Abstract
Cerebellar development occurs mainly postnatally and implies cell proliferation and migration. Hepatocyte growth factor (HGF) and Met are involved in mediating these responses in other tissues and are coexpressed in the cerebellum. Here we show that Met is localized in granule cell precursors and that cultures of these cells respond to HGF with proliferation. To study the role of HGF and Met in the cerebellum in vivo, we produced a viable hypomorphic Met mutant by knocking in the met locus a point mutation to abrogate the receptor Grb2-binding site. A similar mutant was previously described as perinatal lethal. In this “first-generation” knock-in the recombinant locus retained the Neo cassette (Metgrb2/grb2neo+). In the knock-in presented here Neo was Loxed and excised by Cre recombinase, which led to higher tissue levels of Metgrb2 protein, sufficient to rescue viability. In Metgrb2/grb2neo− mice the size of the cerebellum was reduced and foliation defects were evident, especially in the central and posterior half of the vermis. Proliferation of granule precursors in vivo was 25% lower than in controls. In cultures of mutant granule cells HGF-induced microtubule-associated protein kinase activation was reduced and transient. Behavioral tests indicated a balance impairment in Metgrb2/grb2neo− mice. Altogether these data indicate that normal cerebellar development and, possibly, function, require HGF and Met, and that proliferation of granule cells in the cerebellum critically depends on full HGF/Met signaling.
The postnatal development of the cerebellum, which consists of several layers characterized by distinct cell types, involves extensive proliferation of granule cell precursors, their inward migration, and selective loss by apoptosis of excess granule neurons. These events depend on multiple cell–cell interactions and are controlled by diffusible factors, both of autocrine and paracrine origin. In the past few years the identity of some of these molecules has been unraveled. Sonic hedgehog (Shh), a molecule which in early development is involved in cell fate determination, postnatally is made by Purkinje cells and acts as a potent mitogen for granule cell precursors, both in vitro and in vivo (1–3). The neurotrophins neurotrophin-3 and brain-derived neurotrophic factor (BDNF) are both essential for the survival of granule neurons in vivo (4, 5). Furthermore, several growth factors originally identified for their activity outside of the nervous system, such as epidermal growth factor, basic fibroblast growth factor, and insulin-like growth factor-1 (IGF-1), have been reported to stimulate proliferation and/or survival of granule cell precursors in vitro (6–8). Among these factors is also hepatocyte growth factor (HGF), which has been shown to protect cultured rat cerebellar neurons from apoptotic cell death (9).
Several studies (10–12) have shown that HGF and its receptor Met, better known for contributing to the formation of placenta, liver, and muscle during embryogenesis, also participate in neuronal cell development (13). In this work we wanted to establish whether the HGF/Met pair plays a role in cerebellar development and function. Early studies localized HGF protein to Purkinje cells and met mRNA to granule cells of the cerebellum (14). We first reassessed Met expression in postnatal cerebellum and verified the response of cerebellar granule cells to HGF in vitro. To extend our analysis to an in vivo model, we then produced a viable partial loss-of-function Met mutant. This mutant was obtained by knocking in the met locus a point mutation that interferes with binding of the Grb2 adapter to the receptor, and thus impairs its ability to activate the Ras/microtubule-associated protein (MAP)-kinase cascade (15–17).
We found that Met is expressed in proliferating cells of the external granule layer (EGL) of the cerebellum and that primary cultures of granule cells respond to HGF with an increase in proliferation. In the mutant with a partial loss of Met function the cerebellum was smaller than in controls and showed abnormal foliation. Furthermore, EGL granule cells proliferation was reduced, and a test for cerebellar function indicated a balance impairment. We conclude that HGF and Met are necessary to promote proliferation of granule cell precursors during postnatal development and may be involved in mediating cerebellar function.
Methods
Immunofluorescence.
Cerebella were removed from the skulls, embedded in OCT, and rapidly fresh-frozen in isopentane. Sections (15 μm) were fixed in −20°C methanol and saturated in PBS with 5% goat serum, 0.1% Tween. Primary antibodies were added at a dilution of 1:300 for anti-mouse Met (Santa Cruz Biotechnology, rabbit polyclonal) and for anti-proliferating cell nuclear antigen (Santa Cruz Biotechnology, mouse monoclonal), and incubated overnight at 4°C. After washing, Cy3 secondary anti-rabbit antibody (1:1,200, Roche Molecular Biochemicals) or FITC secondary anti-mouse antibody (1:50, Roche Molecular Biochemicals) were added and incubated for 1 h at room temperature.
Western Blot.
Postnatal day (P) 8 cerebella or embryonic day (E) 13.5 embryos were homogenized and lysed in ice-cold RIPA buffer (0.15 mM NaCl/0.05 mM Tris⋅HCl, pH 7.2/1% Triton X-100/1% sodium deoxycholate/0.1% SDS) with Sigma Protease Inhibitor Mixture. Primary cerebellar granule cells were lysed in ice-cold EB buffer (1% Triton/10 mM Tris⋅HCl, pH 7.5/150 mM NaCl/5 mM EDTA/10% glycerol) with Sigma Protease Inhibitor Mixture. Protein concentration was determined by the BioRad DC protein assay and equal amounts of protein were separated on SDS/PAGE. Proteins were transferred to a poly(vinylidene difluoride) membrane (Amersham Pharmacia) followed by blocking in 5% low-fat milk. Membranes were incubated with anti-mouse Met or anti-human Met (1:10,00, Santa Cruz Biotechnology, rabbit polyclonal), anti-phospho-MAP-kinase (1:10,000, Sigma, mouse monoclonal), or anti-actin (1:50,00, Sigma, mouse monoclonal) antibodies overnight at 4°C. After washing, filters were incubated with peroxidase-conjugated secondary anti-mouse or anti-rabbit antibody (1:4,000, Amersham Pharmacia). The signal was revealed by ECL (Amersham Pharmacia) or Supersignal (Pierce).
Dissociated Cerebellar Cell Cultures.
Cerebella were removed from P6 mice, and granule cell cultures were prepared as described in ref. 9. Cells were cultured in serum-containing medium (Basal Medium Eagle supplemented with 10% FCS/25 mM KCl/1 g/liter glucose/2 mM L-glutamine/100 units/ml penicillin/100 units/ml streptomycin) for biochemical analysis, or serum-free medium without KCl for proliferation analysis. Cells were seeded into 35-mm dishes or 96-multiwell plates coated with poly-D-lysine.
