Abstract
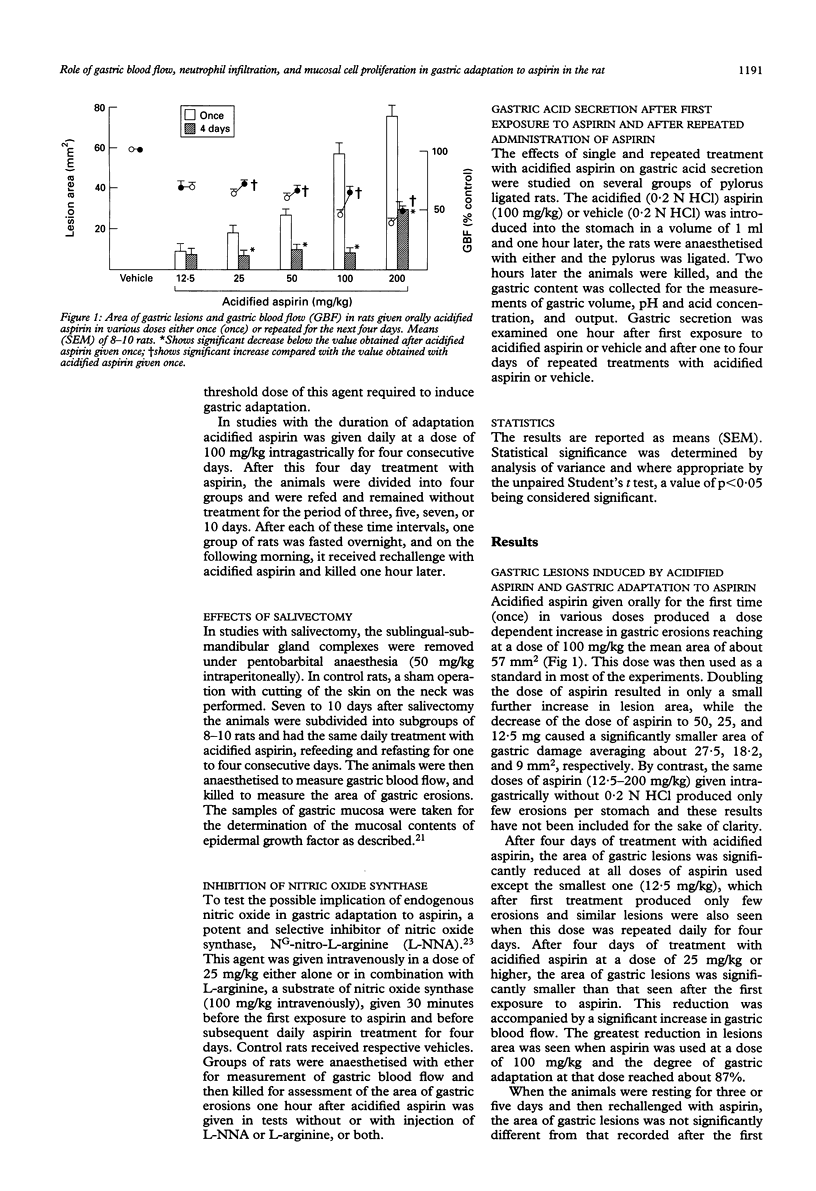
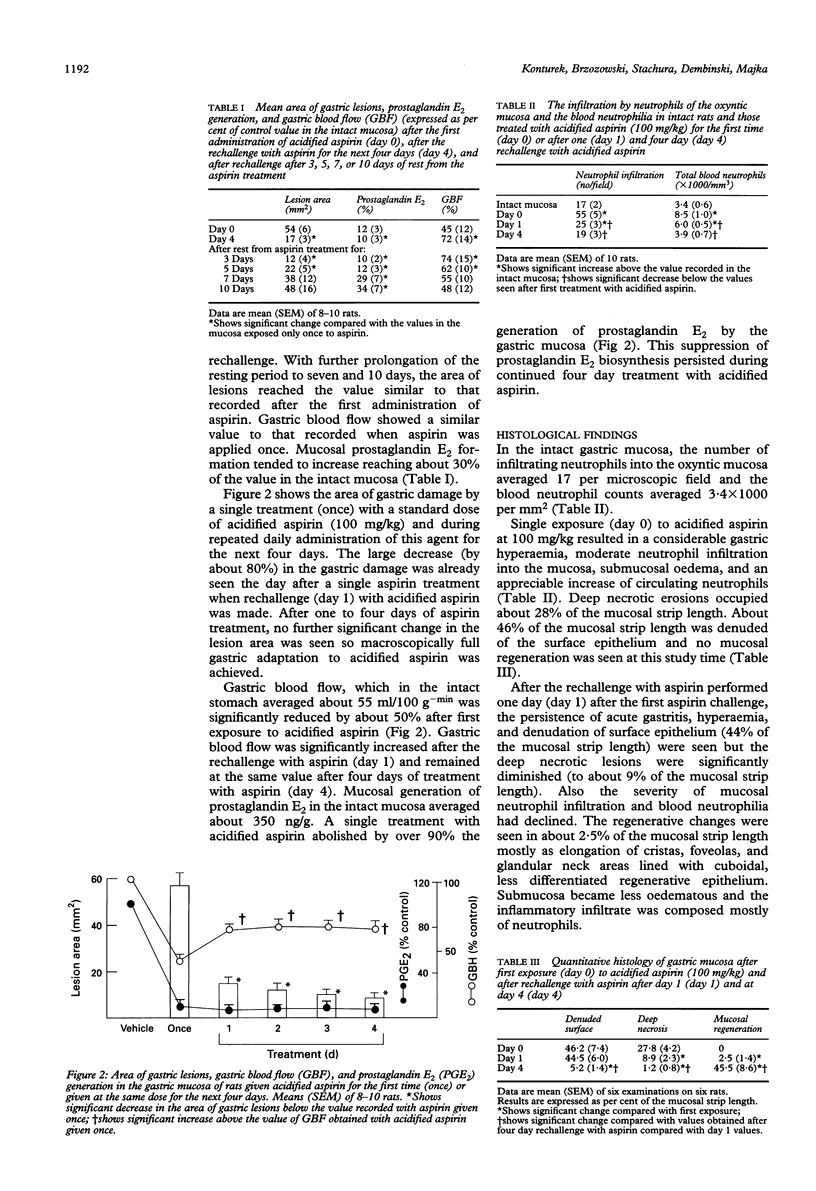
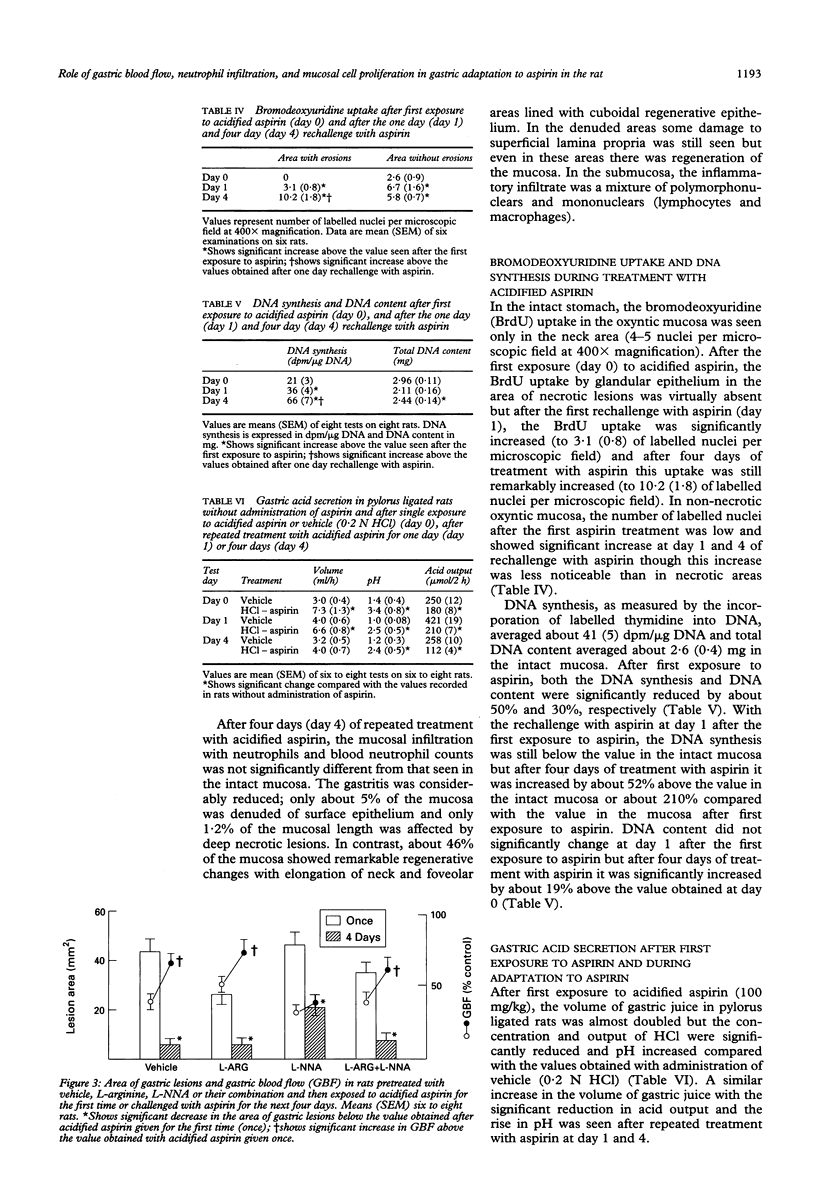
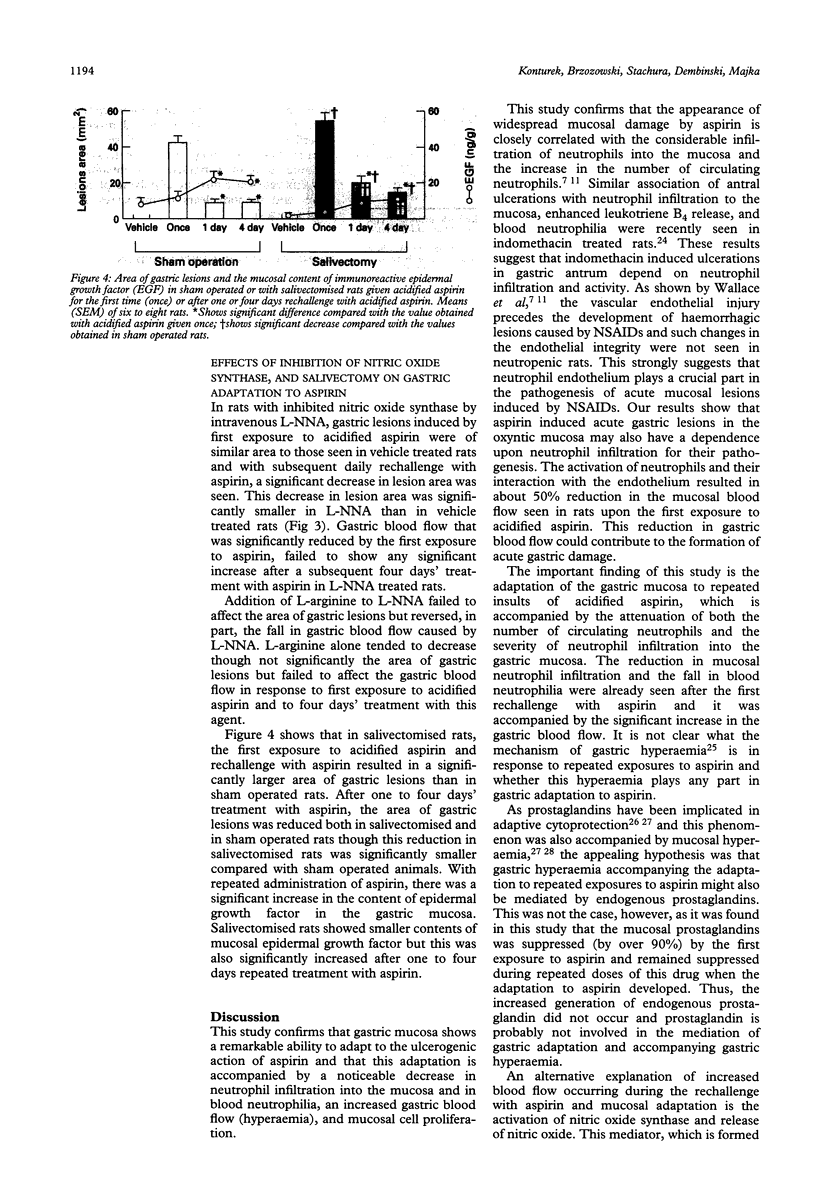
Gastric mucosa exhibits the ability to adapt to ulcerogenic action of aspirin but the mechanism of this phenomenon is unknown. In this study, acute gastric lesions were produced by single or repeated doses of acidified aspirin in rats with intact or resected salivary glands and with intact or suppressed synthase of nitric oxide. A single oral dose of aspirin produced a dose dependent increase in gastric lesions accompanied by considerable blood neutrophilia and mucosal neutrophil infiltration, significant reduction in gastric blood flow, and almost complete suppression of biosynthesis of prostaglandins. After rechallenge with aspirin, the mucosal damage became smaller and progressively declined with repeated aspirin insults. Gastric adaptation to aspirin was accompanied by a significant rise in gastric blood flow, reduction in both blood neutrophilia and mucosal neutrophil infiltration, and a remarkable increase in mucosal cell regeneration and mucosal content of epidermal growth factor. Salivectomy, which reduced the mucosal content of epidermal growth factor, aggravated the initial mucosal damage induced by the first exposure to acidified aspirin but did not prevent the adaptation of this mucosa to repeated aspirin insults. Pretreatment with NG-nitro-L-arginine (L-NNA), a specific inhibitor of nitric oxide synthase, eliminated the hyperaemic response to repeated aspirin but did not abolish the development of adaptation to aspirin showing that the maintenance of the gastric blood flow plays little part