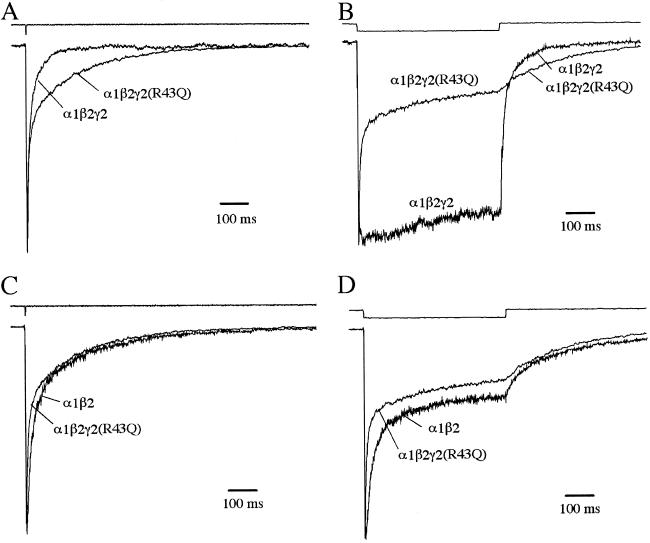
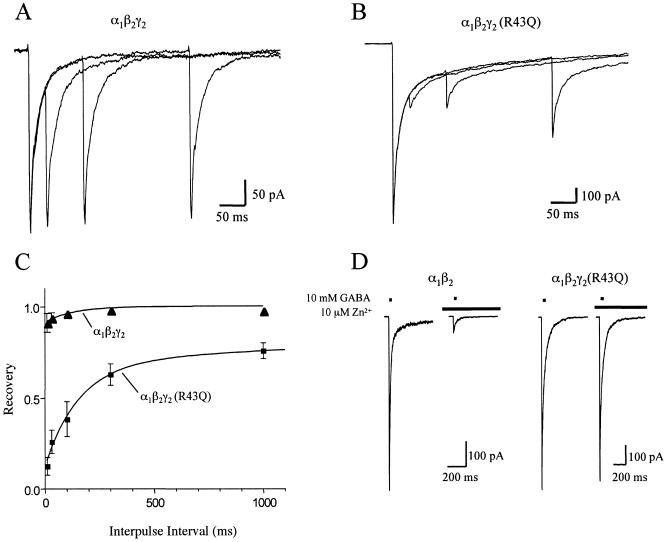
To evaluate the possible effect of the γ2(R43Q) mutation on the kinetics of inhibitory postsynaptic currents, we mimicked synaptic receptor activation by applying brief pulses of GABA (10 mM) to outside-out patches containing WT α1β2γ2 or α1β2γ2(R43Q) receptors (Fig. 1A). Receptors containing the γ2(R43Q) mutation deactivated significantly more slowly than WT receptors. This slow deactivation was accompanied by a markedly increased fast component of desensitization during long pulses of saturating GABA (Fig. 1B). Fast desensitization of α1β2γ2(R43Q) receptors also led to more pronounced depression and slower recovery during paired-pulse experiments (Fig. 2 A–C). Because the kinetics of α1β2γ2(R43Q) receptors resembled those of α1β2 receptors (Fig. 1 C and D; Table 1), we confirmed that the γ2(R43Q) subunit had been incorporated properly into the receptors by examining the sensitivity to block by Zn2+ (23, 24). A low concentration of Zn2+ (10 μM) strongly blocked currents from α1β2 transfections, but those from α1β2γ2 or α1β2γ2(R43Q) transfections were blocked only weakly (Fig. 2D), indicating that failure to incorporate the γ2-subunit cannot account for the slowed deactivation and increased desensitization observed in α1β2γ2(R43Q) receptors. These results are summarized in Table 1. Because transition through desensitized states can prolong deactivation (25), the changes in deactivation and desensitization caused by the γ2(R43Q) mutation potentially could arise by alteration of a single, underlying kinetic process, for example, by stabilization of a desensitized state.
Fig 1.