BrdUrd Incorporation in Vitro.
Cerebellar cells isolated as described above were resuspended in serum-free medium and transferred to poly-D-lysine-coated 96-well plates, at a density of 75,000 cells per well. Growth factors were added immediately, and cells were cultured for 46 h. Cells were pulsed with BrdUrd at 24 h of culture. The amount of BrdUrd incorporation was quantified by using the Cell Proliferation kit (Roche Diagnostics) according to the manufacturer's recommendations.
Generation of Met Mutant Mice.
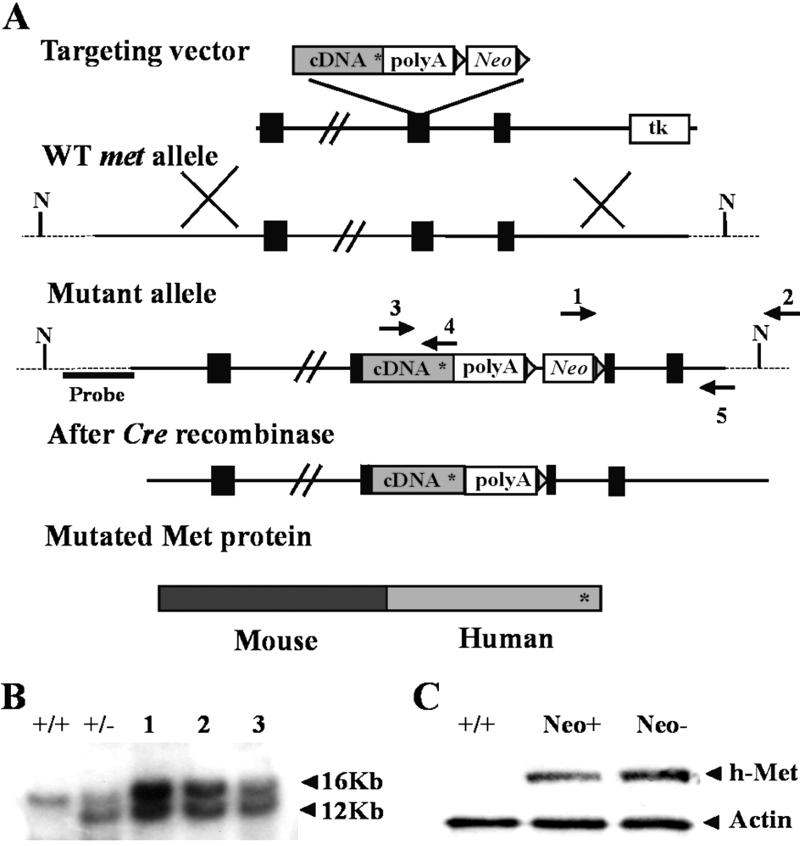
The targeting vector was as described in ref. 12, except that the Neo cassette was flanked by LoxP sites for Cre-mediated excision in vivo (18). Cell culture, electroporation of R1 embryonic stem (ES) cells, and selection with G418 and GANCR were done as described (12). Pools of six individual G418R ES cell clones were tested for homologous recombination by PCR by using primer 1–2 (see Fig. 2). For Southern blot analysis, DNA (20 μg) was digested with NdeI, electrophoresed on a 0.6% agarose gel, and blotted on nylon membranes. Blots were hybridized with a 32P-labeled 1.2-kb probe deriving from genomic sequences located upstream of the 5′ end of the targeting vector (see Fig. 2). Double-selected recombinant ES cells were morula-aggregated in an external facility, and three chimeric animals were obtained, all capable of transmitting the recombinant gene. Mice were genotyped by PCR using primers 3 and 4. Removal of the Neo cassette, after crossing with the Cre Deleter (18), was assessed by PCR using primer 1 in the Neo cassette and primer 5 (see Fig. 2). Neo− mutants were crossed with SV129 mice. Mutants free from Cre recombinase were selected by PCR. Mice used in this work were obtained by successive backcrossing of the F2 generation.
Fig 2.
Knock-in of YVNV→YVHV signaling mutation (Metgrb2) in the met locus. (A) Schematic representation of the knock-in strategy. Black boxes represent exons. N indicates NdeI sites. The Neo cassette was flanked by LoxP sites (triangles). Asterisk indicates the location of the mutation. Arrows indicate primers used for screening of ES (1 and 2) and Neo− alleles (1 and 5), and for genotyping mice heterozygous for the Metgrb2 mutation (3 and 4). (B) Southern blot of NdeI digests of genomic DNA isolated from double-selected R1 ES cell clones [+/+, WT ES; +/−, control recombinant ES cells used for production of first-generation Metgrb2 mice (see text); 1–3, recombinant ES clones]. (C) Western blots of total protein extracts from E13.5 WT, Metgrb2/grb2neo+, and Metgrb2/grb2neo− embryos. h-Met, antibody specific for human Met; Actin, anti-actin (control for protein loading).
Cresyl Violet Staining.
Brains were dissected, fixed for 24 h at 4°C in PBS containing 4% paraformaldehyde, dehydrated, and embedded in paraffin. Sections (6–8 μm) were cut and stained with cresyl violet.
Immunohistochemistry: BrdUrd Incorporation in Vivo.
BrdUrd was injected i.p. 2 or 72 h before death. Brains were dissected and fixed in 4% paraformaldehyde overnight, dehydrated, and embedded in paraffin. Sections (6–8 μm thick) were rehydrated, microwaved for 15 min in 10 mM citrate buffer, pH 6, and treated with 2 M HCl at room temperature for 1 h. They were then saturated in PBS with 5% goat and 5% sheep serum, 0.5% Triton for 30 min, and finally incubated with an anti-BrdUrd mAb (1:50, Roche Molecular Biochemicals) overnight at 4°C. For calbindin staining sections were incubated with anti-calbidin polyclonal antibody (1:3,000, Swant, Bellinzona, Switzerland) overnight at 4°C. For both stains, after extensive washing, sections were incubated with biotinylated goat anti-mouse or anti-rabbit IgG (1:800, Roche Molecular Biochemicals) for 1 h, washed again, and finally incubated with peroxidase-conjugated streptavidin. Staining was visualized with diaminobenzidine (Sigma).
Thin Rod Test.
The stationary horizontal thin rod test was performed as described in ref. 19 on age- and sex-matched animals.
Results
Met Receptor Expression in the Cerebellum.