in this adaptation. In conclusion, the stomach adapts readily to repeated aspirin insults and this is accompanied by a considerable reduction in blood neutrophilia and the severity of neutrophil infiltration and by an extensive proliferation of mucosal cells possibly involving epidermal growth factor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgartner A., Koelz H. R., Halter F. Indomethacin and turnover of gastric mucosal cells in the rat. Am J Physiol. 1986 Jun;250(6 Pt 1):G830–G835. doi: 10.1152/ajpgi.1986.250.6.G830. [DOI] [PubMed] [Google Scholar]
- Dembinski A., Konturek S. J. Effects of E, F, and I series prostaglandins and analogues on growth of gastroduodenal mucosa and pancreas. Am J Physiol. 1985 Feb;248(2 Pt 1):G170–G175. doi: 10.1152/ajpgi.1985.248.2.G170. [DOI] [PubMed] [Google Scholar]
- Eastwood G. L., Quimby G. F. Effect of chronic aspirin ingestion on epithelial proliferation in rat fundus, antrum, and duodenum. Gastroenterology. 1982 May;82(5 Pt 1):852–856. [PubMed] [Google Scholar]
- Endoh K., Kao J., Domek M. J., Leung F. W. Mechanism of gastric hyperemia induced by intragastric hypertonic saline in rats. Gastroenterology. 1993 Jan;104(1):114–121. doi: 10.1016/0016-5085(93)90842-z. [DOI] [PubMed] [Google Scholar]
- Fries J. F., Miller S. R., Spitz P. W., Williams C. A., Hubert H. B., Bloch D. A. Identification of patients at risk for gastropathy associated with NSAID use. J Rheumatol Suppl. 1990 Feb;20:12–19. [PubMed] [Google Scholar]
