Abstract
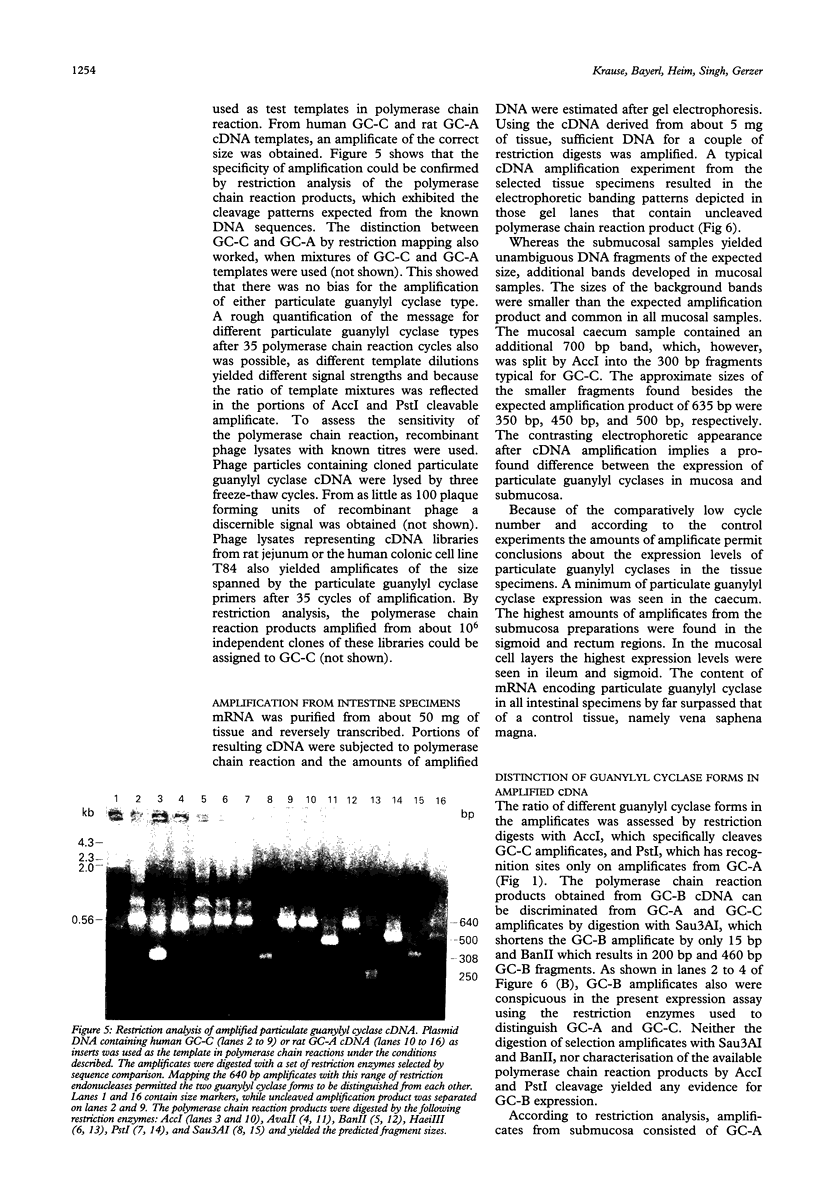
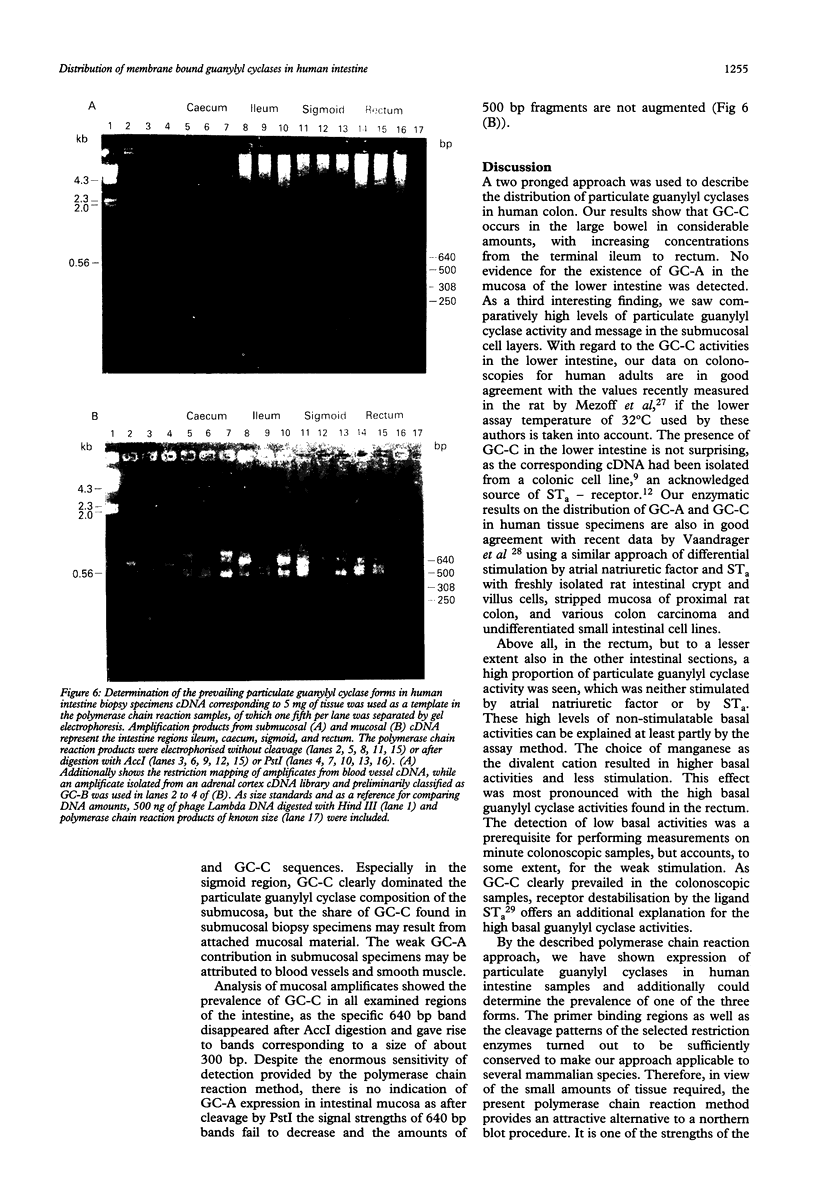
The quantification and distinction of particulate guanylyl cyclases in the human intestine were considered by an enzymatic approach, which comprised the signal transduction from receptor binding to cGMP formation, and, in addition, by showing the expression of an intracellular portion of these transmembrane proteins. Basal guanylyl cyclase (GC) activities were 50 to 80 pmol cGMP formation/min/mg protein and were stimulated up to twofold by heat stable enterotoxin, but were not significantly influenced by atrial natriuretic factor. Enzymatic analysis of colonoscopic specimens pointed to the prevalence of guanylyl cyclase C in the terminal ileum and in the large bowel including colon ascendens, colon descendens, sigmoid, and rectum. The availability of sequence information on human guanylyl cyclases permitted the development of a polymerase chain reaction approach for distinguishing the expression of GC-A and GC-C in human tissue samples. The expression levels of particulate guanylyl cyclases found by polymerase chain reaction in surgical biopsy specimens confirmed the enzymatic data, in that substantial expression of GC-C was found not only in the small intestine but also in the large bowel. According to the restriction mapping of amplificates, GC-C prevailed over GC-A throughout the human intestine, particularly in the mucosal layers.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianchi C., Thibault G., De Léan A., Genest J., Cantin M. Atrial natriuretic factor binding sites in the jejunum. Am J Physiol. 1989 Feb;256(2 Pt 1):G436–G441. doi: 10.1152/ajpgi.1989.256.2.G436. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brunner J., Lübcke R., Barbezat G. O., Yandle T. G., Espiner E. A. Atrial natriuretic peptide and water and electrolyte transport in the human jejunum. Gut. 1991 Jun;32(6):635–639. doi: 10.1136/gut.32.6.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M. S., Lowe D. G., Lewis M., Hellmiss R., Chen E., Goeddel D. V. Differential activation by atrial and brain natriuretic peptides of two different receptor guanylate cyclases. Nature. 1989 Sep 7;341(6237):68–72. doi: 10.1038/341068a0. [DOI] [PubMed] [Google Scholar]
- Chinkers M., Garbers D. L. Signal transduction by guanylyl cyclases. Annu Rev Biochem. 1991;60:553–575. doi: 10.1146/annurev.bi.60.070191.003005. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cohen M. B., Guarino A., Shukla R., Giannella R. A. Age-related differences in receptors for Escherichia coli heat-stable enterotoxin in the small and large intestine of children. Gastroenterology. 1988 Feb;94(2):367–373. doi: 10.1016/0016-5085(88)90423-4. [DOI] [PubMed] [Google Scholar]