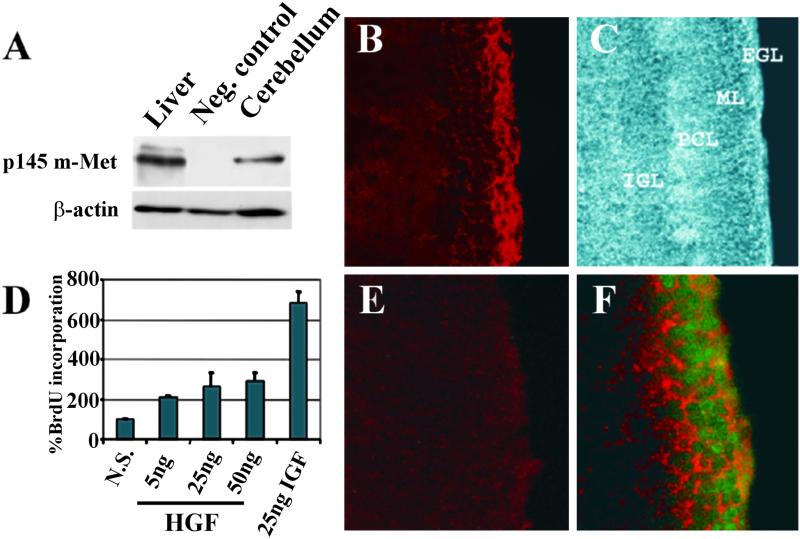
Met and HGF are expressed in the adult cerebellum (14). To determine whether they could play a role during cerebellar development we first examined Met expression at P8, at the peak of granule cell proliferation. Western blots were done on extracts of cerebellum, with liver as a positive control for Met expression. Met was present in both tissues, visualized as a band migrating at ≈145 kDa (the Met β-chain) (Fig. 1A). Its localization was assessed by immunofluorescence. In cerebellar sections from P8 mice, Met was localized in the EGL (Fig. 1 B and C). Double immunofluorescence with antibodies against Met and proliferating cell nuclear antigen revealed colocalization of the two proteins (Fig. 1F). The finding that Met is expressed in proliferating granule cells suggests a role for the HGF/Met pair in cerebellar development.
Fig 1.
Expression and localization of Met in the cerebellum, and proliferative response of granule cell cultures to HGF. (A) Western blot of mouse cerebellum and liver extracts at P8 (p145 m-Met: mouse Met, specifically detected by anti-mouse Met antibody). Neg. control: cerebellum from genetically modified mice expressing a mouse-human Met-chimera (see Fig. 2). (B) Anti-m-Met immunostaining revealing expression in the EGL. (C) Phase contrast showing cerebellar morphology. (E) Pretreatment of the antibody with cognate peptide. (F) Double staining with anti-Met (red) and anti-proliferating cell nuclear antigen (green) showing coexpression. (B, C, and E, ×20; F, ×40.) (D) BrdUrd incorporation in HGF- or IGF-1-treated cerebellar granule cell cultures from P6 mice. Three experiments were performed in quadruplicate and values are shown as mean ± SEM.
HGF Is a Mitogen for Granule Cells in Vitro.
We used primary cultures of cerebellar cells to test whether HGF was capable of stimulating granule cell proliferation in vitro. Cells derived from the cerebellum of P6 mice were incubated for 24 h with or without different amounts of HGF (5–50 ng/ml) and pulsed for 22 h with BrdUrd. IGF-I was used as a positive control. Incorporation of BrdUrd was quantified by an ELISA colorimetric method. As shown in Fig. 1D, HGF caused a 2- to 3-fold increase in proliferation, whereas the effect of IGF-I was more potent. Combined addition of HGF and IGF-1 did not result in a synergistic effect (not shown).
Generation of Partial Loss-of-Function Met Mutant Mice by Homologous Recombination.
Having confirmed that Met is expressed by proliferating cells in postnatal cerebellum at a stage critical for its development, and that granule cells respond to HGF in vitro with increased proliferation, we next wanted to verify these observations in an in vivo model. Both the Met and HGF knockouts are embryonal lethal (10–12), thus we aimed at obtaining a viable hypomorphic mutant by means of a partial loss of Met function. To this end we produced a different version of a targeting vector described in ref. 12, previously used to generate by homologous recombination a knock-in mouse with a point mutation in the Met Grb2 binding site (Metgrb2: Y1356VNV→Y1356VHV). In both the original and our version of the targeting vector, a mutated human MET cDNA fragment coding for the transmembrane and cytoplasmic domain of the receptor, followed by the Neo cassette, was inserted in-frame in a mouse genomic clone (Fig. 2A). In the “first-generation” Metgrb2 mice the Neo cassette remained in the recombinant locus. These mutants were perinatal lethal when homozygous, with rare individuals surviving into adulthood (12). Subsequent work has shown that retention of Neo in the recombinant locus causes a decreased level of mutant Met receptor in some tissues (20). Thus in our version of the targeting vector, LoxP sites were introduced to flank the Neo cassette (Fig. 2A) to allow its excision on crossing of Metgrb2neo+ mice with the Cre Deleter strain (18). Double-selected ES cells were screened by PCR and Southern blot analysis (Fig. 2B). Recombinant ES cells were morula-aggregated to produce chimeric mice. Three chimerae gave germ-line transmission of the recombinant locus. Heterozygous mice were crossed with the Cre Deleter strain (in the SV129 genetic background) for excision of the Neo cassette. Mice without Neo were identified by PCR and crossed with wild-type (WT) SV129 mice. Heterozygous Metgrb2neo− mice without the Cre Deleter gene were selected by PCR.
Homozygous Metgrb2/grb2neo+ mice showed a phenotype similar to first-generation mutants. At birth they were easily distinguishable from littermates because of their hyperflexed forelimbs (due to reduction of extensor muscles; ref. 12), and they died in a few hours. After Neo removal, homozygous Metgrb2/grb2neo− mice had normal forelimbs and were viable. To compare the amount of Met protein in the Metgrb2/grb2neo+ and Metgrb2/grb2neo− mutants, protein extracts of E13.5 embryos were analyzed by Western blot by using an antibody specific for the carboxyl terminus of human Met. Fig. 2C shows that the level of Met receptor was higher in the Metgrb2/grb2neo− embryos, which suggests that the increase in expression of the mutated Met receptor after Neo excision is most likely responsible for the rescue of viability of Metgrb2/grb2neo− mice and indicates that, in the presence of an adequate amount of receptor, the net effect of the mutation (if any) must be very subtle.
