Abstract
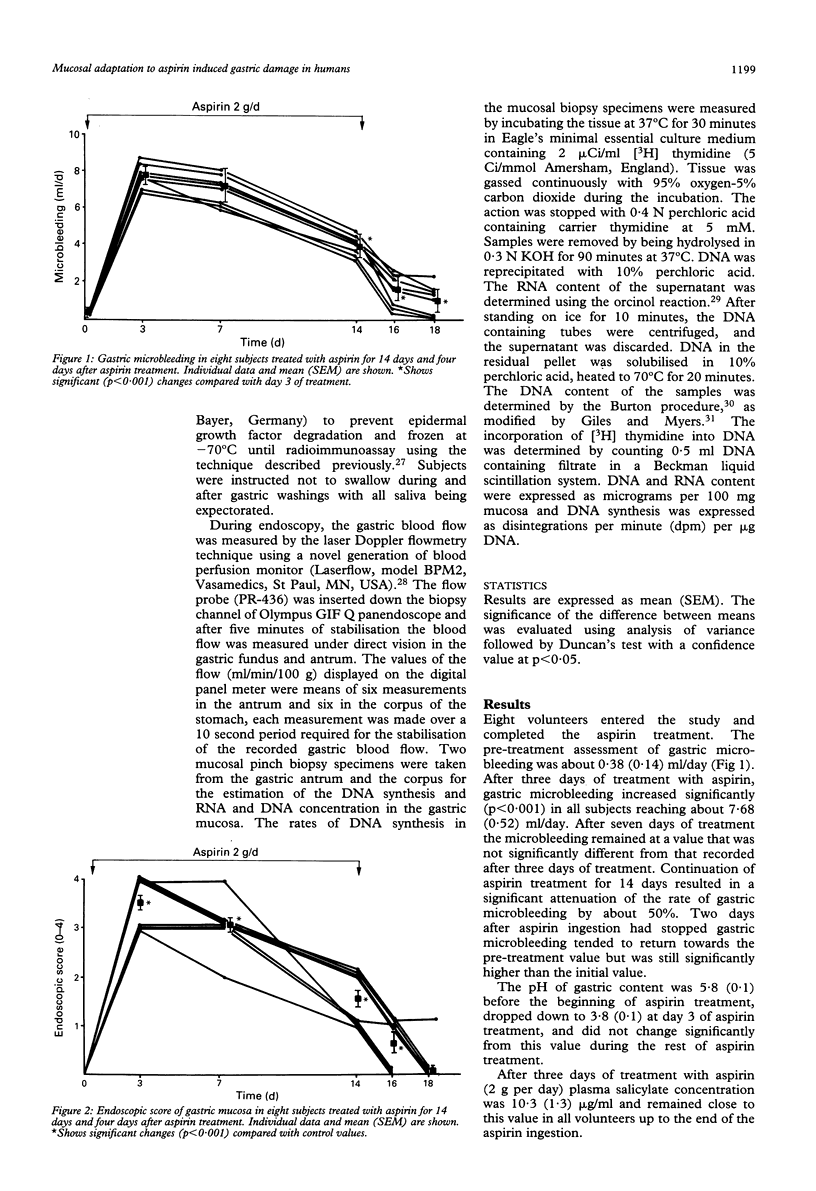
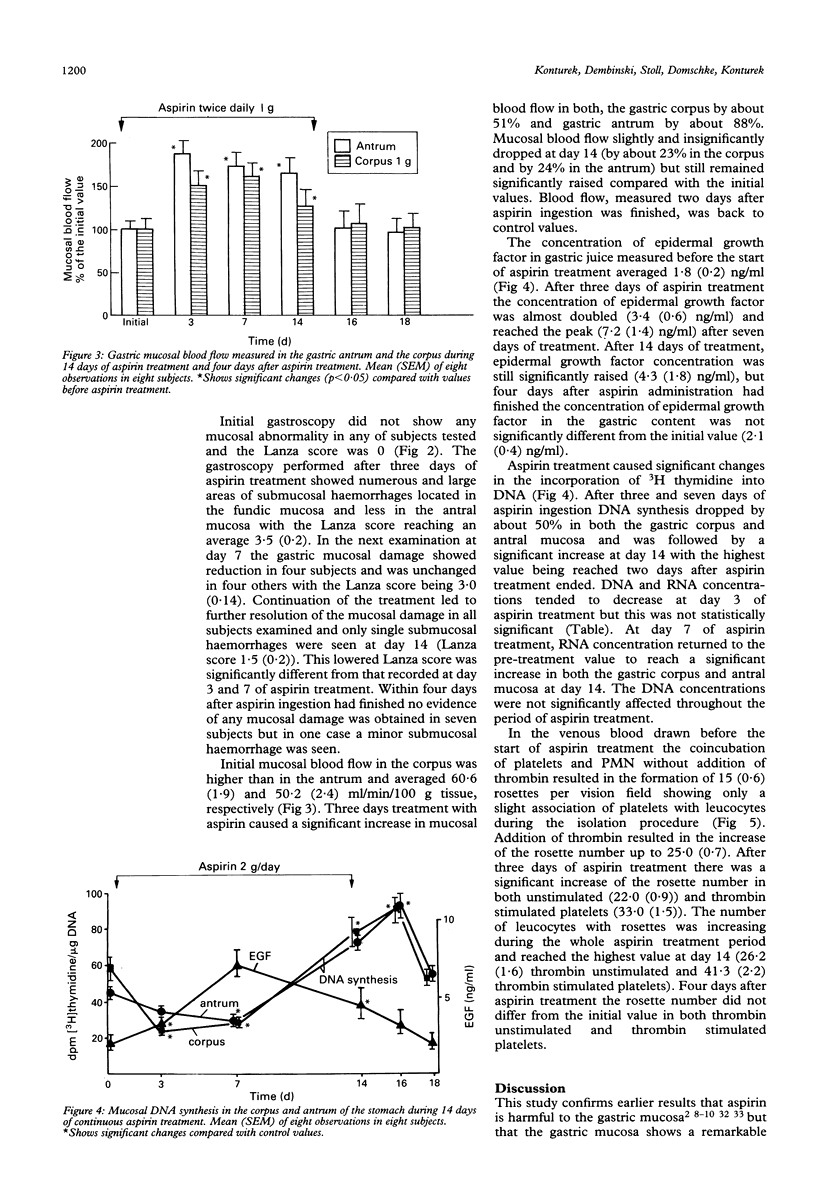
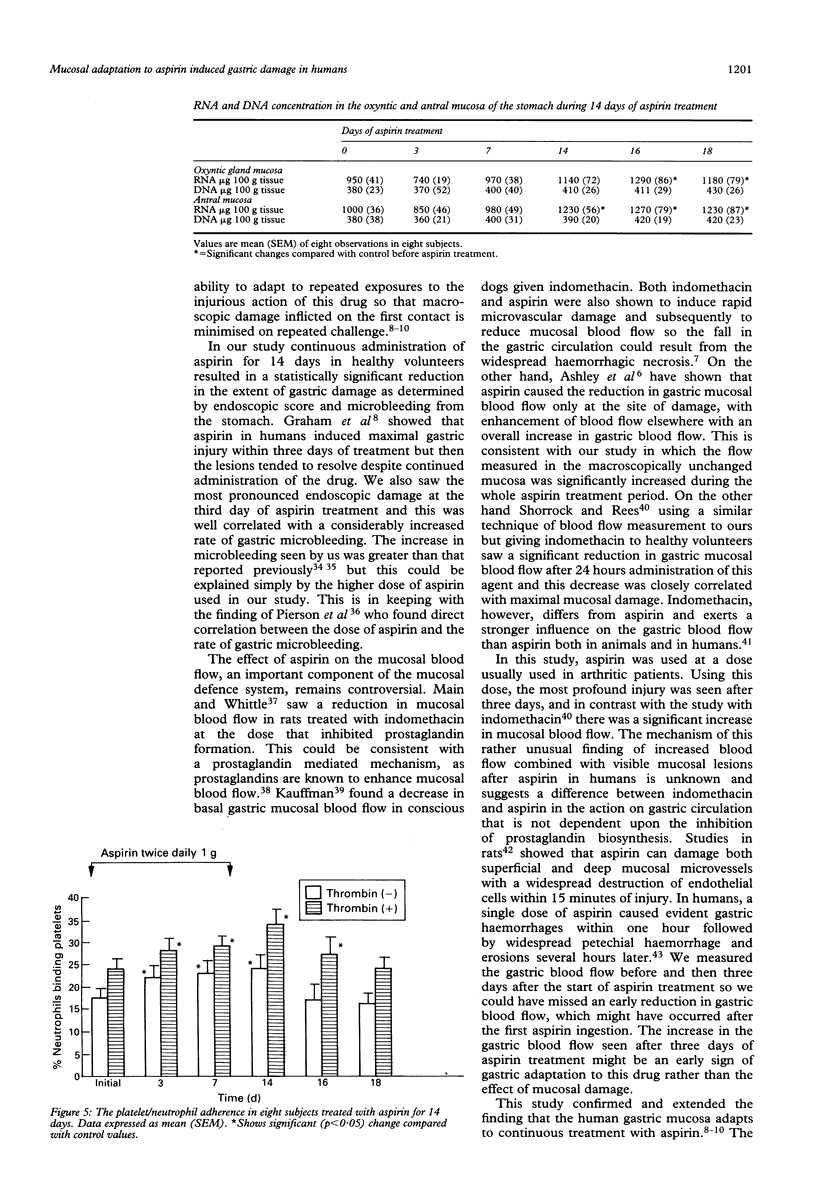
The gastropathy associated with the ingestion of non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin is a common side effect of this class of drugs, but the precise mechanisms by which they cause mucosal damage have not been fully explained. During continued use of an injurious substance, such as aspirin, the extent of gastric mucosal damage decreases and this phenomenon is named gastric adaptation. To assess the extent of mucosal damage by aspirin and subsequent adaptation the effects of 14 days of continuous, oral administration of aspirin (2 g per day) to eight healthy male volunteers was studied. To estimate the rate of mucosal damage, gastroscopy was performed before (day 0) and at days 3, 7, 14 of aspirin treatment. Gastric microbleeding and gastric mucosal blood flow were measured using laser Doppler flowmeter and mucosal biopsy specimens were taken for the estimation of tissue DNA synthesis and RNA and DNA concentration. In addition, the activation of neutrophils in peripheral blood was assessed by measuring their ability to associate with platelets. Aspirin induced acute damage mainly in gastric corpus, reaching at day 3 about 3.5 on the endoscopic Lanza score but lessened to about 1.5 at day 14 pointing to the occurrence of gastric adaptation. Mucosal blood flow increased at day 3 by about 50% in the gastric corpus and by 88% in the antrum. The in vitro DNA synthesis and RNA concentration, an index of mucosal growth, were reduced at day 3 but then increased to reach about 150% of initial value at the end of aspirin treatment. It is concluded that the treatment with aspirin in humans induces gastric adaptation to this agent, which entails the increase in mucosal blood flow, the rise in neutrophil activation, and the enhancement in mucosal growth.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asako H., Kubes P., Wallace J., Wolf R. E., Granger D. N. Modulation of leukocyte adhesion in rat mesenteric venules by aspirin and salicylate. Gastroenterology. 1992 Jul;103(1):146–152. doi: 10.1016/0016-5085(92)91107-f. [DOI] [PubMed] [Google Scholar]