The γ2(R43Q) mutation slows deactivation and enhances fast desensitization. (A) Normalized average currents evoked by applying 2-ms pulses of saturating GABA (10 mM) to outside-out patches. Actual peak currents were 1.1 nA (WT) and 1.3 nA (R43Q). (B) Normalized average currents evoked by 500-ms pulses of saturating GABA. Actual peak currents were 230 pA (WT) and 1.2 nA (R43Q). (C and D) Comparison of kinetics in the mutant with those in receptors lacking the γ2-subunit. Traces from the mutant are the same as those in A and B. The actual peak currents for α1β2 were 305 pA (C) and 270 pA (D). The top traces in each graph are the liquid junction currents recorded at the open pipette tip immediately after each experiment to evaluate the speed of solution exchange (20–80% exchange times were <200 μs). Deactivation time constants (and percent amplitude) were (in mean ± SEM) 9 ± 1 ms (74 ± 3%) and 148 ± 42 ms for α1β2γ2; 8 ± 0.9 ms (52 ± 3.1%), 64 ± 8 ms (30 ± 2%), and 415 ± 49 ms for α1β2γ2(R43Q); 14 ± 0.9 ms (70 ± 3%), 83 ± 6 ms (21 ± 2%), and 664 ± 103 ms for α1β2.
Fig 2.
The γ2(R43Q) mutation increases paired-pulse desensitization. A (WT) and B (R43Q) each show three overlaid responses to pairs of 2-ms pulses of 10 mM GABA separated by 30, 100, or 300 ms. Each trace is the average of five records. (C) Results of paired-pulse experiments plotted as fractional recovery vs. interpulse interval. Recovery is calculated as: (I2 − B2)/(I1 − B2), where I1 and I2 are the peak responses to the first and second GABA pulses and B2 is the current immediately before the second GABA pulse. The data for R43Q receptors were fit with a biexponential curve that had time constants of 0.16 s (57%) and 4.2 s (31%) and a y intercept of 0.12. The data for WT receptors were fit with a monoexponential curve that had a time constant of 0.13 s (9%) and a y intercept of 0.91. (D) Responses of α1β2 and α1β2γ2(R43Q) receptors to 2-ms pulses of 10 mM GABA in the presence or absence of 10 μM Zn2+ applied 500 ms before GABA application. Zn2+ (10 μM) blocks currents from α1β2 receptors strongly but blocks currents from α1β2γ2(R43Q) receptors weakly, indicating efficient incorporation of the γ2(R43Q) subunit when cotransfected with α1- and β2-subunit cDNAs.
Table 1.
Kinetics and Zn2+ sensitivity of α1β2γ2 and α1β2γ2(R43Q) receptors
| Subunits
|
Deactivation | Desensitization | Paired-pulse recovery | 10 μM Zn2+ | |||||
|---|---|---|---|---|---|---|---|---|---|
| τW, ms | n | τW, ms | % at 500 ms | n | % at 100 ms | n | % Block | n | |
| α1β2 | 83 ± 13 | 10 | 40 ± 21 | 26 ± 14 | 9 | ND | 85 ± 6 | 5 | |
| α1β2γ2 | 46 ± 39 | 10 | 371 ± 81 | 65 ± 18 | 7 | 96 ± 3 | 3 | 7 ± 5 | 3 |
| α1β2γ2(R43Q) | 98 ± 39 | 12 | 74 ± 42 | 24 ± 13 | 13 | 39 ± 23 | 6 | 10 ± 5 | 7 |
Note that for every parameter shown, except zinc block, the mutant [α1β2γ2(R43Q)] values are significantly different from wild-type α1β2γ2. One-way ANOVA with Dunnett posttest; ND, not determined; values are mean ± SD. τW is the weighted time constant computed as ∑ Aiτi, where Ai is the amplitude and τiis the time constant of the ith component.
GABA Sensitivity in Oocytes Is Not Altered by the γ2(R43Q) Subunit Mutation.