Abnormal Foliation in Cerebellum of Metgrb2/grb2neo− Mutants.
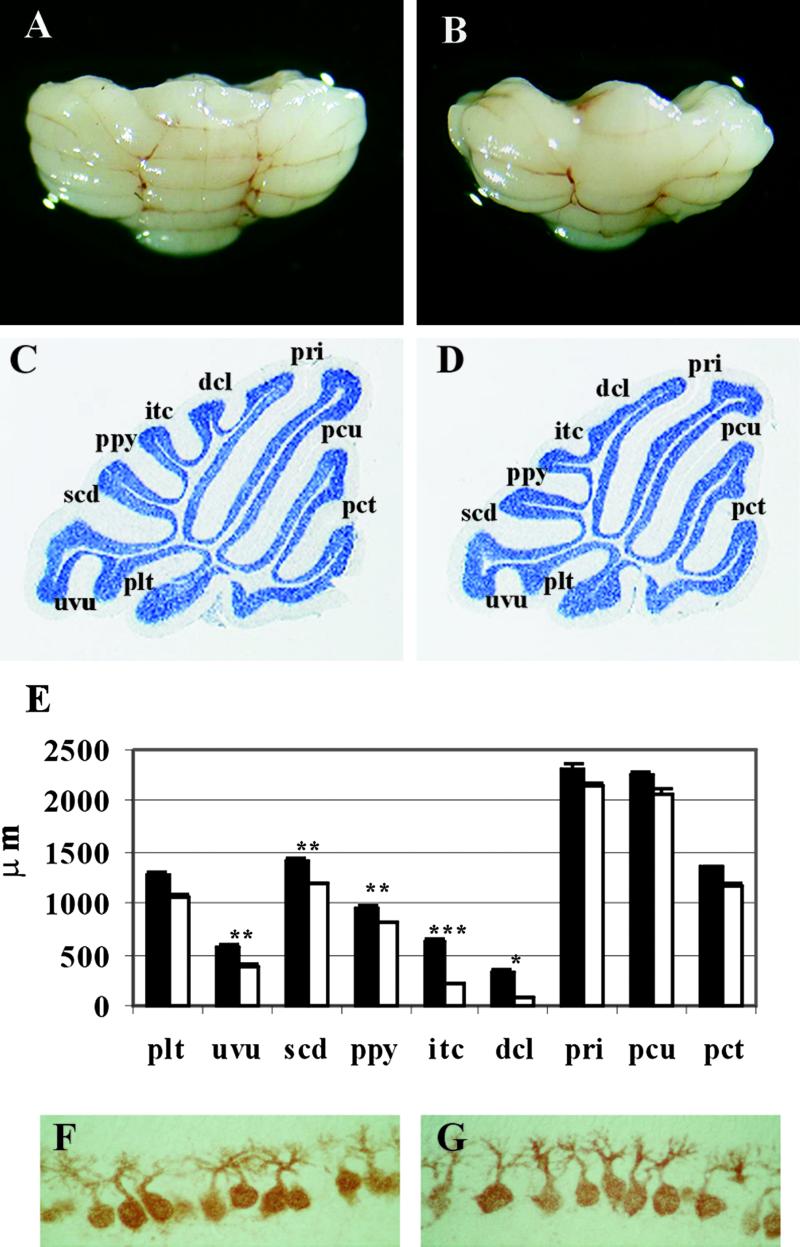
All members of the Met family, Met, Ron, and Sea, share the sequence YVNV in their multidocking site (21). The conservation of the optimal motif for Grb2 binding (YXNX) suggests that a direct link with Grb2 is essential for proper functioning of these receptors. For this reason, although Metgrb2/grb2neo− mice lacked obvious defects, we felt that these mutants deserved to be analyzed in depth, searching for a phenotype which, although weak, could still be informative. The cerebellum of the Metgrb2/grb2neo− mutants was first examined morphologically. Its appearance and organization was compared with that of WT littermates at P23, when development is complete. The cerebellum of mutant mice was significantly smaller than that of controls (Fig. 3 A and B). Cerebellar weight was reduced by ≈20%, whereas no statistically significant difference was found in brain weight (not shown).
Fig 3.
Macroscopic analysis of Metgrb2/grb2neo− cerebellum. (A and B) Cerebellum from WT (A) and Metgrb2/grb2neo− mutant (B) at P23. (C and D) Sagittal sections of cerebellum from P23 WT (C) and mutant mice (D) stained with cresyl violet (×1.6). Cerebellar fissures and sulci: plt, posteriolateral; uvu, uvular; scd, secondary; ppy, prepyramidal; itc, intercrural; dcl, declival; pri, primary; pcu, preculminate; pct, precentral. (E) Fissure depth was analyzed by using the image pro plus system (Media Cybernetics, Silver Spring, MD). Six mutants and five controls were analyzed. Values are shown as mean ± SEM. t test: *, P < 0.02; **, P < 0.01; ***, P < 0.001. (F and G) Calbindin staining of Purkinje cells at P8 in control (F) and Metgrb2/grb2neo− mice (×40).
Histological analysis showed that in Metgrb2/grb2neo− mutants the layers of the cerebellum formed normally, with the appropriate cells types. However, the mutants, compared with their WT littermates, displayed abnormalities in foliation. By P23, WT animals develop a mature foliation pattern within the vermis, with the characteristic folia separated by well-defined fissures (Fig. 3C). In Metgrb2/grb2neo− mutants the fissures in the anterior portion of the cerebellum were normal in size, whereas defects were evident in the median and posterior portions of the cerebellum. The declival sulcus (dcl) and the intercrural fissure (itc) were reduced or absent, whereas the secondary fissure (scd) and the posterolateral (plt) fissure were reduced to a lesser extent (Fig. 3D). Quantitative analysis confirmed that the decrease in depth of each of these fissures in mutant animals at P23 was statistically significant (Fig. 3E). Because recent findings on the cerebellar-deficient folia (cdf) mouse mutant show that abnormal foliation may be caused by a defect intrinsic to a subset of Purkinje cells (resulting in their ectopic placement) rather than to granule cells (22, 23), we verified their location and morphology in the Metgrb2/grb2neo− mutant. Purkinje cells in the mutant mice were correctly positioned and were indistinguishable from controls (Fig. 3 F and G).