- Gana T. J., Huhlewych R., Koo J. Focal gastric mucosal blood flow in aspirin-induced ulceration. Ann Surg. 1987 Apr;205(4):399–403. doi: 10.1097/00000658-198704000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. Y., Smith J. L., Dobbs S. M. Gastric adaptation occurs with aspirin administration in man. Dig Dis Sci. 1983 Jan;28(1):1–6. doi: 10.1007/BF01393353. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Smith J. L., Spjut H. J., Torres E. Gastric adaptation. Studies in humans during continuous aspirin administration. Gastroenterology. 1988 Aug;95(2):327–333. [PubMed] [Google Scholar]
- Kitahora T., Guth P. H. Effect of aspirin plus hydrochloric acid on the gastric mucosal microcirculation. Gastroenterology. 1987 Oct;93(4):810–817. doi: 10.1016/0016-5085(87)90444-6. [DOI] [PubMed] [Google Scholar]
- Konturek P. K., Brzozowski T., Konturek S. J., Dembiński A. Role of epidermal growth factor, prostaglandin, and sulfhydryls in stress-induced gastric lesions. Gastroenterology. 1990 Dec;99(6):1607–1615. doi: 10.1016/0016-5085(90)90464-c. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Brzozowski T., Dembinski A., Warzecha Z., Konturek P. K., Yanaihara N. Interaction of growth hormone-releasing factor and somatostatin on ulcer healing and mucosal growth in rats: role of gastrin and epidermal growth factor. Digestion. 1988;41(3):121–128. doi: 10.1159/000199763. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Brzozowski T., Majka J., Dembinski A., Slomiany A., Slomiany B. L. Transforming growth factor alpha and epidermal growth factor in protection and healing of gastric mucosal injury. Scand J Gastroenterol. 1992 Aug;27(8):649–655. doi: 10.3109/00365529209000134. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Brzozowski T., Majka J., Szlachcic A., Nauert C., Slomiany B. Nitric oxide in gastroprotection by aluminium-containing antacids. Eur J Pharmacol. 1992 Dec 15;229(2-3):155–162. doi: 10.1016/0014-2999(92)90550-n. [DOI] [PubMed] [Google Scholar]
