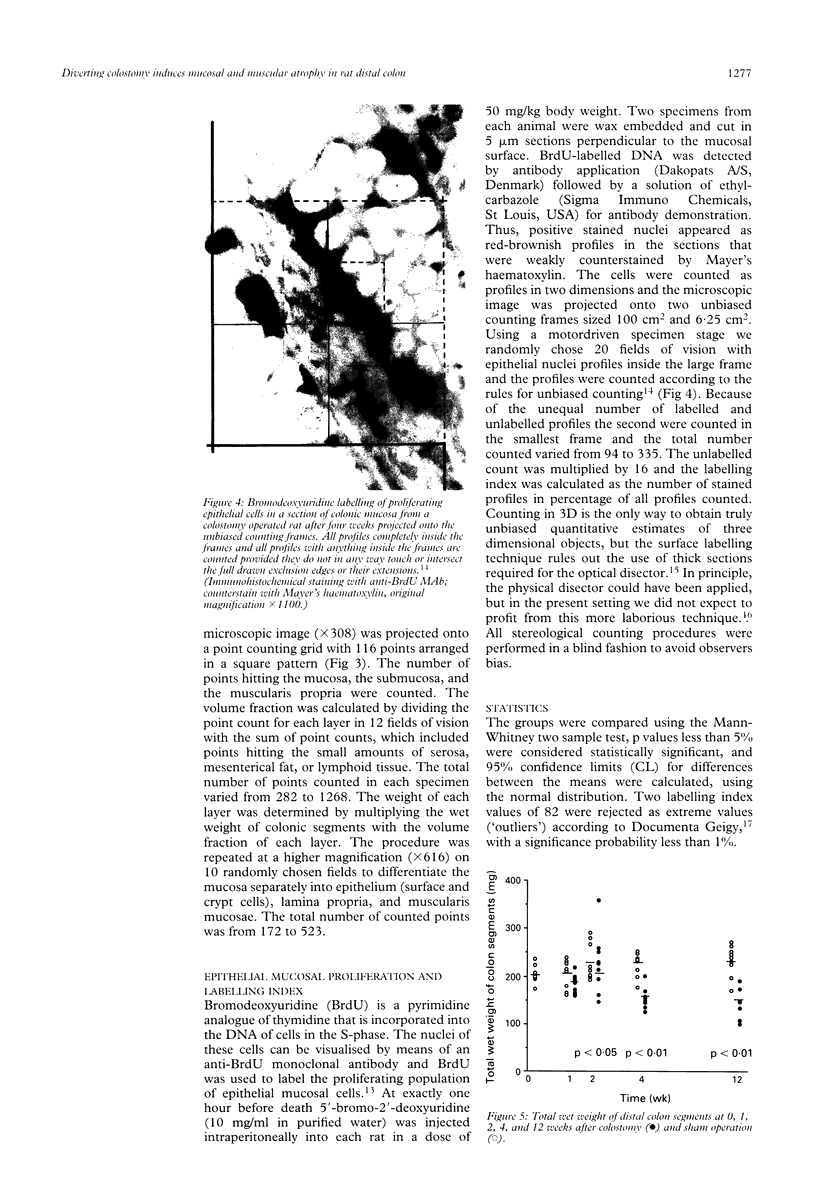
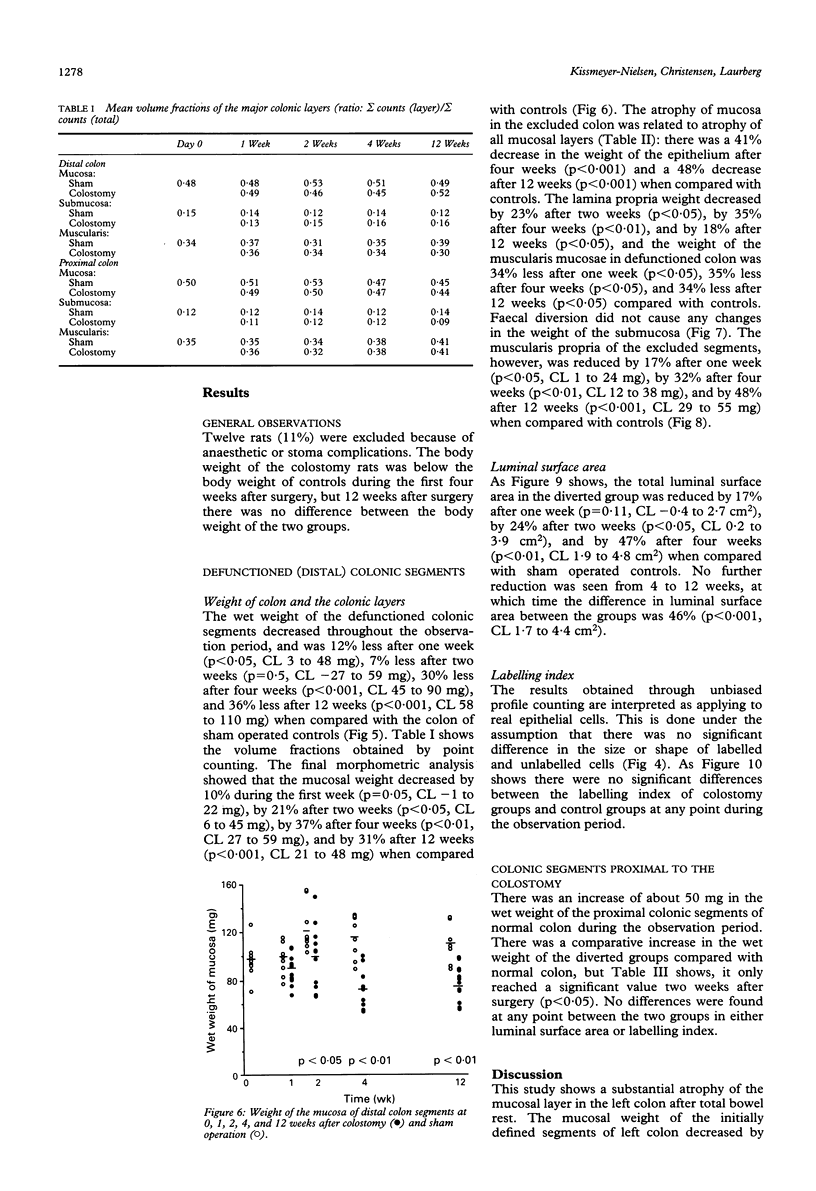
Abstract
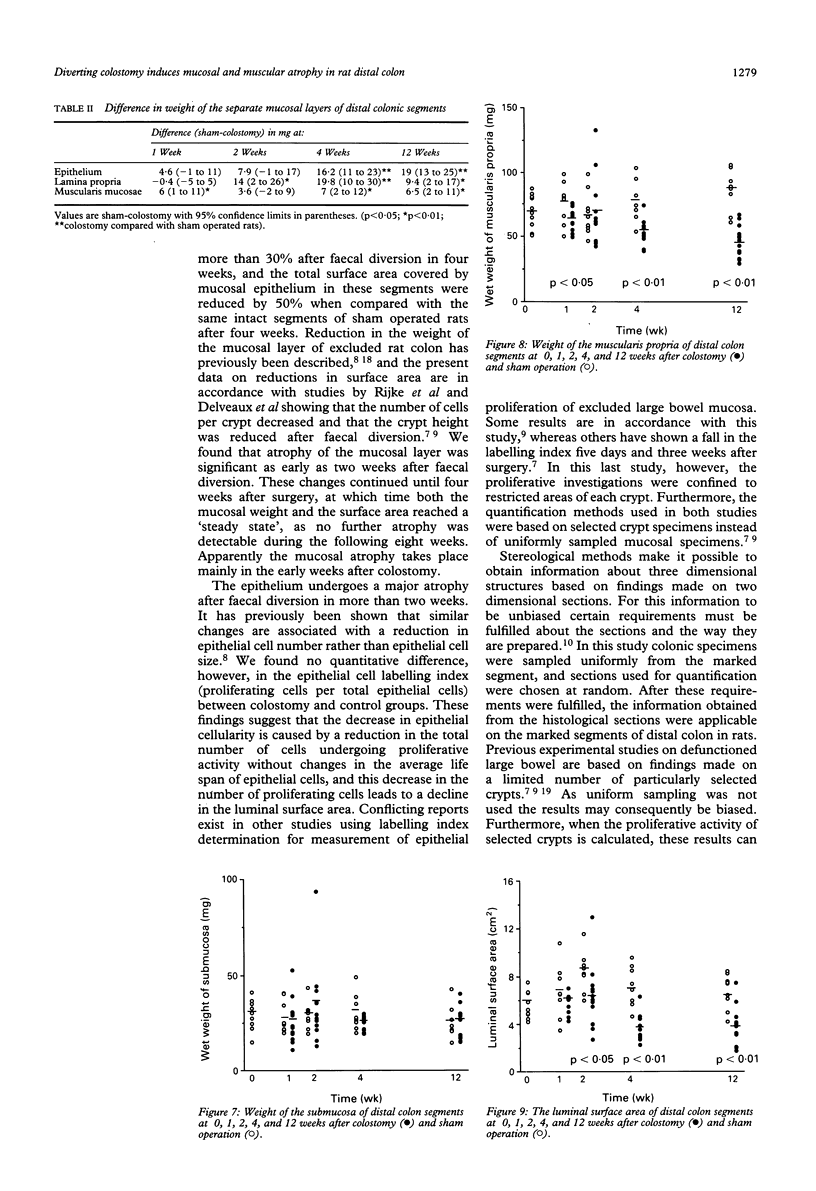
The progress of adaptive changes in the left colon after diverting colostomy was studied in rats using stereological techniques. Standardised segments of left colon proximal and distal to the colostomy was examined after 0, 1, 2, 4, or 12 weeks. In excluded colon the mucosal weight was reduced by 37% (p < 0.01) and the luminal surface area by 47% (p < 0.01) after four weeks and reached a steady state at this point of time, as no further reduction was seen from 4 to 12 weeks. The number of proliferating crypt cells was determined immunohistochemically after in vivo labelling with bromodeoxyuridine and was compared with the total number of colonocytes. Total bowel rest leads to a reduction in the number of proliferating epithelial cells and not to a reduced average life span. The weight of the muscularis propria decreased by 32% after four weeks (p < 0.01) and by 48% after 12 weeks (p < 0.001), whereas the weight of the submucosa was unchanged. No adaptive changes were found in segments proximal to the colostomy. These results show that the wall composition of defunctioned colon in rats is radically changed resulting from a mucosal and muscular atrophy, and from a reduction in luminal surface area.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleton G. V., Williamson R. C. Hypoplasia of defunctioned rectum. Br J Surg. 1989 Aug;76(8):787–789. doi: 10.1002/bjs.1800760807. [DOI] [PubMed] [Google Scholar]