Cerebellar Granule Cell Proliferation Is Decreased in Metgrb2/grb2neo− Mutants.
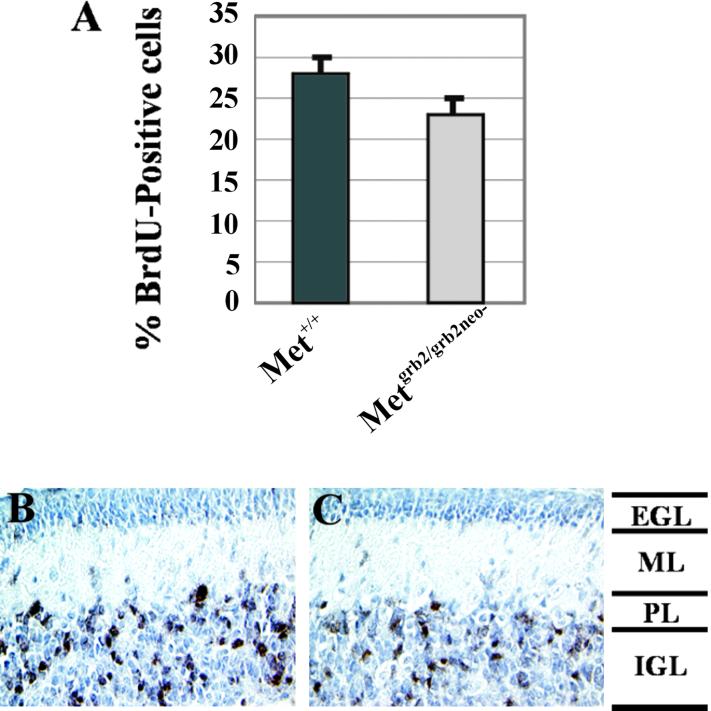
During the early postnatal period cells in the EGL undergo extensive proliferation and generate a large pool of granule cell precursors. To investigate whether HGF affects proliferation of these cells, BrdUrd incorporation, a marker for DNA synthesis, was analyzed in vivo in Metgrb2/grb2neo− mutants. BrdUrd was injected i.p. in P8 or P7 mice, and the animals were killed 2 or 72 h later. The cerebellum was dissected and prepared for histological analysis. The proliferative index in the EGL was determined by counting BrdUrd-positive versus total cells in sections of mutant and control mice killed 2 h after injection. In the mutant mice proliferation was ≈25% reduced with respect to controls (Fig. 4A). Similar results were obtained by evaluating BrdUrd incorporation 72 h after injection (not shown). Analysis at this later time also showed that in the mutants no delay occurred in migration of BrdUrd-positive cells. In fact, BrdUrd-labeled cells were all found in the internal granule layer rather than in the molecular layer, in the mutants as in the controls (Fig. 4 B and C). The reduction in granule cell proliferation was similar throughout the cerebellum, with no significant differences in the areas more heavily affected morphologically.
Fig 4.
BrdUrd incorporation in the cerebellum of Metgrb2/grb2neo− mutants. (A) P8 mice were injected with BrdUrd and killed 2 h later. Sections were stained by immunohistochemistry using anti-BrdUrd antibodies. BrdUrd-positive cells were counted in at least six regions of the EGL (3,000–4,000 cells per cerebellum). Five mutant and three control mice were analyzed and values are shown as mean ± SD. t test: P < 0.0001. (B and C) P7 mice were injected with BrdUrd and killed 72 h later (×20). Black, BrdUrd-positive cells; light blue, hematoxylin-stained nuclei; ML, molecular layer; PL, Purkinje layer; IGL, internal granule layer.
HGF acts as a survival factor for granule cells in vitro (9). To assess whether in Metgrb2/grb2neo− mice granule cell survival was impaired, parasagittal sections of P8 cerebella from mutant and control mice were terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL)-stained. Quantitative analysis of total cell death did not reveal any significant difference between the number of apoptotic cells in mutant and control mice (56 ± 18.3 versus 57.3 ± 13.8 TUNEL-positive cells per section).
These data altogether indicate that HGF induces proliferation in granule cell precursors and suggest that direct recruitment of Grb2 by the Met receptor is important for a full proliferative response in the EGL. Conversely, granule cell migration and survival are either HGF independent or do not require full Met signaling.
HGF-Induced MAP-Kinase Activation Is Impaired in Cerebellar Granule Cells from Metgrb2/grb2neo− Mutants.
The Grb2 adapter is recruited to tyrosine kinase receptors through its Src homology 2 domain and binds the Ras guanine nucleotide exchanger Sos by way of the N-terminal Src homology 2 domain (24). Thus receptors with a Grb2-binding site can activate the Ras/MAP-kinase cascade directly. It has been shown in transfected fibroblasts that uncoupling Met from Grb2 lowers the HGF-dependent MAP-kinase response, and that full activation of the Ras pathway is necessary to promote cell proliferation (15, 17).