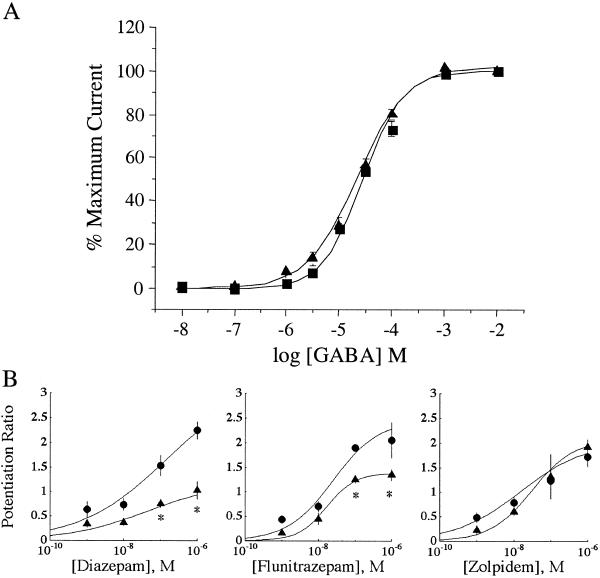
Mutant and WT γ2-subunits were expressed together with WT α1- and β2-subunits in Xenopus oocytes to assess the effects of the γ2(R43Q) subunit mutation on sensitivity to GABA and modulating ligands. Two to 3 days after mRNA injection, GABA-induced currents of γ2(R43Q) were compared with WT γ2 by using the two-electrode voltage-clamp technique (Fig. 3A). There was no significant difference in the affinity (EC50) or the slope (n) of the GABA dose-response curve between WT (EC50 = 24 μM, n = 0.88) and γ2(R43Q) (EC50 = 28 μM, n = 1.0). Thus, the γ2(R43Q) subunit assembles and, when measured in oocytes, displays a dose-response relation similar to WT.
Fig 3.
(A) Responsiveness to GABA is not altered in oocytes expressing the γ2(R43Q) subunit. GABA dose-response curves for oocytes expressing WT (•) and R43Q (▴) γ2-subunits with WT α1- and β2-subunits in a 1:1:10 molar ratio. Data points represent the mean current ± SE from five or more oocytes from two or more frogs. Data were fitted to a curve following the equation % max = min + (Max − Min)/(1+10[(logEC50−X)⋅n]), where Max is the maximal current, Min is the current at 0.01 μM GABA, X is the log of the GABA concentration, EC50 is the half-maximal current response, and n is the Hill coefficient. The vertical, dashed lines show the GABA concentrations used in our earlier (1 μM) and present (10 μM) studies of benzodiazepine potentiation. (B) Oocytes expressing γ2(R43Q) have reduced sensitivity to potentiation by the classical benzodiazepines diazepam and flunitrazepam but not zolpidem. Dose-response curves were constructed for oocytes expressing WT (•) and R43Q (▴) γ2-subunits in conjunction with WT α1- and β2-subunits in a 1:1:10 molar ratio. Currents were elicited with 10 μM GABA. Each data point is the median ratio of the current in the presence of GABA alone and GABA plus BDZ of at least four oocytes from two or more frogs (error bars indicate SEM). Predicted curves are drawn through the points based on the median fitted parameters; the asterisk marks where the predicted curves diverge with 95% confidence. The median EC50 values were 140 nM (WT) and 44 nM (R43Q) for diazepam, 24 nM (WT) and 16 nM (R43Q) for flunitrazepam, and 15 nM (WT) and 30 nM (R43Q) for zolpidem. Hill slopes were not significantly different from unity.
Reduced Sensitivity to Benzodiazepines in Oocytes Expressing the γ2(R43Q) Subunit Mutation.
Our previous studies revealed that whereas the response to GABA (1 μM) was indistinguishable in oocytes expressing WT or γ2(R43Q) subunits, potentiation of GABA (1 μM) induced currents by 1 μM diazepam was absent in receptors containing the γ2(R43Q) mutant subunit (18). These results suggest that R43 either may stabilize the BZD-binding site via nonlocal effects or, alternatively, may be critical for transducing BZD binding into potentiation. We have extended these investigations by determining dose-response curves for the benzodiazepines diazepam and flunitrazepam, as well as the imidazopyridine zolpidem, at a concentration of GABA (10 μM) 10-fold-higher than we used in our previous study, to assess the effects of GABAA receptor modulators at higher levels of receptor occupancy (Fig. 3B). Interestingly, with this higher GABA concentration, we now found that diazepam and flunitrazepam potentiated currents in oocytes expressing the γ2(R43Q) subunit although to a significantly lesser extent than in those expressing WT γ2-subunit. Previous reports investigating amino acids involved in benzodiazepine binding and allosteric modulation have found differences in the residues and receptor subunits involved in binding benzodiazepines and imidazopyridines such as zolpidem (26, 27), suggesting they act via different binding and perhaps transduction mechanisms. Therefore, we examined the ability of zolpidem to modulate γ2(R43Q)-containing GABA receptors. Responses to zolpidem in the presence of 10 μM GABA in γ2(R43Q) oocytes were not significantly different than those seen in the WT γ2-subunit-expressing oocytes, suggesting that the binding and transduction pathways activated by zolpidem are not impaired by the presence of the R43Q mutation of the γ2-subunit. In summary, benzodiazepine sensitivity is reduced significantly in receptors containing the γ2(R43Q) subunit but is not abolished completely.
