Abstract
Studies using functional neuroimaging and patient populations have demonstrated that distinct brain regions subserve semantic knowledge for different classes of inanimate objects (e.g., tools, musical instruments, and houses). What this work has yet to consider, however, is how conceptual knowledge about people may be organized in the brain. In particular, is there a distinct functional neuroanatomy associated with person knowledge? By using event-related functional magnetic resonance imaging (fMRI), we measured neural activity while participants made semantic judgments about people or objects. A unique pattern of brain activity was associated with person judgments and included brain regions previously implicated in other aspects of social-cognitive functioning: medial prefrontal cortex, superior temporal cortex, intraparietal sulcus, and fusiform gyrus. These regions were generally marked by relatively little change from baseline brain activity for person judgments along with significant deactivations for object judgments. Together, these findings support the notion that person knowledge may be functionally dissociable from other classes of semantic knowledge within the brain.
Among the most intriguing findings in cognitive neuroscience is that different categories or classes of objects are often associated with distinct neuroanatomical regions. Both neuropsychological and functional neuroimaging research have converged on the observation that perception of, and semantic knowledge about, particular classes of inanimate stimuli (e.g., tools, musical instruments, and houses) are subserved by distinct areas of the human brain (1–8). Although the exact basis of this neuroanatomical localization remains open to debate, most researchers concur that the brain contains some kind of category-specific neural architecture. Indeed, this observation has prompted some theorists to suggest that the mind may have evolved dedicated neural circuits to deal with knowledge pertaining to certain categories of objects; specifically, objects that have biological relevance or significance to people (e.g., conspecifics, tools, and plants) (4). The benefits of such a modular system reside in the rapid and relatively error-free manner in which semantic knowledge can be selected and deployed. Were distinct classes of information to share a similar neuroanatomical location, interitem competition might compromise (e.g., slow down) the selection process.
In the current experiment, we examined the neural substrates of a class of semantic knowledge that earlier work on category specificity has largely ignored, namely, other people. Although neural regions that subserve the perception of persons (e.g., body parts and faces) have been characterized (9–16), research has yet to investigate how the brain represents general knowledge about the internal, unobservable attributes of social agents. Person knowledge differs from knowledge about inanimate objects in a number of potentially important respects. Most obviously, the attributes used to describe persons differ substantially from those used to describe inanimate objects. Whereas a person may be described as anxious or devious, inanimate objects rarely engender such a description. One basic feature of person knowledge is that it frequently refers to the mental states of others, states that cannot be directly observed but may instead require generalization from one's own internal psychological properties (i.e., theory of mind). Finally, the application of person knowledge demands a flexibility that is typically unnecessary for most classes of object knowledge (e.g., people must frequently track interactions among independent agents acting in complex social settings).
To the extent that: (i) conspecifics are arguably the most important stimulus class to humans; and (ii) person knowledge differs in several important ways from semantic knowledge about inanimate objects, we expect the representation of person knowledge in the human brain to conform to the category-specific neural organization observed in object semantics. To this end, we used event-related functional MRI (fMRI) to test the prediction that the brain represents person knowledge in a distinct manner from knowledge about inanimate objects. Adopting a paradigm from related research on the organization of semantic memory, the current experiment compared the brain activity associated with semantic judgments about people with that associated with comparable judgments about inanimate objects.
Materials and Methods
Fourteen paid volunteers from the Dartmouth College community (7 male and 7 female; age range, 18–27) participated in this experiment. All participants were right-handed, native English speakers with no history of neurological problems. All gave informed consent according to the procedures approved by the Committee for the Protection of Human Subjects at Dartmouth College. Data from one female participant were discarded because of problems with the acquisition of images during the functional scans.
Imaging Procedure.
Imaging was conducted by using a 1.5-tesla GE Signa scanner. An Apple Powerbook G3 computer running psyscope v.1.2.5 (17) controlled stimulus presentation and recorded participants' behavioral responses by means of a keypress interfaced with a PSYSCOPE button box (Carnegie Mellon University, Pittsburgh). Stimuli were projected onto a screen at the end of the magnet bore that participants viewed by way of a mirror mounted on the head coil. A pillow and foam cushions were placed within the head coil to minimize head movements.
We first collected a high-resolution T1-weighted structural scan (SPGR) followed by four functional runs of 250 axial scans (20 slices; 5 mm thick; 1 mm skip). Functional images were collected by using a gradient spin-echo echo-planar pulse sequence (repetition time = 2,000 ms; echo time = 35 ms; flip angle = 90°, 3.75 × 3.75 in-plane resolution). The duration of each functional run was 8 min and 20 s.
Behavioral Procedure.