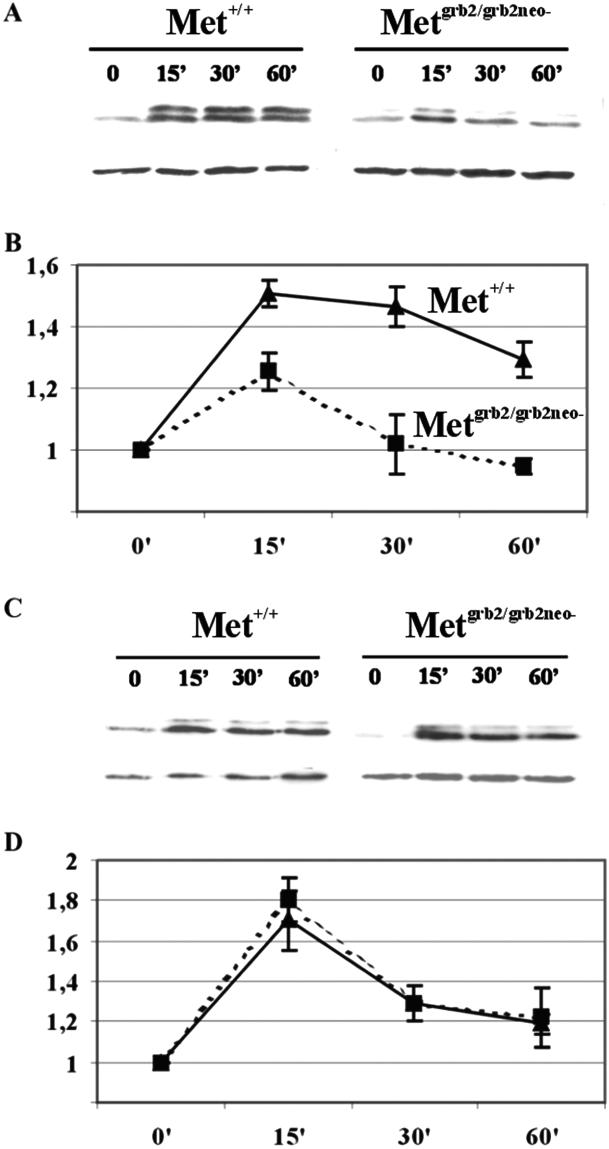
To investigate whether in cerebellar granule cells from Metgrb2/grb2neo− mice the Ras/MAP-kinase cascade is impaired, primary granule cell cultures from P6–P8 mutant and WT mice were treated with HGF or BDNF, used as control, for different times. Activation of the Ras pathway was assessed by evaluating phosphorylation of the downstream effector MAP kinase. As shown in Fig. 5A, HGF-induced MAP-kinase phosphorylation was weaker in mutant granules than in control cells. Densitometric analysis of the blots showed that in WT cerebellar cultures MAP kinase activity peaked 15 min after stimulation, and was sustained for more than an hour. In granule cells obtained from Metgrb2/grb2neo− mutants, the peak at 15 min was reduced and MAP-kinase activation was transient. Conversely, the pattern of BDNF-induced MAP-kinase phosphorylation was the same in WT and Metgrb2/grb2neo− granule cells (Fig. 5 C and D). These data show that in primary cultures of mutant granule cells HGF-mediated activation of the Ras/MAP-kinase cascade is decreased. Because this effect is specific for HGF stimulation it must be a consequence of the mutated Met protein and is likely to be caused by the loss of the Grb2-binding site.
Fig 5.
HGF-dependent MAP-kinase phosphorylation in Metgrb2/grb2neo−. (A and C) Representative Western blots of extracts of cerebellar granule cell cultures from P6 WT and mutant mice, stimulated with HGF (20 ng/ml) (A) or BDNF (100 ng/ml) (C) for the indicated times. Blots were probed with antiphospho-MAP kinase, stripped, and reprobed with an antibody against β-actin for normalization. (B and D) Densitometric analysis of the Western blots. Scanned films were analyzed with the IMAGE MASTER TOTAL LAB V100 program (Amersham Pharmacia) and plotted with Microsoft EXCEL. Values were obtained from eight different experiments and are presented as mean ± SEM.
Impaired Cerebellar Function in Metgrb2/grb2neo− Mutants.
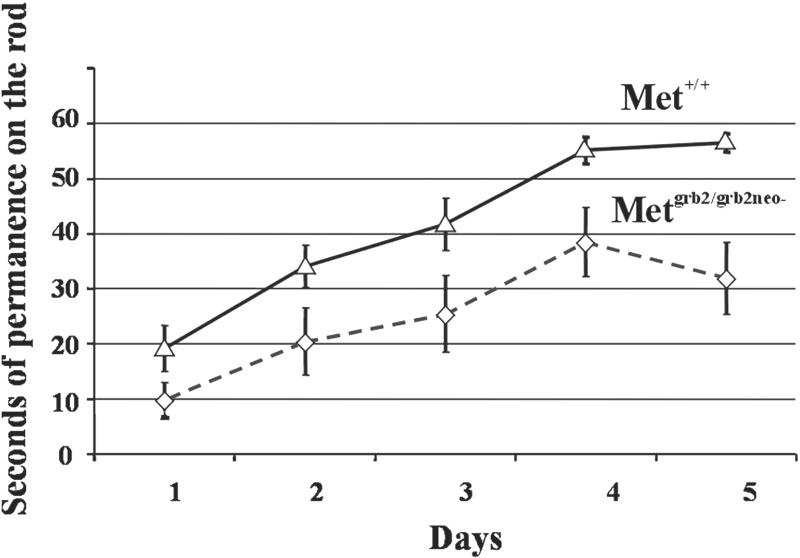
Metgrb2/grb2neo− mutants and WT littermates were indistinguishable with respect to behavior when observed in their normal cage environment. However, subtle defects in balance control can emerge if animals are subjected to tests such as the thin-rod horizontal stationary test (19). In this test mice are put on a suspended rod and the time of permanence on it is measured. Three consecutive trials were performed for 5 days. WT and Metgrb2/grb2neo− mutants showed clear differences in performance (Fig. 6). However, although the average permanence time was reduced in Metgrb2/grb2neo− mutant mice, the slope of the learning curve was comparable, suggesting no major defects in cerebellar learning.
Fig 6.
Cerebellar function in Metgrb2/grb2neo− mutant mice. WT and mutant mice were tested for equilibrium and coordination by the thin-rod test. The time spent on the rod up to a limit of 60 s was measured. Nine animals for each genotype were tested in a total of 15 sessions. Values are shown as mean ± SEM.
Discussion
Cerebellar development involves a proliferative and a migratory wave during which granule cell precursors actively divide and subsequently, when postmitotic, migrate inward to their final destination. Some of the molecules that regulate these and other events, such as protection from apoptotic death, which occurs to eliminate supranumerary cells, are just beginning to be identified. Among these molecules are a number of neurotrophins and growth factors, such as neurotrophin-3, BDNF, epidermal growth factor, IGF-1, and basic fibroblast growth factor, which bind to receptors of the tyrosine kinase superfamily. The pattern of expression and biological activity of another member of this family, the Met receptor, which in response to HGF controls proliferation and/or migration of a vast array of cell types (25), suggest that this ligand–receptor pair could contribute to cerebellar development as well.
In this work we first confirmed that Met is indeed expressed by granule cell precursors in the EGL and that cultured granule cells respond to HGF. Previous work by others had shown that HGF prevents apoptotic death in cultured cerebellar granule (9). Because several other growth factors, such as epidermal growth factor, IGF-I, and Shh, can induce granule cell proliferation in vitro (1–3, 6, 7), we focused on the proliferative response. Addition of HGF to primary granule cell cultures caused a 2- to 3-fold increase in the number of BrdUrd-positive cells. In our experimental conditions the proliferative effect of HGF was significant, but relatively small compared with that observed with IGF-1. In other systems, such as cultures of motor or sensory neurons, HGF requires the presence of ciliary neurotrophic factor or nerve growth factor to efficiently promote survival (26, 27). In our cultures we tried combining it with IGF-1, without any synergistic effect. This result leaves open the possibility that in vivo, where HGF could act in combination with other factors, its effects on granule cells could be more dramatic.