- Asako H., Kubes P., Wallace J., Wolf R. E., Granger D. N. Modulation of leukocyte adhesion in rat mesenteric venules by aspirin and salicylate. Gastroenterology. 1992 Jul;103(1):146–152. doi: 10.1016/0016-5085(92)91107-f. [DOI] [PubMed] [Google Scholar]
- Ashley S. W., Sonnenschein L. A., Cheung L. Y. Focal gastric mucosal blood flow at the site of aspirin-induced ulceration. Am J Surg. 1985 Jan;149(1):53–59. doi: 10.1016/s0002-9610(85)80009-x. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner A., Koelz H. R., Halter F. Indomethacin and turnover of gastric mucosal cells in the rat. Am J Physiol. 1986 Jun;250(6 Pt 1):G830–G835. doi: 10.1152/ajpgi.1986.250.6.G830. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- CERIOTTI G. Determination of nucleic acids in animal tissues. J Biol Chem. 1955 May;214(1):59–70. [PubMed] [Google Scholar]
- Chung R. S., Bruch D., Dearlove J. Endoscopic measurement of gastric mucosal blood flow by laser Doppler velocimetry: effect of chronic esophageal variceal sclerosis. Am Surg. 1988 Feb;54(2):116–120. [PubMed] [Google Scholar]
- Dembińska-Kieć A., Zmuda A., Wenhrynowicz O., Stachura J., Peskar B. A., Gryglewski R. J. Selectin-P-mediated adherence of platelets to neutrophils is regulated by prostanoids and nitric oxide. Int J Tissue React. 1993;15(2):55–64. [PubMed] [Google Scholar]
- Eastwood G. L., Quimby G. F. Effect of chronic aspirin ingestion on epithelial proliferation in rat fundus, antrum, and duodenum. Gastroenterology. 1982 May;82(5 Pt 1):852–856. [PubMed] [Google Scholar]
- Fries J. F., Miller S. R., Spitz P. W., Williams C. A., Hubert H. B., Bloch D. A. Identification of patients at risk for gastropathy associated with NSAID use. J Rheumatol Suppl. 1990 Feb;20:12–19. [PubMed] [Google Scholar]