Participants responded to visually presented noun–adjective pairs (4,000 ms duration) by pressing one of two response buttons if the adjective could ever be true of the noun (left forefinger) or another button if it could not (right forefinger). Nouns were the name of a person (e.g., David, Emily) or an object from the categories clothing (e.g., glove, shirt) and fruit (e.g., grape, mango). Half of the adjectives could appropriately describe a person (e.g., assertive, energetic, fickle, nervous) but not any of the objects, whereas the remaining half of the adjectives could describe one class of objects, but not persons or the other class of objects (e.g., clothing: patched, threadbare; fruit: sundried, seedless). To ensure that participants made use of general semantic knowledge about different classes of targets, they were further instructed to decide the appropriateness of the adjective for hypothetical exemplars of the noun (e.g., for a hypothetical person named David, not an individual they might know with that name). Each trial began with a fixation cross presented for 250 ms. A target noun was then presented alone for 1,000 ms (36-point New York font), after which an adjective was also presented (36-point Helvetica font). The noun–adjective pair remained onscreen for an additional 2,750 ms, during which the participant's behavioral response was recorded. Each fMRI run consisted of 50 trials in which the adjective was appropriate to the noun and 50 trials in which it was not. A pseudorandom order of trial types and a variable interstimulus interval (250–6,000 ms) was used to optimize estimation of the event-related fMRI response (18). During interstimulus intervals, participants passively viewed a fixation crosshair, which defined the baseline.
MRI Data Analysis.
Preprocessing and statistical analysis of the fMRI data were performed by using spm99 software (Wellcome Department of Cognitive Neurology). To allow the magnetic field to reach equilibrium, the first four time points (8 s) of each functional run were discarded. Preprocessing included slice timing and motion correction, normalization to the MN1305 stereotactic space (interpolating to 3-mm cubic voxels), and spatial smoothing with an 8-mm Gaussian kernel. An automated segmentation algorithm (Stanford University) identified gray matter voxels from each participant's T1-weighted anatomical scan, and subsequent statistical modeling was restricted to these voxels. Statistical analyses were performed by using the general linear model in which the event-related design was modeled by using a canonical hemodynamic response function and its temporal derivative. Comparisons of interest were implemented as linear contrasts. This analysis was performed individually for each participant, and contrast images for each participant were used in a second-level analysis treating participants as a random effect. Peak coordinates were identified by using a statistical criterion of at least 19 contiguous voxels exceeding a voxel-wise threshold of P < 0.001. A Monte Carlo simulation (www.wjh.harvard.edu/∼slotnick/scripts.htm) of our brain volume demonstrated that this cluster extent cutoff provided an experiment-wise threshold of P < 0.05, corrected for multiple comparisons.
Regions of interest (ROIs) were defined from clusters that survived these thresholding criteria, and peristimulus hemodynamic time courses were extracted for each of these ROIs on a participant-by-participant basis (representing percent signal change in each condition relative to the fixation baseline). One sample, two-tailed t tests (random effects, threshold of P < 0.01) were used to test whether signal change at the time point corresponding to the peak response differed significantly from baseline in each region.
Results
Analysis of the reaction time data showed that participants made semantic judgments about Persons significantly faster than comparable judgments about Objects [means, Ms: 900 ms vs. 1,019 ms, t(12) = 3.92, P < 0.002, r = 0.49]. In addition, faster responses were returned on “yes” than “no” trials [Ms: 870 ms vs. 980 ms, t(12) = 6.29, P < 0.0001, r = 0.67]; however, because this response type factor did not impact on the fMRI data, imaging analyses were collapsed across “yes” and “no” trials.
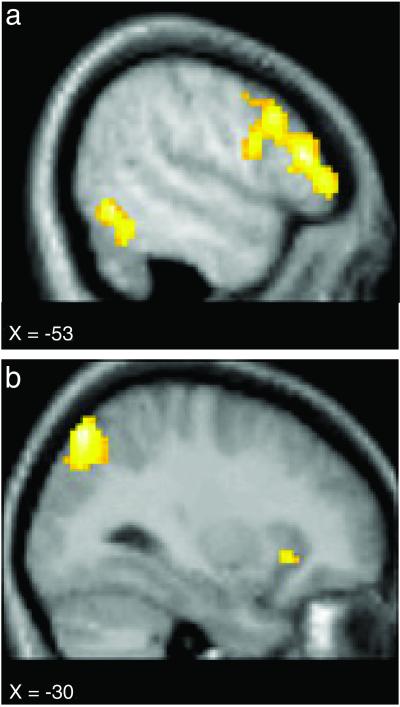
To examine whether judgments about persons and objects were associated with different patterns of neural activity, we compared the event-related BOLD (blood oxygen level-dependent) signal associated with Person trials to that associated with Object trials. This comparison yielded distinct patterns of brain activity for each type of target. Object > Person comparisons (Table 1 and Fig. 1) demonstrated greater activity in left inferior frontal gyrus (LIFG), left inferotemporal (IT) cortex, left posterior parietal cortex, left superior frontal gyrus, and bilateral insula cortex. LIFG modulation was observed in multiple regions extending the entire extent of the inferior frontal gyrus. No significant activation differences were observed between fruits and items of clothing.