Functional Uncoupling of γ2(R43Q) Subunit-Containing Receptors May Involve Disruption of a Salt Bridge Between Neighboring Subunits.
The incorporation of γ2(R43Q) subunits into GABA receptors results in the selective loss of properties typically conferred by the WT γ2-subunit. WT α1β2γ2 receptors displayed rapid deactivation and little fast desensitization or paired-pulse depression and high sensitivity to diazepam and flunitrazepam. In contrast, α1β2γ2(R43Q) receptors somewhat resembled α1β2 receptors, having a large, slow component of deactivation, pronounced fast desensitization, and strong, paired-pulse depression with slow recovery and reduced sensitivity to diazepam and flunitrazepam. However, the resemblance in functional properties of α1β2γ2(R43Q) and α1β2 receptors is not a result of failure to incorporate the γ2-subunit because, unlike α1β2 receptors, α1β2γ2 and α1β2γ2(R43Q) were insensitive to blocking by low concentrations of Zn2+. Thus, the γ2(R43Q) mutation may disrupt interactions between the γ2-subunit and other subunits that modulate kinetics and benzodiazepine actions.
By analogy with the crystal structure of the homologous acetylcholine-binding protein (28), R43 may be located at the interface between γ2 and another subunit (probably β2), on the side of the γ2-subunit opposite from that that forms the BZD-binding interface with the α-subunit. Therefore, R43 is not likely to be part of the BZD-binding site (22). Modeling suggests further that γ2(R43) could form a salt bridge with γ2(E178), which may be involved in a second salt bridge across the subunit interface with β2(R117) (29). This network of salt bridges could be important in transmitting conformational changes between subunits. Replacement of an arginine with glutamine retains a hydrophilic side chain while reducing the side-chain size slightly but, importantly, it removes a positive charge and would disrupt any salt bridge interactions. We, therefore, hypothesize that the γ2(R43Q) mutation results in the loss of a critical salt bridge involved in transmitting conformational changes between subunits, causing a partial “functional uncoupling” of the γ2-subunit.
Possible Effects of γ2(R43Q) Subunit-Containing GABAA Receptors on the Function of Inhibitory Synapses.
During inhibitory synaptic transmission, the slow deactivation caused by the γ2(R43Q) mutation predicts that inhibitory postsynaptic currents would be prolonged. However, the mutation also greatly increases the rate and degree of desensitization and dramatically slows the recovery from desensitization after a brief GABA pulse. Therefore, rapid and repetitive stimulation of inhibitory synapses may cause α1β2γ2(R43Q) receptors to accumulate in desensitized states, resulting in a progressive loss of inhibition. The reduced sensitivity to benzodiazepines seen in the present study is consistent with our previous results (3) and suggests that, if endogenous benzodiazepines exist, then an impairment of benzodiazepine modulation of γ2(R43Q)-containing GABA receptors also may contribute to a reduction in synaptic strength.
Disruption of GABA Receptor Function and Epilepsy in Humans Carrying the γ2(R43Q) Subunit Mutation.
The family identified with the γ2(R43Q) mutation had epilepsy phenotypes of childhood absence epilepsy, febrile seizures, and GEFS+. Absence seizures and their electroencephalogram signature of generalized spike-wave discharges are generated by aberrations in normal thalamocortical rhythms involving the network of pyramidal neurons in cerebral cortex, thalamic relay nuclei, and the reticular thalamic nucleus (for review, see refs. 30 and 31). GABA is a major neurotransmitter in the thalamocortical network, with complex effects involving fast inhibition via GABAA and slower inhibition via GABAB receptors. Activation of GABAA receptors in the reticular thalamic nucleus inhibits absences, and blockade of these receptors induces them (31, 32). Because the reticular thalamic nucleus is the “pacemaker” of normal and aberrant thalamocortical rhythms, the observed accumulation of desensitized GABAA receptors in the experiments reported here could lead to facilitation of abnormal spike-wave discharges. The physiological generators of convulsions seen in patients with febrile seizures and GEFS+ phenotypes are understood less clearly but likely involve cortex with or without involvement of thalamus and other subcortical structures (31, 32). Pharmacological blockade of GABAA receptors by systemic administration potently induces generalized convulsions that are generated primarily in the cortex. Thus, desensitization of GABAA receptors in the cortex by this mutation might generate such convulsive seizures, in addition to the effects on the thalamocortical network posited above. The generation of genetically modified mice carrying the γ2(R43Q) mutation should provide further insights into the cellular and network mechanisms underlying various forms of seizures associated with this mutation.