- Graham D. Y., Smith J. L., Dobbs S. M. Gastric adaptation occurs with aspirin administration in man. Dig Dis Sci. 1983 Jan;28(1):1–6. doi: 10.1007/BF01393353. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Smith J. L., Spjut H. J., Torres E. Gastric adaptation. Studies in humans during continuous aspirin administration. Gastroenterology. 1988 Aug;95(2):327–333. [PubMed] [Google Scholar]
- Hattori R., Hamilton K. K., Fugate R. D., McEver R. P., Sims P. J. Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J Biol Chem. 1989 May 15;264(14):7768–7771. [PubMed] [Google Scholar]
- Hsu-Lin S., Berman C. L., Furie B. C., August D., Furie B. A platelet membrane protein expressed during platelet activation and secretion. Studies using a monoclonal antibody specific for thrombin-activated platelets. J Biol Chem. 1984 Jul 25;259(14):9121–9126. [PubMed] [Google Scholar]
- Hudson N., Hawthorne A. B., Cole A. T., Jones P. D., Hawkey C. J. Mechanisms of gastric and duodenal damage and protection. Hepatogastroenterology. 1992 Feb;39 (Suppl 1):31–36. [PubMed] [Google Scholar]
- Ivey K. J. Mechanisms of nonsteroidal anti-inflammatory drug-induced gastric damage. Actions of therapeutic agents. Am J Med. 1988 Feb 22;84(2A):41–48. doi: 10.1016/0002-9343(88)90253-7. [DOI] [PubMed] [Google Scholar]
- Jungi T. W., Spycher M. O., Nydegger U. E., Barandun S. Platelet-leukocyte interaction: selective binding of thrombin-stimulated platelets to human monocytes, polymorphonuclear leukocytes, and related cell lines. Blood. 1986 Mar;67(3):629–636. [PubMed] [Google Scholar]
- Kitahora T., Guth P. H. Effect of aspirin plus hydrochloric acid on the gastric mucosal microcirculation. Gastroenterology. 1987 Oct;93(4):810–817. doi: 10.1016/0016-5085(87)90444-6. [DOI] [PubMed] [Google Scholar]
- Kitahora T., Guth P. H. Effect of aspirin plus hydrochloric acid on the gastric mucosal microcirculation. Gastroenterology. 1987 Oct;93(4):810–817. doi: 10.1016/0016-5085(87)90444-6. [DOI] [PubMed] [Google Scholar]