- Konturek S. J. Mechanisms of gastroprotection. Scand J Gastroenterol Suppl. 1990;174:15–28. doi: 10.3109/00365529009091926. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Radecki T., Brzozowski T., Piastucki I., Dembiński A., Dembińska-Kieć A., Zmuda A., Gryglewski R., Gregory H. Gastric cytoprotection by epidermal growth factor. Role of endogenous prostaglandins and DNA synthesis. Gastroenterology. 1981 Sep;81(3):438–443. [PubMed] [Google Scholar]
- Lacy E. R., Cowart K. S., Hund P., 3rd Effects of chronic superficial injury on the rat gastric mucosa. Gastroenterology. 1992 Oct;103(4):1179–1191. doi: 10.1016/0016-5085(92)91502-u. [DOI] [PubMed] [Google Scholar]
- Lacy E. R., Ito S. Microscopic analysis of ethanol damage to rat gastric mucosa after treatment with a prostaglandin. Gastroenterology. 1982 Sep;83(3):619–625. [PubMed] [Google Scholar]
- Lacy E. R., Kuwayama H., Cowart K. S., King J. S., Deutz A. H., Sistrunk S. A rapid, accurate, immunohistochemical method to label proliferating cells in the digestive tract. A comparison with tritiated thymidine. Gastroenterology. 1991 Jan;100(1):259–262. doi: 10.1016/0016-5085(91)90610-w. [DOI] [PubMed] [Google Scholar]
- Leung F. W., Robert A., Guth P. H. Gastric mucosal blood flow in rats after administration of 16,16-dimethyl prostaglandin E2 at a cytoprotective dose. Gastroenterology. 1985 Jun;88(6):1948–1953. doi: 10.1016/0016-5085(85)90024-1. [DOI] [PubMed] [Google Scholar]
- Levi S., Goodlad R. A., Lee C. Y., Walport M. J., Wright N. A., Hodgson H. J. Effects of nonsteroidal anti-inflammatory drugs and misoprostol on gastroduodenal epithelial proliferation in arthritis. Gastroenterology. 1992 May;102(5):1605–1611. doi: 10.1016/0016-5085(92)91720-o. [DOI] [PubMed] [Google Scholar]