Table 1.
Significant peak locations in Object > Person
| Anatomic label
|
x
|
y
|
z
|
t value | |
|---|---|---|---|---|---|
| Object | Person | ||||
| R. insula | 36 | 23 | −6 | 5.70 | 3.20 |
| L. insula | −33 | 24 | −6 | 6.42 | 4.40 |
| L. inf. frontal gyrus | −53 | 30 | 12 | 3.67 | 1.34 |
| −50 | 19 | 27 | 4.80 | 3.22 | |
| −50 | 41 | −2 | 3.65 | 1.40 | |
| −50 | 7 | 22 | 5.26 | 3.15 | |
| −50 | 24 | 4 | 4.34 | 1.64 | |
| −48 | 8 | 36 | 4.11 | 2.56 | |
| −59 | 8 | 38 | 4.29 | 3.41 | |
| −45 | −1 | 33 | 4.48 | 3.04 | |
| L. inf. temporal | −53 | −59 | −5 | 2.98 | 0.31 |
| −50 | −53 | −10 | 4.53 | 2.18 | |
| L. post. parietal | −30 | −67 | 50 | 5.00 | 3.21 |
| L. sup. frontal gyrus | −9 | 17 | 43 | 3.96 | 2.88 |
Coordinates are from the Talairach and Tournoux atlas (49). Object and Person columns display the t value associated with the area's peak hemodynamic response relative to passive baseline for Object and Person trials, respectively;
, P < 0.01;
, P < 0.001; R, right; L, left; inf, inferior; post, posterior; sup, superior.
Fig 1.
Activation maps show brain areas to be more active during Object trials than during Person trials. Regions of modulation included the left inferior prefrontal cortex and the left IT cortex (a), as well as the left posterior parietal and the left insula cortex (b). See Table 1 for the Talairach and Tournoux (49) atlas coordinates.
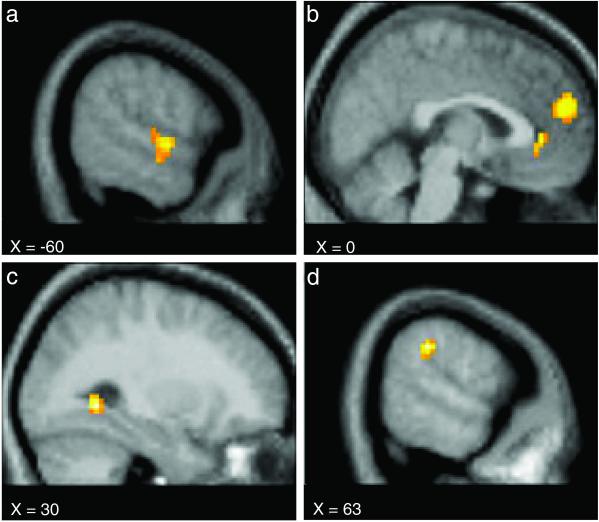
In sharp contrast, Person > Object comparisons (Table 2 and Fig. 2) were associated with modulation in a very different set of brain areas that included dorsal and ventral aspects of the medial prefrontal cortex (MPFC), right intra-parietal sulcus (IPS), right fusiform gyrus (FuG), left superior temporal (ST) and medial temporal (MT) cortex, left motor cortex, and regions of the occipital cortex bilaterally.
Table 2.
Significant peak locations in Person > Object
| Anatomic label
|
x
|
y
|
z
|
t value | |
|---|---|---|---|---|---|
| Object | Person | ||||
| Dorsal MPFC | 0 | 54 | 21 | −5.40 | −1.36 |
| Ventral MPFC | 3 | 39 | 0 | −5.55 | −3.41 |
| 12 | 36 | 0 | −4.39 | −2.81 | |
| R. fusiform | 30 | −51 | −3 | −2.08 | −0.92 |
| R. intraparietal sulcus | 63 | −33 | 33 | −3.19 | −0.89 |
| 60 | −33 | 21 | −3.67 | −0.46 | |
| R. occipital | 48 | −63 | 12 | −3.70 | −1.64 |
| L. sup. temporal | −60 | −6 | −3 | −0.65 | 1.61 |
| −60 | −12 | −12 | −0.45 | 1.37 | |
| L. med. temporal | −66 | −24 | −12 | −1.55 | 0.25 |
| −66 | −18 | −15 | −0.78 | 0.39 | |
| L. motor | −45 | −27 | 63 | −1.57 | −2.22 |
| −30 | −39 | 60 | −0.41 | −1.49 | |
| −33 | −36 | 69 | −1.12 | −1.71 | |
| −30 | −27 | 69 | −1.46 | −2.29 | |
| L. occipital | −51 | −75 | 21 | −3.07 | −0.80 |
| −15 | −99 | 21 | −2.10 | −0.96 | |
Coordinates are from the Talairach and Tournoux atlas (49). Object and Person columns display the t value associated with the area's peak hemodynamic response relative to a passive baseline for Object and Person trials, respectively. Negative t values represent deactivations relative to baseline;
, P < 0.01;
, P < 0.001; L, left; R, right; MPFC, medial prefrontal cortex; sup, superior; Ed, medial.