- Kitchingman G. K., Prichard P. J., Daneshmend T. K., Walt R. P., Hawkey C. J. Enhanced gastric mucosal bleeding with doses of aspirin used for prophylaxis and its reduction by ranitidine. Br J Clin Pharmacol. 1989 Nov;28(5):581–585. doi: 10.1111/j.1365-2125.1989.tb03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konturek J. W., Bielanski W., Konturek S. J., Bogdal J., Oleksy J. Distribution and release of epidermal growth factor in man. Gut. 1989 Sep;30(9):1194–1200. doi: 10.1136/gut.30.9.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konturek S. J., Kwiecien N., Obtulowicz W., Kiec-Dembinska A., Polanski M., Kopp B., Sito E., Oleksy J. Effect of carprofen and indomethacin on gastric function, mucosal integrity and generation of prostaglandins in men. Hepatogastroenterology. 1982 Dec;29(6):267–270. [PubMed] [Google Scholar]
- Konturek S. J., Kwiecień N., Obtułowicz W., Polański M., Kopp B., Oleksy J. Comparison of prostaglandin E2 and ranitidine in prevention of gastric bleeding by aspirin in man. Gut. 1983 Feb;24(2):89–93. doi: 10.1136/gut.24.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza F. L. A review of mucosal protection by synthetic prostaglandin E analogs against injury by non-steroidal anti-inflammatory agents. Scand J Gastroenterol Suppl. 1989;163:36–43. doi: 10.3109/00365528909091173. [DOI] [PubMed] [Google Scholar]
- Lanza F. L. Endoscopic studies of gastric and duodenal injury after the use of ibuprofen, aspirin, and other nonsteroidal anti-inflammatory agents. Am J Med. 1984 Jul 13;77(1A):19–24. doi: 10.1016/s0002-9343(84)80014-5. [DOI] [PubMed] [Google Scholar]
- Lee M., Aldred K., Lee E., Feldman M. Aspirin-induced acute gastric mucosal injury is a neutrophil-dependent process in rats. Am J Physiol. 1992 Dec;263(6 Pt 1):G920–G926. doi: 10.1152/ajpgi.1992.263.6.G920. [DOI] [PubMed] [Google Scholar]
- Lev R., Siegel H. I., Glass G. B. Effects of salicylates on the canine stomach: a morphological and histochemical study. Gastroenterology. 1972 May;62(5):970–980. [PubMed] [Google Scholar]
