Abstract
Background
Intercostal muscles are richly innervated by mechanoreceptors. In vivo studies of cat intercostal muscle have shown that there are 3 populations of intercostal muscle mechanoreceptors: primary muscle spindles (1°), secondary muscle spindles (2°) and Golgi tendon organs (GTO). The purpose of this study was to determine the mechanical transduction properties of intercostal muscle mechanoreceptors in response to controlled length and velocity displacements of the intercostal space. Mechanoreceptors, recorded from dorsal root fibers, were localized within an isolated intercostal muscle space (ICS). Changes in ICS displacement and the velocity of ICS displacement were independently controlled with an electromagnetic motor. ICS velocity (0.5 – 100 μm/msec to a displacement of 2,000 μm) and displacement (50–2,000 μm at a constant velocity of 10 μm/msec) parameters encompassed the full range of rib motion.
Results
Both 1° and 2° muscle spindles were found evenly distributed within the ICS. GTOs were localized along the rib borders. The 1° spindles had the greatest discharge frequency in response to displacement amplitude followed by the 2° afferents and GTOs. The 1° muscle spindles also possessed the greatest discharge frequency in response to graded velocity changes, 3.0 spikes·sec-1/μm·msec-1. GTOs had a velocity response of 2.4 spikes·sec-1/μm·msec-1 followed by 2° muscle spindles at 0.6 spikes·sec-1/μm·msec-1.
Conclusion
The results of this study provide a systematic description of the mechanosenitivity of the 3 types of intercostal muscle mechanoreceptors. These mechanoreceptors have discharge properties that transduce the magnitude and velocity of intercostal muscle length.
Background
The location and numbers of intercostal muscle mechanoreceptors suggest that they mediate the transduction of chest wall contractile mechanics [1-3]. Intercostal muscle spindles have been identified histologically [3]. These afferents increase their discharge frequency during inspiration and decrease their activity during expiration [3]. Intercostal muscle mechanoreceptors enter the spinal cord through the thoracic dorsal roots [3]. These chest wall mechanoreceptors are responsible for segmental and intersegmental proprioceptive feedback from the intercostal muscles. Intercostal afferents have been shown to alter inspiratory muscle motor activity [4-7]. The activity of respiratory muscle afferents provides essential muscle mechanical information during ventilation [3]. This is supported by previous reports that have shown these receptors to be sensitive to changes in muscle length and stretch velocity and are active during spontaneous breathing [1-3,8].
There have been relatively few studies investigating the mechanical transduction properties of intercostal muscle mechanoreceptors, with mechanical stimulation confined to the tidal breathing range [1,2]. Hindlimb muscle preparations [9-11] and similar studies have shown that there are two basic types of muscle spindles receptors, 1° and 2° endings. The 1° muscle spindles were shown to be more responsive to the velocity of stretch and the 2° afferents were sensitive to static length changes. A third type of mechanoreceptor, the Golgi tendon organ (GTO), was also responsive to muscle movement (contraction). Von Euler & Peretti [2] found that all three types of mechanoreceptors (GTOs, 1° and 2° muscle spindles) increase their discharge frequency in response to changes in muscle length and stretch velocity in the tidal breathing range. This is supported by intra-axonal recording of 1° muscle spindles demonstrating increased discharge with inspiration during tidal breathing in the cat [3]. While it is known that intercostal mechanoreceptors are sensitive to muscle motion, the specific afferent response to independently controlled changes in muscle displacement and velocity over the tidal volume range of rib motion is unknown. It was hypothesized that intercostal muscle 1° muscle spindles, 2° muscle spindles and GTO's transduce muscle length changes with increased length associated with increased discharge frequency. It was further hypothesized that 1° intercostal muscle spindles and GTO's also code muscle velocity changes with increased velocity associated with an increase in discharge frequency.
Results
Fifty-six intercostal muscle mechanoreceptors were localized. Muscle spindles accounted for 21 of these mechanoreceptors, 10 were identified as Golgi tendon organs, and 25 mechanoreceptors were not characterized. The receptive field locations of these afferents were distributed throughout the intercostal muscle (Fig. 1). The receptive fields of the isolated mechanoreceptors were discretely localized within a 3 – 6 mm circumferential area. The muscle spindles were distributed evenly along the dorsal-ventral extent of the 7th intercostal space. The Golgi tendon organs were located primarily along the caudal edge of the fixed cranial rib with a few isolated along the cranial edge of the caudal rib.
Figure 1.