Fig 2.
Activation maps show brain areas to be more active during Person trials than during Object trials. Regions of modulation included the left temporal sulcus (a), the dorsal and ventral MPFC (b), the right FuG (c), and the right parietal temporal-occipital junction (d). See Table 2 for the Talairach and Tournoux (49) atlas coordinates.
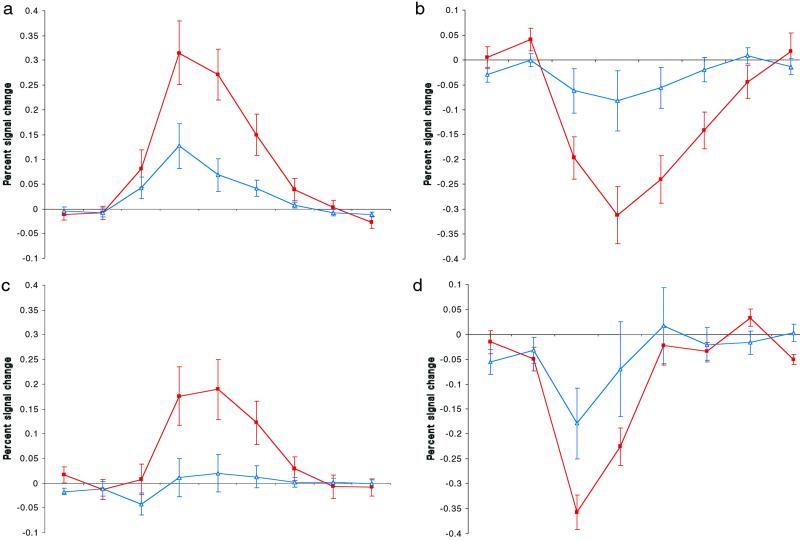
To further investigate the neural response in regions modulated by Object and Person judgments, we examined the hemodynamic time course in each of the regions described above (Fig. 3). Beyond the observed neuroanatomical differences between Object and Person judgments, these two sets of brain regions were associated with qualitatively different hemodynamic responses. Whereas regions identified from Object > Person comparisons uniformly produced signal changes above baseline (Table 1 and Fig. 3 a and b), regions identified from Person > Object comparisons were generally marked by nonsignificant or modest changes from baseline in response to Person targets, along with significant deactivations in response to Objects (Table 2 and Fig. 3 c and d). Indeed, the brain regions associated with activations above baseline for Person trials were almost entirely a subset of those demonstrating activations for Object trials, except for some activations unique to Person trials in bilateral basal ganglia, bilateral occipital lobe, and left cerebellum. In all cases, these unique Person trial activations were in voxels adjacent to Object trial activations and appeared generally to be serving the motoric and perceptual demands of the experimental task.
Fig 3.
The hemodynamic time courses in the left inferior prefrontal (a) and the left inferior temporal cortex (c) were characterized by activations above baseline for Object trials (filled red squares) and either modest or nonsignificant activations for Person trials (open blue triangles). In contrast, the dorsal medial prefrontal (b) and right lateral parietal cortices (d) were characterized by significant deactivations for Object trials, along with no significant modulations for Person trials, a pattern typical for areas identified in Person > Object comparisons. Time courses were calculated by collapsing across multiple clusters within a neuroanatomical region (except for the dorsal medial prefrontal cortex, for which only one cluster was identified). The scale is seconds (one second per hash mark). Error bars display the standard error of the mean.