Note Added in Proof.
While this manuscript was in review, another paper (33) appeared examining the γ2 (R43Q) mutation. This study did not find any differences in kinetics or diazepam sensitivity between wild-type and the γ2(R43Q) mutant.
Acknowledgments
We thank Craig Morton for assistance with the molecular modeling. This work was supported by the National Health and Medical Research Council of Australia, Bionomics Ltd., and the National Institutes of Health (D.W. and C.C.). M.V.J. and D.A.W. were supported by grants from the Epilepsy Foundation of America.
Abbreviations
GABAA, γ-aminobutyric acid type A (receptor)
GEFS+, generalized epilepsy with febrile seizures plus
HEK, human embryonic kidney
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Berkovic S. F. & Scheffer, I. E. (2001) Epilepsia 42, 16-23. [DOI] [PubMed] [Google Scholar]
- 2.Lerche H., Jurkat-Rott, K. & Lehmann-Horn, F. (2001) Am. J. Med. Genet. 106, 146-159. [DOI] [PubMed] [Google Scholar]
- 3.Wallace R. H., Scheffer, I. E., Barnett, S., Richards, M., Dibbens, L., Desai, R. R., Lerman-Sagie, T., Lev, D., Mazarib, A., Brand, N., et al. (2001) Am. J. Hum. Genet. 68, 859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallace R. H., Wang, D. W., Singh, R., Scheffer, I. E., George, A. L., Jr., Phillips, H. A., Saar, K., Reis, A., Johnson, E. W., Sutherland, G. R., et al. (1998) Nat. Genet. 19, 366-370. [DOI] [PubMed] [Google Scholar]
- 5.Charlier C., Singh, N. A., Ryan, S. G., Lewis, T. B., Reus, B. E., Leach, R. J. & Leppert, M. (1998) Nat. Genet. 18, 53-55. [DOI] [PubMed] [Google Scholar]
- 6.Lerche H., Biervert, C., Alekov, A. K., Schleithoff, L., Lindner, M., Klinger, W., Bretschneider, F., Mitrovic, N., Jurkat-Rott, K., Bode, H., et al. (1999) Ann. Neurol. 46, 305-312. [DOI] [PubMed] [Google Scholar]
- 7.Biervert C., Schroeder, B. C., Kubisch, C., Berkovic, S. F., Propping, P., Jentsch, T. J. & Steinlein, O. K. (1998) Science 279, 403-406. [DOI] [PubMed] [Google Scholar]
- 8.Biervert C. & Steinlein, O. K. (1999) Hum. Genet. 104, 234-240. [DOI] [PubMed] [Google Scholar]
- 9.Singh N. A., Charlier, C., Stauffer, D., DuPont, B. R., Leach, R. J., Melis, R., Ronen, G. M., Bjerre, I., Quattlebaum, T., Murphy, J. V., et al. (1998) Nat. Genet. 18, 25-29. [DOI] [PubMed] [Google Scholar]
- 10.Phillips H. A., Scheffer, I. E., Berkovic, S. F., Hollway, G. E., Sutherland, G. R. & Mulley, J. C. (1995) Nat. Genet. 10, 117-118. [DOI] [PubMed] [Google Scholar]
- 11.Phillips H. A., Favre, I., Kirkpatrick, M., Zuberi, S. M., Goudie, D., Heron, S. E., Scheffer, I. E., Sutherland, G. R., Berkovic, S. F., Bertrand, D., et al. (2001) Am. J. Hum. Genet. 68, 225-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips H. A., Marini, C., Scheffer, I. E., Sutherland, G. R., Mulley, J. C. & Berkovic, S. F. (2000) Ann. Neurol. 48, 264-267. [PubMed] [Google Scholar]
- 13.Steinlein O. K., Magnusson, A., Stoodt, J., Bertrand, S., Weiland, S., Berkovic, S. F., Nakken, K. O., Propping, P. & Bertrand, D. (1997) Hum. Mol. Genet. 6, 943-947. [DOI] [PubMed] [Google Scholar]
- 14.Steinlein O. K., Mulley, J. C., Propping, P., Wallace, R. H., Phillips, H. A., Sutherland, G. R., Scheffer, I. E. & Berkovic, S. F. (1995) Nat. Genet. 11, 201-203. [DOI] [PubMed] [Google Scholar]
- 15.Barnard E. A., Skolnick, P., Olsen, R. W., Mohler, H., Sieghart, W., Biggio, G., Braestrup, C., Bateson, A. N. & Langer, S. Z. (1998) Pharmacol. Rev. 50, 291-313. [PubMed] [Google Scholar]
- 16.Mehta A. K. & Ticku, M. K. (1999) Brain Res. Brain Res. Rev. 29, 196-217. [DOI] [PubMed] [Google Scholar]
- 17.Chang Y. & Weiss, D. S. (2000) in GABA in the Nervous System: The View at Fifty Years, eds. Martin, D. L. & Olsen, R. W. (Lippincott, Philadelphia), pp. 127–139.