- Levi S., Goodlad R. A., Lee C. Y., Stamp G., Walport M. J., Wright N. A., Hodgson H. J. Inhibitory effect of non-steroidal anti-inflammatory drugs on mucosal cell proliferation associated with gastric ulcer healing. Lancet. 1990 Oct 6;336(8719):840–843. doi: 10.1016/0140-6736(90)92341-e. [DOI] [PubMed] [Google Scholar]
- Levi S., Goodlad R. A., Lee C. Y., Walport M. J., Wright N. A., Hodgson H. J. Effects of nonsteroidal anti-inflammatory drugs and misoprostol on gastroduodenal epithelial proliferation in arthritis. Gastroenterology. 1992 May;102(5):1605–1611. doi: 10.1016/0016-5085(92)91720-o. [DOI] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Main I. H., Whittle B. J. Investigation of the vasodilator and antisecretory role of prostaglandins in the rat gastric mucosa by use of non-steroidal anti-inflammatory drugs. Br J Pharmacol. 1975 Feb;53(2):217–224. doi: 10.1111/j.1476-5381.1975.tb07351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D. M. NSAID-induced gastrointestinal damage. A critical review of prophylaxis and therapy. J Clin Gastroenterol. 1990;12 (Suppl 2):S13–S20. [PubMed] [Google Scholar]
- Miura S., Suematsu M., Tanaka S., Nagata H., Houzawa S., Suzuki M., Kurose I., Serizawa H., Tsuchiya M. Microcirculatory disturbance in indomethacin-induced intestinal ulcer. Am J Physiol. 1991 Aug;261(2 Pt 1):G213–G219. doi: 10.1152/ajpgi.1991.261.2.G213. [DOI] [PubMed] [Google Scholar]
- O'Laughlin J. C., Hoftiezer J. W., Ivey K. J. Effect of aspirin on the human stomach in normals: endoscopic comparison of damage produced one hour, 24 hours, and 2 weeks after administration. Scand J Gastroenterol Suppl. 1981;67:211–214. [PubMed] [Google Scholar]
- PIERSON R. N., Jr, HOLT P. R., WATSON R. M., KEATING R. P. Aspirin and gastrointestinal bleeding. Chromate blood loss studies. Am J Med. 1961 Aug;31:259–265. doi: 10.1016/0002-9343(61)90114-0. [DOI] [PubMed] [Google Scholar]
- Rot A. Endothelial cell binding of NAP-1/IL-8: role in neutrophil emigration. Immunol Today. 1992 Aug;13(8):291–294. doi: 10.1016/0167-5699(92)90039-A. [DOI] [PubMed] [Google Scholar]