Finally, because Person trials were associated with significantly faster reaction times than Object trials, we conducted a secondary analysis to rule out the possibility that time course differences were spuriously produced by differences in the relative difficulty of the Person and Object judgments. Specifically, for each functional scan we selected 20 Object trials and then selected a subset of 20 Person trials matched to the Object trials for reaction time. The resulting average reaction time for Object trials (1,051 ms) was nearly identical to that for Person trials (1,047 ms). We then reanalyzed the fMRI data by using these trials and subsequently compared the peak signal change for time courses associated with Person and Object trials in each of the regions obtained in the primary analysis. Of the 17 peak activations observed in Person > Object comparisons, only a single region did not continue to demonstrate a significant difference (P < 0.05) after matching for time on task. This was the left MT region centered at −66, −18, −15, which was only marginally significant, P < 0.11. Of the 14 peak activations observed in Object > Person comparisons, all regions continued to demonstrate a significant difference after matching for time on task. Differences were particularly stable in MPFC (for Person > Object) and LIFG (for Object > Person), where we obtained a significance level of P < 0.01 for all comparisons after matching for time on task.
Discussion
The results of this experiment demonstrate a qualitative dissociation between the brain areas subserving semantic judgments about people and inanimate objects. This dissociation was evidenced both by the neuroanatomical localization of different brain areas modulated by Object and Person judgments (Figs. 1 and 2) as well as by qualitative differences in the nature of the hemodynamic time courses underlying these modulations (Fig. 3).
Neuroanatomically, Object > Person comparisons yielded regions (LIFG, IT, and posterior parietal cortex) that were highly consistent with earlier neuroimaging research on semantic memory and object recognition. LIFG activation has consistently been observed across a range of tasks that require use of semantic knowledge, including object naming and categorization, exemplar generation, abstract/concrete word decisions, and tests of factual knowledge (19–22). In addition, activation in the IT region was almost identical to that observed in a previous study in which participants were required to make semantic judgments about tools versus animals (8), and similar activations throughout IT have been associated with a range of object perception/naming tasks (5–7). Finally, earlier work has also implicated posterior parietal cortex during the maintenance of object information before a behavioral response (23) and during tactile object recognition tasks (24).
In the same way, Person > Object comparisons identified regions (dorsal and ventral MPFC, the IPS, the ST region, and FuG) that converge with earlier work suggesting that these areas participate in a range of social-cognitive tasks (25). For example, a number of studies have observed modulation in dorsal MPFC in tasks that require self-monitoring or the attribution of mental states to others (26–28), as well as during retrieval of personally relevant memories (29, 30). In addition, patient studies have linked ventral MPFC to activities that involve the rapid, flexible use of social knowledge, such as gender stereotyping (31) and the integration of emotion with thoughts and behavior (32–35). Similarly, the IPS, ST, and FuG have consistently been linked to the perception of socially relevant stimuli, such as eye gaze, biological motion, body parts, and faces. For instance, several recent studies have observed IPS modulation when people assume the physical perspective of another individual (36) or perceive targets displaying direct eye gaze (15, 37), tasks that have been linked to theory of mind and its associated cognitive functions. Likewise, the ST region (the superior temporal sulcus and adjacent areas of the superior and inferior temporal gyri) has been shown to play an important role in the perception of social stimuli, including head and mouth movements, changes in eye gaze, body parts, and emotional expression (9–12). Finally, the modulation we observed in FuG corresponds closely to a region, dubbed the fusiform face area (FFA), that previous research suggests is selectively responsive to perception of, imagery for, and identification of faces (13–16, 38). We note that, although participants never reported engaging in visual imagery for the person trials, these IPS, ST, and FuG modulations may nevertheless have been associated with participants' spontaneous generation of mental images of body parts and faces.
Importantly, Object and Person trials were also associated with qualitatively different hemodynamic time courses. Whereas Object > Person modulations took the form of activations above the baseline, modulations associated with Person > Object comparisons were typically deactivations. More specifically, these Person > Object modulations were produced by consistent deactivations for Object trials along with nonsignificant or modest modulations for Person trials. As such, these data raise a question about how to interpret the observation that Person judgments consistently produced little change from a passive baseline condition.
One framework in which to consider these results originates in the observation that, when at rest, some brain regions are characterized by relatively high rates of metabolic activity (29, 39, 40). That is, the resting brain consistently assumes a preferred configuration of metabolic activity, with some regions routinely displaying higher levels of activity than others. Interestingly, of the four neuroanatomical regions with notably high resting metabolic rates, three were observed in the current study for Person > Object comparisons: dorsal MPFC, ventral MPFC, and lateral parietal areas that include the IPS (medial parietal/precuneus regions constitute the fourth high-metabolism region, which we did not observe across comparisons in this experiment).