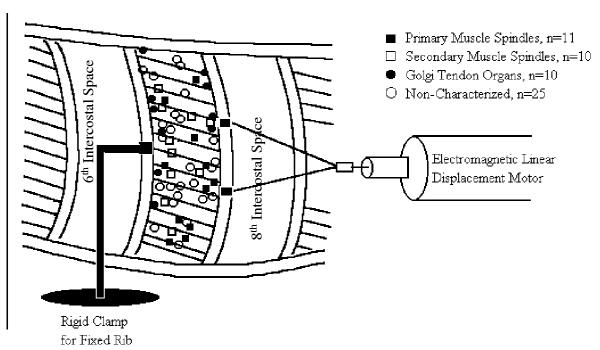
A schematic representation of the experimental preparation. The cranial rib of the isolated rib space is fixed to a rigid clamp. The caudal rib is connected to the electromagnetic displacement motor. The receptive field locations of the intercostal muscle mechanoreceptors are shown. The 1° muscle spindles are represented by the open circles. The 2° muscle spindles by the closed circles, the Golgi tendon organs by the open squares, and the uncharacterized mechanoreceptors by the closed squares. A total of 56 mechanoreceptors in 35 cats were found.
Eleven receptors were identified as 1° muscle spindles and 10 were characterized as 2° muscle spindles. The 1° afferents possessed higher conduction velocities (CV = 68.5 ± 8.7 m/sec) than 2° endings (CV = 43.8 ± 6.2 m/sec), although there was an overlap between the two (Fig. 3). The 10 mechanoreceptors classified as Golgi tendon organs had a mean CV of 41.9 ± 8.8 m/sec.
Figure 3.
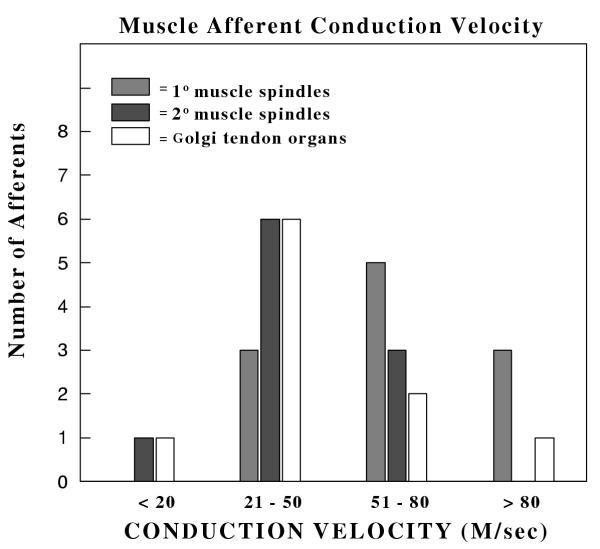
Distribution of conduction velocities for muscle mechanoreceptors. The mean conduction velocity of 1° and 2° afferents was 68.5 ± 8.7 and 43.8 ± 6.2 m/sec, respectively. The mean conduction velocity for Golgi tendon organs was 41.9 ± 8.8 m/sec.
Muscle spindles were studied with and without intact ventral roots. There was no significant difference in the Linit static discharge frequencies after the ventral roots were severed indicating there was no appreciable activity in gamma motor axons. Increasing ICS displacement resulted in an increased discharge frequency in all 1° muscle spindles (Fig. 4). The Linit static discharge frequency for all 1° muscle spindles was 16 ± 2 spikes/sec. The slope of static displacement discharge frequency was found to be 8.8 spikes/sec/mm (Fig. 4). The range of static displacement discharge frequency for 1° afferents was found to be 18.8 ± 5 spikes/sec at 50 μm displacement to 34.5 ± 5 spikes/sec at 2000 μm displacement. The peak instantaneous frequency of 1° muscle spindles occurred during the ramp phase of displacement. The peak instantaneous frequency also increased with increasing velocity of stretch. 1° muscle spindles had a characteristic adaptation of discharge frequency at the end of the ramp phase of stretch. The Linit static discharge and static hold phase discharge frequencies were unaffected by ventral root section.
Figure 4.
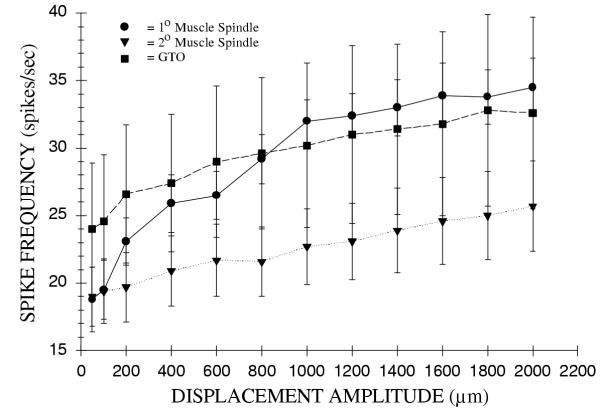
The peak instantaneous discharge frequencies during changes in static displacement for intercostal muscle mechanoreceptors. The discharge frequencies are for: a) 1° muscle spindles (n = 11); b) 2° muscle spindles (n = 10); and c) GTO (n = 10). The mean discharge frequencies, ± S.E. are plotted.