In vitro experiments may be highly suggestive, but unequivocal proof for function comes only from in vivo studies. Loss-of-function models for growth factors or their receptors are frequently lethal in homozygosity, and lethality poses a problem when, as in the cerebellum, the events under study occur postnatally. The HGF and Met knockouts are lethal in midgestation and thus uninformative in terms of cerebellar development. A knock-in mouse with a point mutation in the Met receptor that impairs signal transduction through the Ras/MAP-kinase pathway (Metgrb2) was described (12). This study also included a knock-in expressing the WT receptor as a control (Metwt). Although Metwt/wt mice were normal, Metgrb2/grb2 mice had a muscle phenotype (affecting limbs, tongue, and diaphragm) milder than the knockouts, but still perinatal lethal (12). Occasionally a few Metgrb2/grb2 mice developed into adults, suggesting that this model was borderline for survival. We later showed that retention of the Neo cassette in the met locus (as in these genetically modified mice) results in reduced level of Met protein in some tissues (20). The lack of phenotype in Metwt/wt mice suggests that reduced level is without effect in the presence of a normal Met receptor. However, such reduction could well “sensitize” the system, exacerbating the effect of a signaling mutation. In the present work we describe a “second-generation” Metgrb2/grb2neo− mutant, obtained by removing the Neo cassette by Cre recombinase. In Metgrb2/grb2neo− embryos the level of Metgrb2 protein was indeed higher than in Metgrb2/grb2neo+ embryos, which was sufficient to rescue their muscle phenotype and viability, thus allowing analysis of postnatal cerebellar development.
Morphological analysis showed that the cerebellum of Metgrb2/grb2neo− mice was small and with an abnormal pattern of foliation. The foliation defect was much more evident in the middle and posterior regions of the cerebellum. Previous work has shown a relation between foliation and changes in granule cell proliferation and/or viability. Hyperproliferation of granule cells in transgenic mice overexpressing Zipro1 or IGF-I results in excess foliation (28, 29), whereas enhanced apoptosis of granule neurons in BDNF or neurotrophin-3 mutant mice results in foliation defects similar to those observed in the Metgrb2/grb2neo− mutant (4, 5). We thus asked whether proliferation and/or apoptosis were altered in the cerebellum of Metgrb2/grb2neo− mice. Our results showed that in these mutants abnormal foliation is associated with a 25% reduction of granule cell precursors proliferation in the EGL.
In our mutants, altered cerebellar foliation seems not to be caused by an increase in cell death, because quantification of apoptotic cells by TUNEL staining did not reveal significant differences compared with WT mice. A previous report showed that in vitro HGF-induced survival of granule neurons depends on phosphatidylinositol 3-kinase rather than on MAP-kinase activation (9).
It has been shown that HGF may have a role as motogenic factor for interneurons of the cerebral cortex. In fact, in u-PAR (urokinase-type plasminogen activator receptor) null mice, which have a low level of Met and HGF expression, the migration of interneurons is altered (30). In tPA (tissue plasminogen activator) null mice, a delay occurs in granule cell migration during cerebellar development (31). Both u-PA and tPA are proteases that in vitro can cleave pro-HGF to the mature biologically active two-chain form. In our Metgrb2/grb2neo− mutant we did not observe any delay in granule cell migration. The same number of BrdUrd-positive cells were still traversing the molecular layer (en route toward the internal granule layer) 72 h after injection in the mutant and the WT control.
In the Metgrb2/grb2neo− mutant the Met receptor cannot recruit the Grb2 adaptor directly. Grb2 is associated with the Ras exchanger Sos, and thus allows the receptor to activate the Ras/MAP-kinase pathway. We provide biochemical evidence that in primary granule cell cultures obtained from Metgrb2/grb2neo− mice, MAP-kinase phosphorylation after HGF stimulation was lower and more transient than in controls. These data are consistent with those previously obtained in transfected cells showing that a direct link of Grb2 with Met is necessary to activate fully the Ras/MAP-kinase cascade and to promote proliferation (15–17). The decrease in MAP-kinase activation and/or its transient quality is likely to be responsible for the reduction of granule cell precursors proliferation observed in vivo. However, the MAP-kinase pathway may not be the only pathway whose response is impaired downstream of the Metgrb2/grb2neo− mutant, because Grb2 also contributes to linking Met with the large adapter protein Gab-1, which in turns amplifies the Met signal by activating several effectors (reviewed in ref. 32). Furthermore, because we did not produce a Metwt/wtneo− control we cannot formally exclude that the observed subtle defects could be caused by the fact that the recombinant Met receptor is a mouse/human chimera, working as an hypomorph independent of the Grb2 mutation.
Metgrb2/grb2neo− mutant mice were indistinguishable, with respect to behavior, from WT littermates when observed in the cage environment. However, we noticed that they had difficulties in complex exercises like swimming (not shown), and when tested in an exercise more specifically devised to assess cerebellar function such as the thin-rod test. This result suggests an altered control of balance and complex movements. We do not know if these defects are a consequence of the observed alterations in cerebellar development or whether they also indicate a role for HGF/Met in cerebellar function. Further studies, such as production of an inducible cerebellar-specific Met knockout are necessary to clarify this aspect.
In conclusion, our data, gathered from experiments on cerebellar cell cultures and a partial loss of function Met knock-in mouse, indicate that HGF is involved in postnatal development of the cerebellum. The role of HGF seems to be to promote proliferation of granule cell precursors in the EGL. Most likely the Met receptor mediates this effect by recruitment of Grb2, which is necessary to promote a full proliferative response. Conversely, a direct link of Met with Grb2 does not seem to be necessary to promote migration of granule cells in the internal granule layer, or their survival. However, we cannot exclude that HGF and Met contribute in vivo to migration and/or survival of granule cells. In fact, our Met mutant confers only a partial loss of function and the requirement for survival or migration may be fulfilled by its residual signaling potential.
Acknowledgments
This work is dedicated to the memory of Adolfo Boroli, whose family has supported A.I.'s Ph.D. fellowship. We thank Flavio Maina for providing the modified targeting vector, Miguel Torres for morula aggregations, Ferdinando Rossi for helpful discussion, and Bob Milne for revising the manuscript. The continuing support of the Compagnia di San Paolo and Fondazione Cassa di Risparmio di Torino to C.P.'s laboratory is gratefully acknowledged.