Recently, Raichle and colleagues (28, 40, 41) have argued that such tonically high metabolic rates may reflect high levels of spontaneous mental processing that take place during rest. To the extent that metabolic activity in a brain region corresponds to the engagement of mental operations subserved by that region, high metabolic rates suggest that some brain regions engage in continuous, active processing during resting states. Intriguingly, three of the four highest resting metabolic rates are found in brain regions, dorsal and ventral MPFC and lateral parietal cortex (including the IPS), that have consistently been linked to social-cognitive processes such as the simulation of other minds, the flexible use of social and moral knowledge, self-referent memory, emotion regulation, and the perception of socially relevant stimuli (15, 25–27, 30–37, 40, 43–48). Whereas these studies have typically reported MPFC and/or lateral parietal increases, it is important to note that these increases have consistently been observed relative to some other active task, thereby obscuring whether any changes occur relative to a resting baseline (see refs. 30, 40, and 47 for exceptions in MPFC). That social thought and perception appear to be subserved by areas with high resting metabolic rates suggests that social-cognitive processes constitute an important component of the brain's resting state of activity.
Because regions with high metabolic rates are actively engaged during resting states (such as the passive fixation conditions that often define the fMRI baseline), they consistently deactivate across a range of active cognitive tasks (39). When obliged to perform an active task, the brain typically suspends baseline processes, producing deactivations in the regions subserving those processes. However, because researchers have focused almost exclusively on the functional significance of activations above baseline, relatively little is known about the exact nature of the default baseline processes taking place during rest. The limits imposed by restricting interpretation to activations above baseline suggests that a different analytic approach is required when investigating a region associated with high resting metabolic rate and consistent deactivations across tasks. Because such regions are tonically engaged in the processes they subserve, they are unlikely to produce activations above a resting baseline; one may imagine a neural ceiling effect, of sorts. Instead, if a region is known to deactivate during many active tasks, then identifying conditions that produce no change from baseline can help to characterize the kind of processing operations that may occur spontaneously during rest. More specifically, if one experimental condition produces the kind of deactivation typical for a particular region whereas a second condition produces little or no deviation from baseline, one may tentatively conclude that the processes uniquely engaged by the second task overlap (at least in part) with the ongoing, default processes of that region.
The results of the current study demonstrate exactly such a pattern in dorsal MPFC, ventral MPFC, and lateral parietal regions. That is, Object trials produced significant deactivations across all three of these regions, replicating the deactivations that are consistently observed in these regions. In contrast, Person trials did not significantly modulate activity in either dorsal MPFC or lateral parietal cortex/IPS and only modestly modulated activity in ventral MPFC. Taken together, these two characteristics of the observed MPFC and lateral parietal modulations suggest that the processes subserving semantic judgments about social targets overlap considerably with default processes engaged during resting baseline. By yielding a significant deactivation below baseline, ventral MPFC only partly conformed to the idealized pattern of results. However, the observation that Person judgments did not overlap considerably with baseline ventral MPFC activity may not be terribly surprising. Whereas ventral MPFC has been linked to the use of social knowledge in real time, its role in representing abstract social knowledge seems to be minimal. Indeed, patients with ventral MPFC lesions can usually articulate social traits and the norms that govern social life, although they appear impaired at making appropriate use of such explicit information in their everyday life (31).
In summary, the results of the present investigation suggest that distinct networks of brain regions subserve the representation of semantic knowledge about people and objects. Although the areas participating in the representation of object knowledge have been characterized extensively in both neuropsychological and neuroimaging work, the current study is, to the best of our knowledge, the first to identify a network of regions that specifically subserve person knowledge. One interesting future direction will be to disentangle whether person-sensitive regions respond simply to the presence of socially relevant stimuli or whether they subserve some specialized cognitive operations brought to bear when thinking about people (e.g., representing the internal states of other intentional agents). We speculate that the semantic system for person knowledge may prove to rely on a neural architecture structurally similar to that underlying object knowledge: temporal areas such as the ST region and FuG may subserve perception and identification of socially relevant stimuli in the environment (as IT areas do for object perception), whereas MPFC areas may represent more elaborative semantic information about the descriptive characteristics or internal mental states of social agents (akin to the role of LIFG in nonsocial semantics). Person knowledge may also have a unique contribution from the lateral parietal/IPS region, which may partly support theory of mind representations (15, 37). While this initial study points out the functional neuroanatomical dissociation between the systems supporting object and person knowledge, continuing research guided by these hypotheses will certainly be needed to characterize the nature of these systems more fully.
Acknowledgments
We thank Lila Davachi, Souheil Inati, Orville Jackson, Bill Kelley, Tammy Laroche, Anat Maril, Rebecca Saxe, Scott Slotnick, Anthony Wagner, and Carrie Wyland for their advice and assistance. This work was supported by National Science Foundation Grant BCS 0072861 and predoctoral National Research Service Award F31 NH65053.