The 2° muscle spindles were also found to increase their discharge frequency with increasing amplitudes of intercostal space displacement. The Linit static discharge frequency of the 2° muscle spindles was not affected by ventral root section. The response of 2° muscle spindles to increasing displacement amplitudes had a slope of 3.7 spikes/sec/mm. The mean Linit static discharge of these afferents was 17 ± 2 spikes/sec (Fig. 4). The range for displacement frequencies for 2° muscle spindles was 19 ± 2 at 50 μm to 26 ± 3 spikes/sec at 2000 μm.
Golgi tendon organs increased their discharge frequency with increasing displacement amplitudes. Most of the GTO's had only small increases in frequency with static changes in intercostal muscle length. The slope of the curve was 2.7 spikes/sec/mm. The mean Linit static discharge frequency for GTO's was 15 ± 4 spikes/sec (Fig. 4). The range of displacement discharge frequencies was 24 ± 5 to 33 ± 7 spikes/sec.
The group mean Linit static discharge frequency for 1° afferents was 16 ± 2 spikes/sec. The range of peak instantaneous frequencies for 1° afferents was 41 ± 3 spikes/sec at 0.5 μm/msec velocity to 221 ± 29 spikes/sec at 100 μm/msec velocity (Fig. 5). The curve was sinusoidal so the slope of the middle, linear range of the sinusoidal curve (between stretch velocities of 5 to 50 μm/msec) was 3.0 spikes·sec-1/μm·msec-1. The static discharge hold phase frequency for 1° muscle spindles was 35 ± 3 spikes/sec.
Figure 5.
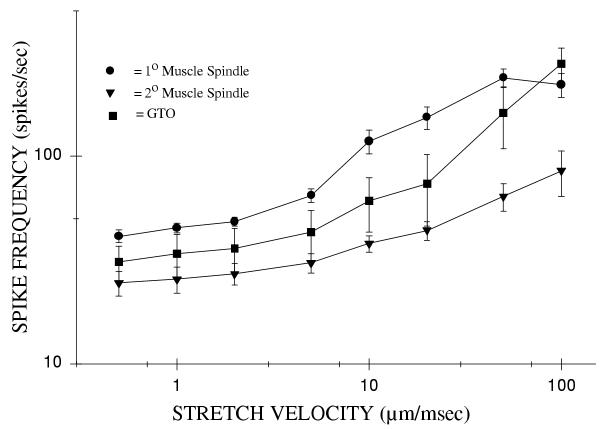
The peak instantaneous discharge frequencies during changes in displacement velocity for intercostal muscle mechanoreceptors. The discharge frequencies are for: a) 1° muscle spindles (n = 11); b) 2° muscle spindles (n = 10); and c) GTO (n = 10). The mean discharge frequencies, ± S.E. are plotted. The axes are plotted on log-log scale.
2° muscle spindles increased peak instantaneous frequencies with increasing intercostal muscle stretch velocity (Fig. 5). The range of discharge frequency was 25 ± 3 spikes/sec at 0.5 μm/msec to 85 ± 21 spikes/sec at 100 μm/msec intercostals muscle stretch velocity. The slope of the frequency response for 2° afferents was 0.6 spikes-sec-1/μm·msec-1 and was taken from stretch velocities between 5 and 100 μm/msec. The static hold phase frequency was 24 ± 3 spikes/sec and the Linit static discharge frequency was 17 ± 3 spikes/sec. 2° muscle spindles were found to have the lowest velocity sensitivity of the three types of mechanoreceptors studied.
Golgi tendon organs mean Linit static discharge frequencies was 16 ± 2 spikes/sec (Fig. 5). The peak instantaneous frequency range for GTO's was from 31 ± 6 to 278 ± 52 spikes/sec as velocities were increased from 0.5 – 100 μm/msec. GTO's were found to possess a sensitivity of 2.4 spikes·sec-1/μm·msec-1 between stretch velocities of 5 and 100 μm/msec. The GTOs had a mean static hold phase frequency of 26 ± 4 spikes/sec.
Discussion
The results of this study provide a systematic description of the mechanosenitivity of the three types of intercostal muscle mechanoreceptors, 1° muscle spindles, 2° muscle spindles, and Golgi tendon organs. These mechanoreceptors have discharge properties that transduce the magnitude and velocity of intercostal muscle length changes into an afferent code that is transmitted to the central nervous system via their spinal dorsal roots. These afferents were located throughout the intercostal muscles. All the mechanoreceptors studied increased their frequency of discharge with increased velocities of intercostal space stretch. With the exception of three Golgi tendon organs, all of the mechanoreceptors increased their frequency of discharge with increased magnitudes of intercostal space displacement. These afferents enter the spinal cord through the thoracic dorsal spinal roots and provide essential information to the central nervous system on intercostal muscle mechanics.