- 18.Wallace R. H., Marini, C., Petrou, S., Harkin, L. A., Bowser, D. N., Panchal, R. G., Williams, D. A., Sutherland, G. R., Mulley, J. C., Scheffer, I. E., et al. (2001) Nat. Genet. 28, 49-52. [DOI] [PubMed] [Google Scholar]
- 19.Harkin L. A., Bowser, D. N., Dibbens, L. M., Singh, R., Phillips, F., Wallace, R. H., Richards, M. C., Williams, D. A., Mulley, J. C., Berkovic, S. F., et al. (2002) Am. J. Hum. Genet. 70, 530-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baulac S., Huberfeld, G., Gourfinkel-An, I., Mitropoulou, G., Beranger, A., Prud'homme, J. F., Baulac, M., Brice, A., Bruzzone, R. & LeGuern, E. (2001) Nat. Genet. 28, 46-48. [DOI] [PubMed] [Google Scholar]
- 21.Clements J. D. (1996) Trends Neurosci. 19, 163-171. [DOI] [PubMed] [Google Scholar]
- 22.Boileau A. J., Kucken, A. M., Evers, A. R. & Czajkowski, C. (1998) Mol. Pharmacol. 53, 295-303. [DOI] [PubMed] [Google Scholar]
- 23.Draguhn A., Verdorn, T. A., Ewert, M., Seeburg, P. H. & Sakmann, B. (1990) Neuron 5, 781-788. [DOI] [PubMed] [Google Scholar]
- 24.Smart T. G., Moss, S. J., Xie, X. & Huganir, R. L. (1991) Br. J. Pharmacol. 103, 1837-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones M. V. & Westbrook, G. L. (1995) Neuron 15, 181-191. [DOI] [PubMed] [Google Scholar]
- 26.Wingrove P. B., Thompson, S. A., Wafford, K. A. & Whiting, P. J. (1997) Mol. Pharmacol. 52, 874-881. [DOI] [PubMed] [Google Scholar]
- 27.Buhr A., Baur, R. & Sigel, E. (1997) J. Biol. Chem. 272, 11799-11804. [DOI] [PubMed] [Google Scholar]
- 28.Brejc K., van Dijk, W. J., Klaassen, R. V., Schuurmans, M., van Der Oost, J., Smit, A. B. & Sixma, T. K. (2001) Nature 411, 269-276. [DOI] [PubMed] [Google Scholar]
- 29.Cromer B. A., Morton, C. J. & Parker, M. W. (2002) Trends Biochem. Sci. 27, 280-287. [DOI] [PubMed] [Google Scholar]
- 30.Steriade M., McCormick, D. A. & Sejnowski, T. J. (1993) Science 262, 679-685. [DOI] [PubMed] [Google Scholar]
- 31.McCormick D. A. & Contretas, D. (2001) Annu. Rev. Physiol. 63, 815-846. [DOI] [PubMed] [Google Scholar]
- 32.Snead O. C., Depaulis, A., Vergnes, M. & Marescaux, C. (1999) Adv. Neurol. 79, 253-278. [PubMed] [Google Scholar]
- 33.Bianchi M. T., Song, L., Zhang, H. & Macdonald, R. L. (2002) J. Neurosci. 13, 5321-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]