- McCarthy D. M. Nonsteroidal anti-inflammatory drugs--the clinical dilemmas. Scand J Gastroenterol Suppl. 1992;192:9–16. doi: 10.3109/00365529209095974. [DOI] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Polk W. H., Jr, Dempsey P. J., Russell W. E., Brown P. I., Beauchamp R. D., Barnard J. A., Coffey R. J., Jr Increased production of transforming growth factor alpha following acute gastric injury. Gastroenterology. 1992 May;102(5):1467–1474. doi: 10.1016/0016-5085(92)91703-7. [DOI] [PubMed] [Google Scholar]
- Rainsford K. D. Microvascular injury during gastric mucosal damage by anti-inflammatory drugs in pigs and rats. Agents Actions. 1983 Aug;13(5-6):457–460. doi: 10.1007/BF02176417. [DOI] [PubMed] [Google Scholar]
- Rainsford K. D., Willis C. Relationship of gastric mucosal damage induced in pigs by antiinflammatory drugs to their effects on prostaglandin production. Dig Dis Sci. 1982 Jul;27(7):624–635. doi: 10.1007/BF01297219. [DOI] [PubMed] [Google Scholar]
- Shorrock C. J., Rees W. D. Mucosal adaptation to indomethacin induced gastric damage in man--studies on morphology, blood flow, and prostaglandin E2 metabolism. Gut. 1992 Feb;33(2):164–169. doi: 10.1136/gut.33.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John D. J., Yeomans N. D., McDermott F. T., De Boer W. G. Adaptation of the gastric mucosa to repeated administration of aspirin in the rat. Am J Dig Dis. 1973 Oct;18(10):881–885. doi: 10.1007/BF01073339. [DOI] [PubMed] [Google Scholar]