Abbreviations
fMRI, functional MRI
LIFG, left inferior frontal gyrus
MPFC, medial prefrontal cortex
ST, superior temporal
MT cortex, medial temporal cortex
IT cortex, inferotemporal cortex
FuG, fusiform gyrus
IPS, intraparietal sulcus
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Caramazza A. & Shelton, J. R. (1998) J. Cognit. Neurosci. 10, 1-34. [DOI] [PubMed] [Google Scholar]
- 2.Martin A., Wiggs, C. L., Ungerleider, L. G. & Haxby, J. V. (1996) Nature 379, 649-652. [DOI] [PubMed] [Google Scholar]
- 3.Martin A. (2001) in Handbook of Functional Neuroimaging of Cognition, eds. Cabeza, R. & Kingstone, A. (MIT Press, Cambridge, MA), pp. 153–186.
- 4.Shelton J. R. & Caramazza, A. (2001) in The Handbook of Cognitive Neuropsychology: What Deficits Reveal About the Human Mind, ed. Rapp, B. (Psychology Press/Taylor & Francis, Philadelphia), pp. 423–443.
- 5.Haxby J. V., Ishai, A., Chao, L., Ungerleider, L. G. & Martin, A. (2000) Trends Cognit. Sci. 4, 3-4. [DOI] [PubMed] [Google Scholar]
- 6.Haxby J. V., Gobbini, M. I., Furey, M. L., Ishai, A., Schouten, J. L. & Pietrini, P. (2001) Science 293, 2425-2430. [DOI] [PubMed] [Google Scholar]
- 7.Ishai A., Ungerleider, L. G., Martin, A., Schouten, J. L. & Haxby, J. V. (1999) Proc. Natl. Acad. Sci. USA 96, 9379-9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao L. L., Haxby, J. V. & Martin, A. (1999) Nat. Neurosci. 2, 913-919. [DOI] [PubMed] [Google Scholar]
- 9.Allison T., Puce, A. & McCarthy, G. (2000) Trends Cognit. Sci. 4, 267-278. [DOI] [PubMed] [Google Scholar]
- 10.Perrett D. I., Harries, M. H., Mistlin, A. J., Hietanen, J. K., Benson, P. J., Bevan, R., Thomas, S., Oram, M. W., Ortega, J. & Brierley, K. (1990) Int. J. Comp. Psychol. 4, 25-55. [Google Scholar]
- 11.Puce A., Allison, T., Bentin, S., Gore, J. C. & McCarthy, G. (1998) J. Neurosci. 18, 2188-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narumoto J., Okada, T., Sadato, N., Fukui, K. & Yonekura, Y. (2001) Brain Res. Cognit. Brain Res. 12, 225-231. [DOI] [PubMed] [Google Scholar]
- 13.Kanwisher N., McDermott, J. & Chun, M. M. (1997) J. Neurosci. 17, 4302-4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCarthy G., Puce, A., Gore, J. C. & Allison, T. (1997) J. Cognit. Neurosci. 9, 605-610. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman E. A. & Haxby, J. V. (2000) Nat. Neurosci. 3, 80-84. [DOI] [PubMed] [Google Scholar]
- 16.Halgren E., Dale, A. M., Sereno, M. I., Tootell, R. B., Marinkovic, K. & Rosen, B. R. (1999) Hum. Brain Mapp. 7, 29-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen J., MacWhinney, B., Flatt, M. & Provost, J. (1993) Behavioral Research Methods, Instruments, and Computers 25, 257-271. [Google Scholar]
- 18.Dale A. M. (1999) Hum. Brain Mapp. 8, 109-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabeza R. & Nyberg, L. (2000) J. Cognit. Neurosci. 12, 1-47. [DOI] [PubMed] [Google Scholar]
- 20.Maril A., Wagner, A. D. & Schacter, D. L. (2001) Neuron 31, 653-660. [DOI] [PubMed] [Google Scholar]
- 21.Wagner A. D., Schacter, D. L., Rotte, M., Koustaal, W., Maril, A., Dale, A. M., Rosen, B. R. & Buckner, R. L. (1998) Science 281, 1188-1191. [DOI] [PubMed] [Google Scholar]
- 22.Gabrieli J. D. E., Poldrack, R. A. & Desmond, J. E. (1998) Proc. Natl. Acad. Sci. USA 95, 906-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mecklinger A., Bosch, V., Gruenewald, C., Bentin, S. & von Cramon, D. Y. (2000) Hum. Brain Mapp. 11, 146-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deibert E., Kraut, M., Kremen, S. & Hart, J. (1999) Neurology 52, 1413-1417. [DOI] [PubMed] [Google Scholar]
- 25.Adolphs R. (2001) Curr. Opin. Neurobiol. 11, 231-239. [DOI] [PubMed] [Google Scholar]
- 26.Castelli F., Happé, F., Frith, U. & Frith, C. (2000) NeuroImage 12, 314-325. [DOI] [PubMed] [Google Scholar]
- 27.Frith C. D. & Frith, U. (1999) Science 286, 1692-1695. [DOI] [PubMed] [Google Scholar]
- 28.Gusnard D. A. & Raichle, M. E. (2001) Nat. Rev. Neurosci. 2, 685-694. [DOI] [PubMed] [Google Scholar]
- 29.Maguire E. A. & Mummery, C. J. (1999) Hippocampus 9, 54-61. [DOI] [PubMed] [Google Scholar]
- 30.Kelley W. M., Macrae, C. N., Wyland, C. L., Caglar, S., Inati, S. & Heatherton, T. F. (2002) J. Cognit. Neurosci. 14, 785-794. [DOI] [PubMed] [Google Scholar]
- 31.Milne E. & Grafman, J. (2001) J. Neurosci. 21, 1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damasio A. R., Tranel, D. & Damasio, H. C. (1991) in Frontal Lobe Function and Dysfunction, eds. Levin, H. S., Eisenberg, H. M. & Benton, A. L. (Oxford Univ. Press, New York), pp. 217–229.