- Ruppin H., Person B., Robert A., Domschke W. Gastric cytoprotection in man by prostaglandin E2. Scand J Gastroenterol. 1981;16(5):647–652. doi: 10.3109/00365528109182025. [DOI] [PubMed] [Google Scholar]
- Shorrock C. J., Rees W. D. Mucosal adaptation to indomethacin induced gastric damage in man--studies on morphology, blood flow, and prostaglandin E2 metabolism. Gut. 1992 Feb;33(2):164–169. doi: 10.1136/gut.33.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottili M., Sternini C., Brecha N. C., Lezoche E., Walsh J. H. Transforming growth factor alpha receptor binding sites in the canine gastrointestinal tract. Gastroenterology. 1992 Nov;103(5):1427–1436. doi: 10.1016/0016-5085(92)91161-v. [DOI] [PubMed] [Google Scholar]
- St John D. J., Yeomans N. D., McDermott F. T., De Boer W. G. Adaptation of the gastric mucosa to repeated administration of aspirin in the rat. Am J Dig Dis. 1973 Oct;18(10):881–885. doi: 10.1007/BF01073339. [DOI] [PubMed] [Google Scholar]
- Tarnawski A., Stachura J., Gergely H., Hollander D. Gastric microvascular endothelium: a major target for aspirin-induced injury and arachidonic acid protection. An ultrastructural analysis in the rat. Eur J Clin Invest. 1990 Aug;20(4):432–440. doi: 10.1111/j.1365-2362.1990.tb01881.x. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Keenan C. M., Granger D. N. Gastric ulceration induced by nonsteroidal anti-inflammatory drugs is a neutrophil-dependent process. Am J Physiol. 1990 Sep;259(3 Pt 1):G462–G467. doi: 10.1152/ajpgi.1990.259.3.G462. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Pike C., Elia G. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature. 1990 Jan 4;343(6253):82–85. doi: 10.1038/343082a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman R. P., Gates T. S., Boehmer C. G., Mantyh P. W. Epidermal growth factor receptors in the canine antrum. Peptides. 1988 Nov-Dec;9(6):1411–1414. doi: 10.1016/0196-9781(88)90211-2. [DOI] [PubMed] [Google Scholar]