In the present study, intercostal muscle mechanoreceptors were characterized as muscle spindles (primary or secondary) or Golgi tendon organs using criteria based primarily on physiological response patterns (see Methods). It has been shown by others that compared to muscles in the cat hindlimb where spindle classification can be based almost entirely on conduction velocity, non-limb muscles contain primary and secondary muscle spindle afferents that are generally slower conducting and exhibit overlapping bimodal or unimodal conduction velocity spectra [12,13]. These studies have shown that only the very fastest afferents innervate primary endings (>75 m/s) and the negatively adapting discharge frequency during static hold and the abrupt decrease in firing at the end of static stretch are the best criteria for characterization of primary spindle afferents. In the present study, intercostal muscle spindles exhibited a bimodal but overlapping conduction velocity distribution necessitating the use of these physiological criteria.
The maximal displacement amplitude used in this study was 2,000 μm. This magnitude is approximately equal to the change in intercostal muscle length recorded during a tidal breath with artificial and spontaneous respiration [2,8,14,15]. Mechanoreceptors were recorded from the intercostal muscles of the 7th intercos tal space. These are the lower parasternal intercostal muscles and increases in their length can occur during spontaneous and mechanical ventilation. Von Euler & Peretti [2] used a change in intercostal muscle length of 2 mm at a velocity of approximately 1–2 mm/sec to study intercostal mechanoreceptors. The present study used a velocity range of 0.5 mm/sec – 100 mm/sec to span the velocities observed in spontaneously breathing and ventilated animals. The responsiveness of intercostal muscle mechanoreceptors to stretch velocities and displacement amplitudes used in the present study encompassed the physiologic range of intercostal muscle motion.
The intercostal muscle spindles localized in this study show that 1° and 2° afferents could be found with equal frequency along the dorsal – ventral extent of the intercostal space. Golgi tendon organs were also evenly distributed along the intercostal space at the tendinous attachment of the muscle to the rib. None of the receptors localized in these experiments were found in the most ventral regions of the rib cage. This is consistent with the results of Von Euler & Peretti [2], who found intercostal muscle spindles in the cat to be evenly distributed along the dorsal – ventral extent with a slight decrease in the numbers of afferents towards the more ventral aspects. This decrease in the number of afferents ventrally may be related to the fact that all of the receptors studied were recorded from the rostral three dorsal rootlets supplying the 7th intercostal space. These rootlets were used primarily due to their longer length compared to the more caudal rootlets. Intercostal muscle mechanoreceptors are distributed throughout the intercostals muscle with afferent nerve fibers distributed throughout all the dorsal rootlets. The activity of the mechanoreceptors functioning within the more rostral rootlets were randomly recorded in the present study and should be representative for the discharge properties of the afferents throughout the intercostal space.
The static displacement sensitivity for intercostal muscle mechanoreceptors has not been studied previously. 1° muscle spindles were found to possess the highest firing frequency range with displacements to 2000 μm. Cooper [9] recorded muscle spindle response to static displacements in cat soleus muscle and found sensitivities of 10 spikes/sec/mm for 1° spindles and 5 spikes/sec/mm for 2° afferents when the muscle was stretched in 1 mm increments to 4 mm displacement. The results of the present study are similar to the sensitivities reported for the cat soleus muscle but differ from muscles of the digits in cats. These differences are probably due to the significant mechanical variations in the function of these different muscle groups. The intercostal muscle has a unique attachment between the ribs that results in an angle of 50 – 60 degrees between the muscle fibers and the rib. A linear displacement of the rib space will therefore not produce a linear displacement of muscle fibers. The change in fiber length is probably less than the change in space width, resulting in an underestimation of the displacement sensitivity. This is particularly true for changes in tension and the associated discharge of the GTO. The experiments described above show that tendon organs are responsive to static changes in intercostal muscle length, but at a sensitivity lower than that of either the 1° or 2° muscle spindles. Increasing the width of the intercostal space may change the length of the muscle fibers less than space width, producing smaller changes in muscle tension. This would reduce the stimulation of GTOs and their displacement sensitivity would be underestimated. While the absolute sensitivity to fiber displacement may be underestimated for the mechanoreceptors recorded in this study, the above results do provide important quantification of the sensitivity of intercostal mechanoreceptors to changes in the functional displacement of the intercostal space.
The dynamic sensitivities of the receptors were found to correspond to the ranges of discharge frequencies of afferents characterized by other investigators [2,9,11]. Matthews [10] found that de-efferented 1° muscle spindles in the soleus muscle of the cat responded to varied velocity changes (1.2 – 100 mm/sec) in the range of 95 – 400 spikes/sec. Discharge frequencies found for primary muscle spindles in the present study are also similar to those for 1° afferents in other muscles with intact γ-innervation [9,16,17] and without γ-innervation [18-20]. In these studies, the 1° muscle spindles were found to possess the greatest sensitivity to changes in velocity. Our finding of a decrease in the frequency response at the highest velocity of stretch used (100 μm/msec) shows a limitation of the primary muscle spindle in coding for rapid length changes.