- Baddeley A. J., Gundersen H. J., Cruz-Orive L. M. Estimation of surface area from vertical sections. J Microsc. 1986 Jun;142(Pt 3):259–276. doi: 10.1111/j.1365-2818.1986.tb04282.x. [DOI] [PubMed] [Google Scholar]
- Blomquist P., Jiborn H., Zederfeldt B. Effect of diverting colostomy on collagen metabolism in the colonic wall. Studies in the rat. Am J Surg. 1985 Mar;149(3):330–333. doi: 10.1016/s0002-9610(85)80101-x. [DOI] [PubMed] [Google Scholar]
- Bristol J. B., Ghatei M. A., Smith J. H., Bloom S. R., Williamson R. C. Elevated plasma enteroglucagon alone fails to alter distal colonic carcinogenesis in rats. Gastroenterology. 1987 Mar;92(3):617–624. doi: 10.1016/0016-5085(87)90009-6. [DOI] [PubMed] [Google Scholar]
- Delvaux G., Caes F., Willems G. Influence of a diverting colostomy on epithelial cell proliferation in the colon of rats. Eur Surg Res. 1983;15(4):223–229. doi: 10.1159/000128357. [DOI] [PubMed] [Google Scholar]
- Dowling R. H. Cellular and molecular basis of intestinal and pancreatic adaptation. Scand J Gastroenterol Suppl. 1992;193:64–67. doi: 10.3109/00365529209096008. [DOI] [PubMed] [Google Scholar]