- Currie M. G., Fok K. F., Kato J., Moore R. J., Hamra F. K., Duffin K. L., Smith C. E. Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):947–951. doi: 10.1073/pnas.89.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Graf L. H., Jr, Laird W. J., Smith P. L. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2800–2804. doi: 10.1073/pnas.75.6.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Rao M. C., Chang E. B. Intestinal electrolyte transport and diarrheal disease (1). N Engl J Med. 1989 Sep 21;321(12):800–806. doi: 10.1056/NEJM198909213211206. [DOI] [PubMed] [Google Scholar]
- Grotmol T., Rødnes J. T., Buanes T., Christensen G., Landsverk T. Atrial natriuretic factor (ANF) does not affect ion transport in human intestine but does in porcine intestine. Acta Physiol Scand. 1993 Apr;147(4):417–429. doi: 10.1111/j.1748-1716.1993.tb09517.x. [DOI] [PubMed] [Google Scholar]
- Heim J. M., Gottmann K., Weil J., Haufe M. C., Gerzer R. Is cyclic GMP a clinically useful marker for ANF action? Z Kardiol. 1988;77 (Suppl 2):41–46. [PubMed] [Google Scholar]
- Heim J. M., Ivanova K., Gerzer R. Amiloride increases the sensitivity of particulate guanylate cyclase to atrial natriuretic factor. Biochem Biophys Res Commun. 1988 May 16;152(3):1263–1268. doi: 10.1016/s0006-291x(88)80421-2. [DOI] [PubMed] [Google Scholar]
- Hughes J. M., Murad F., Chang B., Guerrant R. L. Role of cyclic GMP in the action of heat-stable enterotoxin of Escherichia coli. Nature. 1978 Feb 23;271(5647):755–756. doi: 10.1038/271755a0. [DOI] [PubMed] [Google Scholar]
- Jakobsen K. S., Breivold E., Hornes E. Purification of mRNA directly from crude plant tissues in 15 minutes using magnetic oligo dT microspheres. Nucleic Acids Res. 1990 Jun 25;18(12):3669–3669. doi: 10.1093/nar/18.12.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M., Raida M., Adermann K., Schulz-Knappe P., Gerzer R., Heim J. M., Forssmann W. G. The circulating bioactive form of human guanylin is a high molecular weight peptide (10.3 kDa). FEBS Lett. 1993 Mar 1;318(2):205–209. doi: 10.1016/0014-5793(93)80022-m. [DOI] [PubMed] [Google Scholar]
- Lowe D. G., Chang M. S., Hellmiss R., Chen E., Singh S., Garbers D. L., Goeddel D. V. Human atrial natriuretic peptide receptor defines a new paradigm for second messenger signal transduction. EMBO J. 1989 May;8(5):1377–1384. doi: 10.1002/j.1460-2075.1989.tb03518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maack T., Suzuki M., Almeida F. A., Nussenzveig D., Scarborough R. M., McEnroe G. A., Lewicki J. A. Physiological role of silent receptors of atrial natriuretic factor. Science. 1987 Oct 30;238(4827):675–678. doi: 10.1126/science.2823385. [DOI] [PubMed] [Google Scholar]