Matthews [10] described a frequency range for two 2° afferents to be approximately 45 – 185 spikes/sec with velocity changes of 1.2 mm/sec to 100 mm/sec to a final length change of 6 mm in the soleus muscle of the cat. The instantaneous burst frequencies obtained in this study for 2° afferents were also similar to those found in other studies [9,11,18]. The results obtained by these investigators were from hindlimb muscles of the cat at 2 – 3 different velocities ranging from 0.5 – 52 mm/sec. Intercostal muscle afferents studied by Critchlow & Von Euler [14] were shown to discharge at rates of 29 – 74 spikes/sec during the inspiratory phase, but were not characterized as being either 1° or 2° muscle spindles. The velocity of intercostal width change was not determined in their study. In the present study, the 2° afferents were found to discharge with a slope of 0.6 spikes·sec-1 /μm·msec-1 and were the least sensitive of the three types of respiratory mechanoreceptors to dynamic changes in intercostal muscle fiber length.
The Golgi tendon organs in this study were found to have a much greater response to the dynamic phase of intercostal muscle stretch compared to the static response. Von Euler & Peretti [2] reported that a few GTOs in cat intercostal muscle could follow vibrational frequencies within the range of 1° muscle spindles. This suggests that the Golgi tendon organs supplying respiratory musculature may be more sensitive to changes in muscle mechanics than those functioning in other muscle groups. Houk & Henneman [21] considered the variable response of GTO discharge to tension changes to be due to differences in motor unit recruitment. These differences, i.e., numbers of muscle fibers activated either parallel to or in-series with the mechanoreceptor, create changes in the afferent receptive field and alter its discharge properties. Passive length changes caused a linear increase in force acting on these receptors. On the other hand, the forces acting on the receptor with active contraction were dependent on the motor units activated. When the motor units in-series with the receptor were activated, there was a linear force applied to the receptor, resulting in increased impulse discharge. However, if the neighboring extrafusal fibers were stimulated to contract, they unload the tendon organ, thus bringing it to a subthreshold level. Houk & Henneman [21] also found the GTOs to exhibit a saturation effect where by the responses to one motor unit and that of a second motor unit did not sum algebraically. The primary role of this type of intercostal mechanoreceptor is hypothesized to be the same as in other muscle groups, i.e. the transduction and coding of muscle tension.
The results of the present study provide a unique demonstration of intercostal muscle mechanoreceptor transduction properties throughout a wide range of intercostal muscle displacement magnitudes and velocities. These afferents transduce the intercostal muscle mechanical events into a neural code that projects to the central nervous system [22] via thoracic spinal dorsal roots.
Conclusions
The results of this study provide a systematic description of the mechanosenitivity of the 3 types of intercostal muscle mechanoreceptors. These mechanoreceptors have discharge properties that transduce the magnitude and velocity of intercostal muscle length. These afferents transduce the intercostal muscle mechanical events into a neural code that projects to the central nervous system.
Methods
These experiments were performed on 35 cats of either sex weighing 3.1 ± 0.8 kg. The animal was placed in an anesthesia chamber and anesthesia was induced with inhalation of halothane/oxygen gas. A catheter was placed in the femoral artery to continuously measure blood pressure (Konigsberg Instruments No. P36) and obtain arterial blood for periodic blood gas analysis (Radiometer ABL 30). Arterial PCO2, PO2 and pH were maintained at control levels throughout the experiment. A second catheter was placed in the femoral vein for intravenous access. Body temperature was maintained at 38 ± 1°C by the use of a heating pad (Neco Model 819) and monitored with a rectal temperature probe (YSI Instruments). Following the catheterization procedures, anesthesia was switched from induction gas to α-chloralose (25 mg/kg) administered i.v. A tracheal catheter was inserted and the animal artificially ventilated (Harvard Respiratory Pump). Sodium bicarbonate was administered as necessary for maintenance of arterial pH at 7.4 ± 0.1.
The animal was placed in a stereotaxic apparatus and its head fixed in a non-traumatic head-holder. The spinous processes of T3 and T10 were clamped. A wide skin incision was made on the left flank and the cutaneous tissues dissected away to expose the external intercostal muscles. A laminectomy was performed from T4 to T8 to expose the dorsal spinal cord. The spinal cord was flooded with warm mineral oil and the dura cut longitudinally to expose the dorsal root filaments. A single dorsal root filament supplying the 7th intercostal space (ICS) was severed at its attachment to the spinal cord and placed on a platform lowered into a warm mineral oil pool. The filament was initially placed across bipolar platinum recording electrodes. The signal was amplified (Grass P-5), band pass filtered (100 Hz – 1 kHz) and led to an oscilloscope (Tektronix 5111), audio monitor (Grass AM 8) and FM tape recorder (Vetter Model D) for subsequent analysis. The 7th intercostal space was probed to assure that this intercostal space contained the afferents in the dorsal root filament.