- Svanes K., Gislason H., Guttu K., Herfjord J. K., Fevang J., Grønbech J. E. Role of blood flow in adaptive protection of the cat gastric mucosa. Gastroenterology. 1991 May;100(5 Pt 1):1249–1258. [PubMed] [Google Scholar]
- Tarnawski A., Stachura J., Durbin T., Sarfeh I. J., Gergely H. Increased expression of epidermal growth factor receptor during gastric ulcer healing in rats. Gastroenterology. 1992 Feb;102(2):695–698. doi: 10.1016/0016-5085(92)90123-g. [DOI] [PubMed] [Google Scholar]
- Tarnawski A., Stachura J., Gergely H., Hollander D. Gastric microvascular endothelium: a major target for aspirin-induced injury and arachidonic acid protection. An ultrastructural analysis in the rat. Eur J Clin Invest. 1990 Aug;20(4):432–440. doi: 10.1111/j.1365-2362.1990.tb01881.x. [DOI] [PubMed] [Google Scholar]
- Trevethick M. A., Clayton N. M., Strong P., Harman I. W. Do infiltrating neutrophils contribute to the pathogenesis of indomethacin induced ulceration of the rat gastric antrum? Gut. 1993 Feb;34(2):156–160. doi: 10.1136/gut.34.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. L., Keenan C. M., Granger D. N. Gastric ulceration induced by nonsteroidal anti-inflammatory drugs is a neutrophil-dependent process. Am J Physiol. 1990 Sep;259(3 Pt 1):G462–G467. doi: 10.1152/ajpgi.1990.259.3.G462. [DOI] [PubMed] [Google Scholar]
- Whittle B. J. Temporal relationship between cyclooxygenase inhibition, as measured by prostacyclin biosynthesis, and the gastrointestinal damage induced by indomethacin in the rat. Gastroenterology. 1981 Jan;80(1):94–98. [PubMed] [Google Scholar]
- Wright N. A., Pike C., Elia G. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature. 1990 Jan 4;343(6253):82–85. doi: 10.1038/343082a0. [DOI] [PubMed] [Google Scholar]