- Foster M. E., Leaper D. J., Williamson R. C. Changing patterns in colostomy closure: the Bristol experience 1975-1982. Br J Surg. 1985 Feb;72(2):142–145. doi: 10.1002/bjs.1800720225. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J., Bendtsen T. F., Korbo L., Marcussen N., Møller A., Nielsen K., Nyengaard J. R., Pakkenberg B., Sørensen F. B., Vesterby A. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988 May;96(5):379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc. 1986 Jul;143(Pt 1):3–45. [PubMed] [Google Scholar]
- Komuro T. The lattice arrangement of the collagen fibres in the submucosa of the rat small intestine: scanning electron microscopy. Cell Tissue Res. 1988 Jan;251(1):117–121. doi: 10.1007/BF00215455. [DOI] [PubMed] [Google Scholar]
- Kripke S. A., Fox A. D., Berman J. M., Settle R. G., Rombeau J. L. Stimulation of intestinal mucosal growth with intracolonic infusion of short-chain fatty acids. JPEN J Parenter Enteral Nutr. 1989 Mar-Apr;13(2):109–116. doi: 10.1177/0148607189013002109. [DOI] [PubMed] [Google Scholar]
- Parks S. E., Hastings P. R. Complications of colostomy closure. Am J Surg. 1985 May;149(5):672–675. doi: 10.1016/s0002-9610(85)80153-7. [DOI] [PubMed] [Google Scholar]
- Pearce N. W., Scott S. D., Karran S. J. Timing and method of reversal of Hartmann's procedure. Br J Surg. 1992 Aug;79(8):839–841. doi: 10.1002/bjs.1800790844. [DOI] [PubMed] [Google Scholar]