- 33.Damasio A. R., (1994) Descartes' Error (Grosset/Putnam, New York).
- 34.Anderson S. W., Bechara, A., Damasio, H., Tranel, D. & Damasio, A. R. (1999) Nat. Neurosci. 2, 1032-1037. [DOI] [PubMed] [Google Scholar]
- 35.Bechara A., Dolan, S., Denburg, N., Hindes, A., Anderson, S. W. & Nathan, P. E. (2001) Neuropsychologia 39, 376-380. [DOI] [PubMed] [Google Scholar]
- 36.Zacks J., Rypma, B., Gabrieli, J. D. E., Tversky, B. & Glover, G. H. (1999) Neuropsychologia 37, 1029-1040. [DOI] [PubMed] [Google Scholar]
- 37.Calder A. J., Lawrence, A. D., Keane, J., Scott, S. K., Owen, A. M., Christoffels, I. & Young, A. W. (2002) Neuropsychologia 40, 1129-1138. [DOI] [PubMed] [Google Scholar]
- 38.O'Craven K. & Kanwisher, N. (2000) J. Cognit. Neurosci. 12, 1013-1023. [DOI] [PubMed] [Google Scholar]
- 39.Shulman G. L., Fiez, J. A., Corbetta, M., Buckner, R. L., Miezen, F. M., Raichle, M. E. & Petersen, S. E. (1997) J. Cognit. Neurosci. 9, 648-663. [DOI] [PubMed] [Google Scholar]
- 40.Gusnard D. A., Akbudak, E., Shulman, G. L. & Raichle, M. E. (2001) Proc. Natl. Acad. Sci. USA 98, 4259-4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raichle M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A. & Shulman, G. L. (2001) Proc. Natl. Acad. Sci. USA 98, 676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fletcher P. C., Happé, F., Frith, U., Baker, S. C., Dolan, R. J., Frackowiak, R. S. J. & Fritch, C. D. (1995) Cognition 57, 109-128. [DOI] [PubMed] [Google Scholar]
- 43.Gallagher H. L., Happé, F., Brunswick, N., Fletcher, P. C., Frith, U. & Frith, C. D. (2000) Neuropsychologia 38, 11-21. [DOI] [PubMed] [Google Scholar]
- 44.George M. S., Ketter, T. A., Parekh, P. I., Herscovitch, P. & Post, R. M. (1996) Biol. Psychiatry 40, 859-871. [DOI] [PubMed] [Google Scholar]
- 45.Goel V., Grafman, J., Sadato, N. & Hallet, M. (1995) NeuroReport 6, 1741-1746. [DOI] [PubMed] [Google Scholar]
- 46.Moll J., de Oliveira-Souza, R., Eslinger, P. J., Bramati, I. E., Mourao-Miranda, J., Andreiuolo, P. A. & Pessoa, L. (2002) J. Neurosci. 22, 2730-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pardo J. V., Pardo, P. J. & Raichle, M. E. (1993) Am. J. Psychiatry 150, 713-719. [DOI] [PubMed] [Google Scholar]
- 48.Reiman E. M., Lane, R. D., Ahern, G. L., Schwartz, G. E., Davidson, R. J., Friston, K. J., Yun, L.-S. & Chen, K. (1997) Am. J. Psychiatry 154, 918-925. [DOI] [PubMed] [Google Scholar]
- 49.Talairach J. & Tournoux, P., (1988) Coplanar Stereotaxic Atlas of the Human Brain (Thieme, New York).