Initial muscle length (Linit) was measured at functional residual capacity (FRC) with calipers. The intercostal muscles of the rostral (6th) and caudal (8th) rib spaces were severed, isolating the 7th intercostal space to be studied. Lung inflation was maintained with a positive end expiratory pressure of 3 cmH2O. The rostral rib of the isolated space was fixed with a rigid clamp (Fig. 1). The caudal rib was attached by two clamps to the armature of the displacement motor (Ling V203). A regulated voltage was applied to the displacement motor which moved the armature and delivered muscle stretch at controlled velocities and magnitudes. Except during mechanical stimulation of the 7th intercostals space, the wound and exposed intercostal muscles were covered with gauze soaked with warm saline.
The isolated ICS was probed for muscle mechanoreceptor activity. When activity was observed, the dorsal root filament was subdivided until the activity of a single intercostal muscle mechanoreceptor was isolated as described previously [23]. The receptive field was localized by probing with a fine tipped glass rod. The localized respiratory muscle afferent (RMA) was characterized by the muscle spindle "silent period" test [24,25]. Conduction velocity (CV) was obtained by electrically stimulating the afferent receptive field and measuring the distance between receptive field and recording electrodes [10]. Further characterization was performed during analysis of RMA response to stretch (see below). The rib space was set to Linit and baseline RMA discharge frequency recorded. The displacement protocol was then initiated. A square wave pulse of controlled duration triggered a trapezoid generator (Frederick Haer & Co. #2337) that delivered a controlled trapezoidal waveform to the displacement motor. The motor, in turn, produced stretch of the intercostal space with varying displacements and velocities.
Displacement protocol
The response to intercostal muscle displacement was determined by applying graded amplitudes of stretch to the intercostal space. The intercostal space was set at Linit, the width measured at FRC. The displacement amplitudes were applied above Linit. The amplitudes were: 50, 100, 200, 400, 600, 800, 1000, 1200, 1400, 1600, 1800, and 2000 μm. These displacements were presented at a constant velocity of 10 μm/msec. Each displacement test was initiated by activating the displacement motor which pulled the caudal rib at a constant velocity to the predetermined amplitude. The stretch was then maintained for 3 seconds, after which the displacement motor returned the rib to Linit. There was a 10 second rest period before the next displacement test was initiated. The discharge of the afferent was recorded throughout the stretch.
The displacement protocol began with at least 3 displacement stretches of 2,000 μm to provide a constant stretch history in the muscle. The ICS was displaced at each amplitude 3 times in an ascending order of amplitudes. The response of the mechanoreceptors to graded displacement of the ICS was determined by measuring the frequency of discharge at two different points during the applied stretch. The first measurement was taken at Linit. This frequency was the baseline discharge of the respiratory muscle afferent. The second discharge frequency was determined 2 seconds after the end of the ramp phase (Fig. 2). The discharge frequency at this point was termed the static hold phase frequency.
Figure 2.
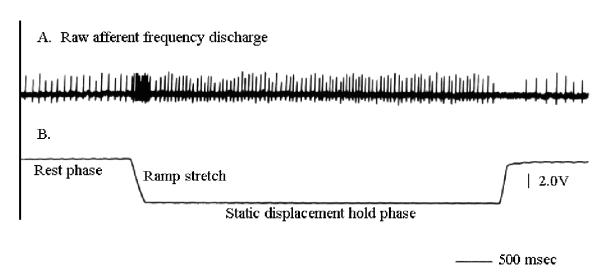
Intercostal muscle spindle response to controlled intercostal space displacement. (a) Afferent spike activity, (b) The intercostal space displacement was to a plateau of 2,000 μm at 10 μm/msec ramp stretch. The calibration for the length change in this trace is: 2.0 V = 1000 μm displacement.
Velocity protocol
The response of RMAs to dynamic changes in intercostal muscle length was determined by varying the stretch velocities of the intercostal space. This experimental trial followed the displacement test. The intercostal space was set at Linit and the velocities applied to displacements above Linit The velocities tested were: 0.5, 1.0, 2.0, 5.0, 10, 20, 50, and 100 μm/msec. Each velocity test was initiated by activating the displacement motor to deliver a 2000 μm displacement at the predetermined velocity. The displacement was maintained for 3 seconds after the completion of the velocity ramp. The rib was then returned to Linit and the muscle allowed to rest for 15 seconds. The next velocity test was then initiated. Each velocity was tested 3 times. The discharge of the afferent was recorded throughout the stretch. The velocity protocol again began with a series of 3 displacement stretches to 2,000 μm to provide a constant stretch history.