- Rainey J. B., Davies P. W., Bristol J. B., Williamson R. C. Adaptation and carcinogenesis in defunctioned rat colon: divergent effects of faeces and bile acids. Br J Cancer. 1983 Oct;48(4):477–484. doi: 10.1038/bjc.1983.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijke R. P., Gart R., Langendoen N. J. Epithelial cell kinetics in the descending colon of the rat. II. The effect of experimental bypass. Virchows Arch B Cell Pathol Incl Mol Pathol. 1979;31(1):23–30. doi: 10.1007/BF02889919. [DOI] [PubMed] [Google Scholar]
- Ryan G. P., Dudrick S. J., Copeland E. M., Johnson L. R. Effects of various diets on colonic growth in rats. Gastroenterology. 1979 Oct;77(4 Pt 1):658–663. [PubMed] [Google Scholar]
- Sterio D. C. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984 May;134(Pt 2):127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Terpstra O. T., Dahl E. P., Williamson R. C., Ross J. S., Malt R. A. Colostomy closure promotes cell proliferation and dimethylhydrazine-induced carcinogenesis in rat distal colon. Gastroenterology. 1981 Sep;81(3):475–480. [PubMed] [Google Scholar]
- Udén P., Blomquist P., Jiborn H., Zederfeldt B. Impact of long-term relative bowel rest on conditions for colonic surgery. Am J Surg. 1988 Nov;156(5):381–385. doi: 10.1016/s0002-9610(88)80192-2. [DOI] [PubMed] [Google Scholar]
- Wynford-Thomas D., Williams E. D. Use of bromodeoxyuridine for cell kinetic studies in intact animals. Cell Tissue Kinet. 1986 Mar;19(2):179–182. doi: 10.1111/j.1365-2184.1986.tb00728.x. [DOI] [PubMed] [Google Scholar]