- Petritsch W., Holzer-Petsche U., Hinterleitner T., Krejs G. J. Intravenous atrial natriuretic peptide does not affect water and ion transport in the human small intestine. Eur J Clin Invest. 1989 Jun;19(3):272–277. doi: 10.1111/j.1365-2362.1989.tb00230.x. [DOI] [PubMed] [Google Scholar]
- Schulz S., Chrisman T. D., Garbers D. L. Cloning and expression of guanylin. Its existence in various mammalian tissues. J Biol Chem. 1992 Aug 15;267(23):16019–16021. [PubMed] [Google Scholar]
- Schulz S., Yuen P. S., Garbers D. L. The expanding family of guanylyl cyclases. Trends Pharmacol Sci. 1991 Mar;12(3):116–120. doi: 10.1016/0165-6147(91)90519-x. [DOI] [PubMed] [Google Scholar]
- Singh S., Singh G., Heim J. M., Gerzer R. Isolation and expression of a guanylate cyclase-coupled heat stable enterotoxin receptor cDNA from a human colonic cell line. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1455–1463. doi: 10.1016/0006-291x(91)91736-v. [DOI] [PubMed] [Google Scholar]
- Tremblay J., Gerzer R., Hamet P. Cyclic GMP in cell function. Adv Second Messenger Phosphoprotein Res. 1988;22:319–383. [PubMed] [Google Scholar]
- Vaandrager A. B., Bot A. G., De Vente J., De Jonge H. R. Atriopeptins and Escherichia coli enterotoxin STa have different sites of action in mammalian intestine. Gastroenterology. 1992 Apr;102(4 Pt 1):1161–1169. [PubMed] [Google Scholar]
- Vaandrager A. B., Bot A. G., De Vente J., De Jonge H. R. Atriopeptins and Escherichia coli enterotoxin STa have different sites of action in mammalian intestine. Gastroenterology. 1992 Apr;102(4 Pt 1):1161–1169. [PubMed] [Google Scholar]
- Vaandrager A. B., Schulz S., De Jonge H. R., Garbers D. L. Guanylyl cyclase C is an N-linked glycoprotein receptor that accounts for multiple heat-stable enterotoxin-binding proteins in the intestine. J Biol Chem. 1993 Jan 25;268(3):2174–2179. [PubMed] [Google Scholar]
- Waldman S. A., Rapoport R. M., Murad F. Atrial natriuretic factor selectively activates particulate guanylate cyclase and elevates cyclic GMP in rat tissues. J Biol Chem. 1984 Dec 10;259(23):14332–14334. [PubMed] [Google Scholar]
- Wiegand R. C., Kato J., Currie M. G. Rat guanylin cDNA: characterization of the precursor of an endogenous activator of intestinal guanylate cyclase. Biochem Biophys Res Commun. 1992 Jun 30;185(3):812–817. doi: 10.1016/0006-291x(92)91699-q. [DOI] [PubMed] [Google Scholar]
- Wong S. K., Garbers D. L. Receptor guanylyl cyclases. J Clin Invest. 1992 Aug;90(2):299–305. doi: 10.1172/JCI115862. [DOI] [PMC free article] [PubMed] [Google Scholar]