Abbreviations
Pn, postnatal day n
En, embryonic day n
MAP, microtubule-associated protein
HGF, hepatocyte growth factor
BDNF, brain-derived neurotrophic factor
IGF-1, insulin-like growth factor-1
EGL, external granule layer
ES, embryonic stem
TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Wechsler-Reya R. J. & Scott, M. P. (1999) Neuron 22, 103-114. [DOI] [PubMed] [Google Scholar]
- 2.Wallace V. A. (1999) Curr. Biol. 9, 445-448. [DOI] [PubMed] [Google Scholar]
- 3.Dahmane N. & Ruiz-i-Altaba, A. (1999) Development (Cambridge, U.K.) 126, 3089-3100. [DOI] [PubMed] [Google Scholar]
- 4.Bates B., Rios, B., Trumpp, A., Chen, C., Fan, G., Bishop, J. M. & Jaenisch, R. (1999) Nat. Neurosci. 2, 115-117. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz P. M, Borghesani, P. R., Levy, R. L., Pomeroy, S. L. & Segal, R. A. (1997) Neuron 19, 269-281. [DOI] [PubMed] [Google Scholar]
- 6.Yamada M., Ikeuchi, T. & Hatanaka, H. (1997) Prog. Neurobiol. 51, 1937. [DOI] [PubMed] [Google Scholar]
- 7.Tao Y., Black, I. B. & DiCicco-Bloom, E. (1996) J. Comp. Neurol. 376, 653-663. [DOI] [PubMed] [Google Scholar]
- 8.Lin X. & Bulleit, R. F. (1997) Dev. Brain Res. 99, 234-242. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L., Himi, T., Morita, I. & Murota, S. (2000) J. Neurosci. Res. 59, 489-496. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt C., Bladt, F., Goedecke, S., Brinkmann, V., Zschiesche, W., Sharpe, M., Gherardi, E. & Birchmeier, C. (1995) Nature 373, 699-702. [DOI] [PubMed] [Google Scholar]
- 11.Bladt F., Riethmacher, D., Isenmann, S., Aguzzi, A. & Birchmeier, C. (1995) Nature 376, 768-771. [DOI] [PubMed] [Google Scholar]
- 12.Maina F., Casagranda, F., Audero, E., Simeone, A., Comoglio, P. M., Klein, R. & Ponzetto, C. (1996) Cell 87, 531-542. [DOI] [PubMed] [Google Scholar]
- 13.Maina F. & Klein, R. (1999) Nat. Neurosci. 2, 213-217. [DOI] [PubMed] [Google Scholar]
- 14.Honda S., Kagoshima, M., Wanaka, A., Tohyama, M., Matsumoto, K. & Nakamura, T. (1995) Mol. Brain Res. 32, 197-210. [DOI] [PubMed] [Google Scholar]
- 15.Ponzetto C., Zhen, Z., Audero, E., Maina, F., Bardelli, A., Basile, M. L., Giordano, S., Narsimhan, R. & Comoglio, P. (1996) J. Biol. Chem. 271, 14119-14123. [DOI] [PubMed] [Google Scholar]
- 16.Besser D., Bardelli, A., Didichenko, S., Thelen, M., Comoglio, P. M., Ponzetto, C. & Nagamine, Y. (1997) Oncogene 14, 705-711. [DOI] [PubMed] [Google Scholar]
- 17.Fixman E. D., Fournier, T. M., Kamikura, D. M., Naujokas, M. A. & Park, M. (1996) J. Biol. Chem. 271, 13116-13122. [DOI] [PubMed] [Google Scholar]
- 18.Schwenk F., Baron, U. & Rajewsky, K. (1995) Nucleic Acids Res. 23, 5080-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Airaksinen M. S., Eilers, J., Garaschuk, O., Thoenen, H., Konnerth, A. & Meyer, M. (1997) Proc. Natl. Acad. Sci. USA 94, 1488-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maina F., Pante, G., Helmbacher, F., Andres, R., Porthin, A., Davies, A. M., Ponzetto, C. & Klein, R. (2001) Mol. Cell 7, 1293-1306. [DOI] [PubMed] [Google Scholar]
- 21.Ponzetto C., Bardelli, A., Zhen, Z., Maina, F., dalla Zonca, P., Giordano, S., Graziani, A., Panayotou, G. & Comoglio, P. M. (1994) Cell 77, 261-271. [DOI] [PubMed] [Google Scholar]
- 22.Beierach E., Park, C., Ackerman, S. L., Goldowitz, D. & Hawkes, R. (2001) J. Comp. Neurol. 436, 42-51. [PubMed] [Google Scholar]
- 23.Park C., Finger, J. H., Cooper, J. A. & Hackerman, S. L. (2002) Genesis 32, 32-41. [DOI] [PubMed] [Google Scholar]
- 24.Chardin P., Cussac, D., Maignan, S. & Ducruix, A. (1995) FEBS Lett. 369, 47-51. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto K. & Nakamura, T. (1996) J. Biochem. 119, 591-600. [DOI] [PubMed] [Google Scholar]
- 26.Davey F., Hilton, M. & Davies, A. M. (2000) Mol. Cell. Neurosci. 15, 79-87. [DOI] [PubMed] [Google Scholar]
- 27.Maina F., Hilton, M. C., Andres, R., Wyatt, S., Klein, R. & Davies, A. M. (1997) Genes Dev. 11, 3341-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang X. W., Wynder, C., Doughty, M. L. & Heintz, N. (1999) Nat. Genet. 22, 327-335. [DOI] [PubMed] [Google Scholar]
- 29.Ye P., Xing, Y., Dai, Z. & D'Ercole, A. J. (1996) Dev. Brain Res. 95, 44-54. [DOI] [PubMed] [Google Scholar]
- 30.Powell E. M., Mars, W. M. & Levitt, P. (2001) Neuron 30, 79-89. [DOI] [PubMed] [Google Scholar]
- 31.Seeds N. W., Basham, M. E. & Haffke, S. P. (1999) Proc. Natl. Acad. Sci. USA 96, 14118-14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furge K. A., Zhang, Y. W. & Vande Woude, G. F. (2000) Oncogene 19, 5582-5589. [DOI] [PubMed] [Google Scholar]