The response of the intercostal muscle afferents to graded changes in displacement velocity were then determined. The discharge frequencies measured were the peak instantaneous, static hold phase, and Linit static discharge frequencies. The peak instantaneous frequency was obtained during the ramp stretch of the intercostal muscle and was the maximum frequency of discharge. The static Linit discharge and static hold phase frequencies were taken from positions as described in the displacement protocol.
Data analysis
Afferents were classified as Golgi tendon organs if electrical stimulation of the adjacent extrafusal muscle fibers caused the receptor to increase its discharge frequency. Those afferents that exhibited a pause in discharge frequency as the extrafusal fibers contracted ("silent period") were classified as muscle spindles [24,25]. Primary muscle spindles were differentiated from secondary muscle spindles with the satisfaction of at least two out of three criteria tested on every afferent: 1) a conduction velocity > 75 m/sec; 2) an increase in discharge frequency at 100 μm displacement reflecting a low stretch threshold; 3) an abrupt decrease in frequency discharge at the end of stretch that returned to Linit static discharge levels. In addition to these criteria, the frequency discharge at the end of the dynamic phase of stretch was used as a distinguishing characteristic of 1° and 2° muscle spindles. Those spindles that decreased discharge frequency gradually to static hold phase levels could be identified as primary endings. Secondary afferents reached static hold phase discharge frequency at the end of ramp stretch and showed little adaptation during the static phase. These differentiation criteria have been used by others in studies of non-limb muscles where conduction velocity of only the very fastest spindle fibers can be associated with primary spindle endings [12,13]. The data recorded on magnetic tape was analyzed with the aid of the Spike 2 (Cambridge Electronics Design, Ltd.) computer program. The analog signal from the LVDT transducer connected to the displacement motor provided a measure of length changes and the output was sent from the magnetic tape recorder to a digitizing signal processor (CED 1401). The afferent spikes were passed through a slope/height window discriminator (Frederick Haer & Co.) and then sent to the signal processor as transistor transistor logic (TTL) pulses. The digitizing rate of the afferent signal was 3000 Hz. The program was designed to provide the instantaneous frequency of the afferent spikes at the corresponding amplitudes of the displacement motor that produced the muscle stretch.
The response frequencies used for analysis of the displacement coding properties included the baseline or Linit static discharge and the static hold phase frequency. The velocity protocol recorded these static frequencies in addition to the peak frequency obtained during the dynamic phase of stretch. The data were grouped by afferent population and graphed as means ± Standard Error (S.E.) with the dependent variable being spike frequency and the independent variable being amplitude and/or velocity of muscle stretch. An ANOVA and Scheffe's test for multiple comparisons were used to compare the coding properties of three populations of mechanoreceptors. Statistical significance was recorded at p < 0.05.
Authors' contributions
GAH conceived of the study, carried out the experimental studies, participated in the data analysis and drafted the manuscript. RDJ participated in the design of the study, analysis of the data and manuscript preparation. PWD also conceived of the study, and participated in its design, participated in the data collection, participated in the data analysis participated in study coordination and participated in manuscript preparation.
All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This work was supported by a grant from NIH-NHLBI #HL-37596.
Contributor Information
Gregory A Holt, Email: tsdc2002@aol.com.
Richard D Johnson, Email: johnson@ufbi.ufl.edu.
Paul W Davenport, Email: davenportp@mail.vetmed.ufl.edu.
References
- Newsom-Davis J. The response to stretch of human intercostal muscle spindles studied in vitro. J Physiol Lond. 1975;249:561–579. doi: 10.1113/jphysiol.1975.sp011030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Euler C, Peretti G. Dynamic and static contributions to the rhythmic gamma activation of primary and secondary spindle endings in external intercostal muscle. J Physiol Lond. 1966;187:501–516. doi: 10.1113/jphysiol.1966.sp008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Niwa M, Sasaki S-I, Ichikawa T, Hirai N. Morphology of single primary spindle afferents of the intercostal muscles in the cat. J Comp Neurol. 1998;398:459–472. doi: 10.1002/(SICI)1096-9861(19980907)398:4<459::AID-CNE1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Remmers JE. Inhibition of inspiratory activity by intercostal muscle afferents. Respir Physiol. 1970;10:358–383. doi: 10.1016/0034-5687(70)90055-1. [DOI] [PubMed] [Google Scholar]
- Romaniuk JR, Supinski GS, Dimarco AF. Reflex control of diaphragm activation by thoracic afferents. J Appl Physiol. 1993;75:63–69. doi: 10.1152/jappl.1993.75.1.63. [DOI] [PubMed] [Google Scholar]
- Trippenbach T, Kelly G. Phrenic activity and intercostal muscle EMG during inspiratory loading in newborn kittens. J Appl Physiol: Respirat Environ Exercise Physiol. 1983;54:496–501. doi: 10.1152/jappl.1983.54.2.496. [DOI] [PubMed] [Google Scholar]
- Berdah SV, DeTroyer A. Contribution of spindle reflexes to post-inspiratory activity in the canine external intercostal muscles. J Physiol. 2001;534:873–880. doi: 10.1111/j.1469-7793.2001.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai N, Ichikama T, Ichikawa T, Miyashita M. Activity of the intercostal muscle spindle afferents in the lower thoracic segments during spontaneous breathing in the cat. Neurosci Res. 1996;25:301–304. doi: 10.1016/0168-0102(96)01052-8. [DOI] [PubMed] [Google Scholar]
- Cooper S. The responses of the primary and secondary endings of muscle spindles with intact motor innervation during applied stretch. Quart J Exp Physiol. 1961;46:389–398. doi: 10.1113/expphysiol.1961.sp001558. [DOI] [PubMed] [Google Scholar]
- Matthews PBC. The response of de-efferented muscle spindle receptors to stretching at different velocities. J Physiol Lond. 1963;168:660–678. doi: 10.1113/jphysiol.1963.sp007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JJA. Classification of muscle spindle afferents in the peroneus brevis muscle of the cat. Brain Res. 1990;509:62–70. doi: 10.1016/0006-8993(90)90309-y. [DOI] [PubMed] [Google Scholar]
- Kato T, Masuda Y, Hidaka O, Komuro A, Inoue T, Morimoto T. Characteristics of the muscle spindle endings of the masticatory muscles in the rabbit under halothane anesthesia. Brain Res. 1999;833:1–9. doi: 10.1016/S0006-8993(99)01350-5. [DOI] [PubMed] [Google Scholar]
- Richmond FJR, Abrahams VC. Physiological properties of muscle spindles in dorsal neck muscles of the cat. J Neurophysiol. 1979;42:604–617. doi: 10.1152/jn.1979.42.2.604. [DOI] [PubMed] [Google Scholar]
- Critchlow V, Von Euler C. Intercostal muscle spindle activity and its gamma motor control. J Physiol Lond. 1963;168:820–847. doi: 10.1113/jphysiol.1963.sp007225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Stein RB. Length changes of intercostal muscles during respiration in the cat. Respir Physiol. 1989;78:309–322. doi: 10.1016/0034-5687(89)90106-0. [DOI] [PubMed] [Google Scholar]
- Boyd IA, Gladden MH, McWilliam PN, Ward J. Control of dynamic and static nuclear bag fibres and nuclear chain fibres by gamma and beta axons in isolated cat muscle spindles. J Physiol Lond. 1977;265:133–162. doi: 10.1113/jphysiol.1977.sp011709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, Hulliger M, Matthews PBC. The effects of fusimotor stimulation during small amplitude stretching on the frequency-response of the primary ending of the mammalian muscle spindle. J Physiol Lond. 1975;253:175–206. doi: 10.1113/jphysiol.1975.sp011186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennerstrand G. Position and velocity sensitivity of muscle spindles in the cat. I. Primary and secondary endings deprived of fusimotor activation. Acta Physiol Scand. 1968;73:281–299. doi: 10.1111/j.1748-1716.1968.tb04106.x. [DOI] [PubMed] [Google Scholar]
- Proske U, Stuart GJ. The initial burst of impulses in responses of toad muscle spindles during stretch. J Physiol Lond. 1985;368:1–17. doi: 10.1113/jphysiol.1985.sp015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windhorst U, Schmidt J, Meyer-Lohmann J. Analysis of the dynamic responses of deefferented primary muscle spindle endings to ramp stretch. Pflugers Arch. 1976;366:233–240. doi: 10.1007/BF00585883. [DOI] [PubMed] [Google Scholar]
- Houk J, Henneman E. Responses of Golgi tendon organs to active contractions of the soleus muscle of the cat. J Neurophysiol. 1967;30:466–481. doi: 10.1152/jn.1967.30.3.466. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Shannon R, Mercak A, Reep RL, Lindsey BG. Cerebral cortical evoked potentials elicited by cat intercostal muscle mechanoreceptors. J Appl Physiol. 1993;74:799–804. doi: 10.1152/jappl.1993.74.2.799. [DOI] [PubMed] [Google Scholar]
- Johnson RD, Taylor JS, Mendell LM, Munson JB. Rescue of motoneuron and muscle afferent function in cats by regeneration into skin. J Neurophysiol. 1995;73:651–661. doi: 10.1152/jn.1995.73.2.651. [DOI] [PubMed] [Google Scholar]
- Higgins DC, Lieberman JS. The muscle silent period and spindle function in man. Electroenceph Clin Neurophysiol. 1968;25:238–243. doi: 10.1016/0013-4694(68)90021-7. [DOI] [PubMed] [Google Scholar]
- Jansen JKS, Rudjord T. On the silent period and Golgi tendon organs of the soleus muscle of the cat. Acta Physiol Scand. 1964;62:364–379. doi: 10.1111/j.1748-1716.1964.tb10435.x. [DOI] [PubMed] [Google Scholar]


