Abstract
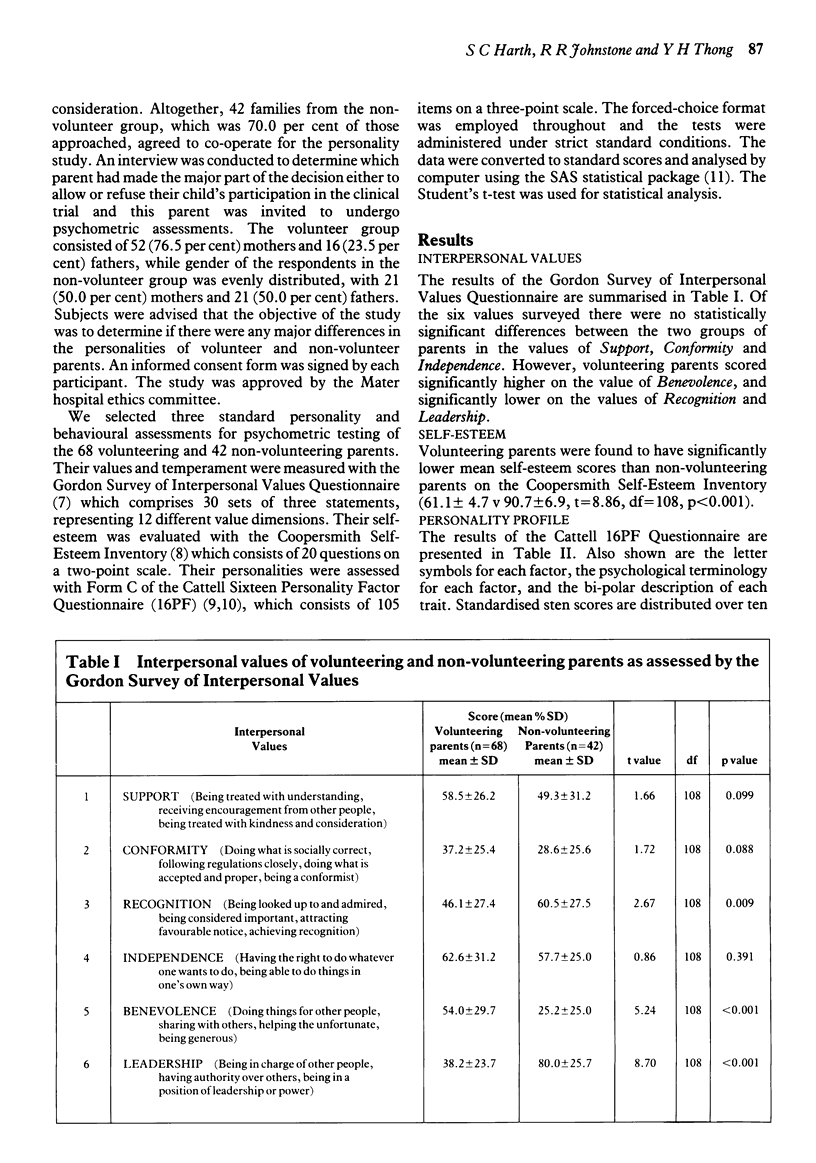
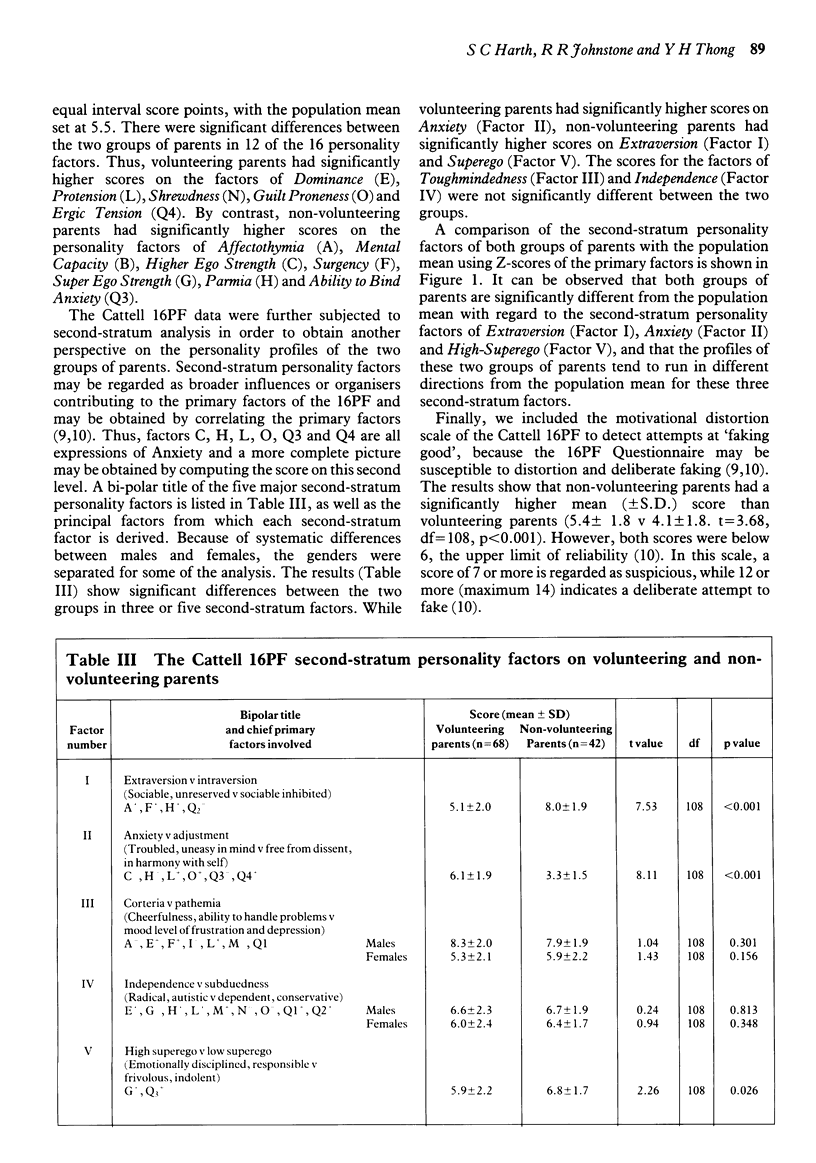
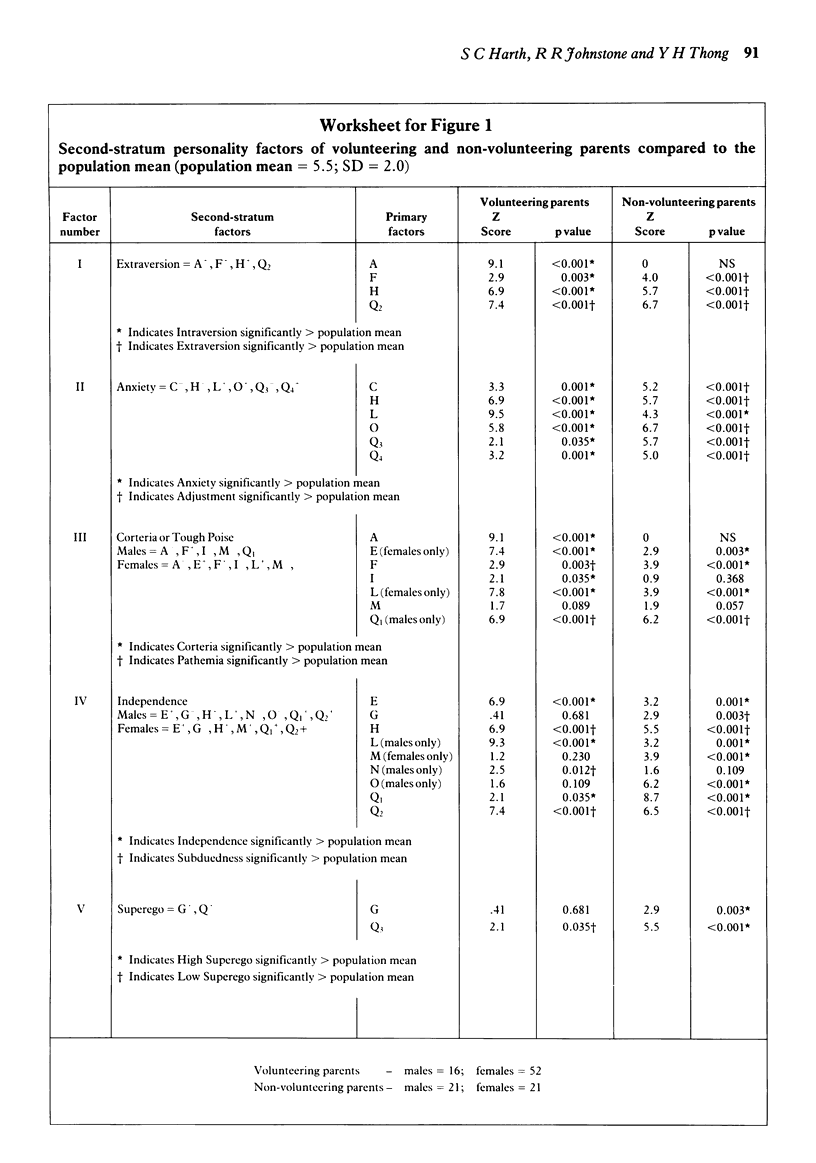
Three standard psychometric tests were administered to parents who volunteered their children for a randomised, double-blind placebo-controlled trial of a new asthma drug and to a control group of parents whose children were eligible for the trial but had declined the invitation. The trial took place at a children's hospital in Australia. The subjects comprised 68 parents who had volunteered their children and 42 who had not, a participation rate of 94 per cent and 70 per cent, respectively. The responses of these parents to the Gordon Survey of Interpersonal Values Questionnaire, the Coopersmith Self-Esteem Inventory and the Cattell Sixteen Personality Factor Questionnaire were analysed by computer. There was a marked difference between the psychological profiles of the two groups of parents. Volunteering parents put more value on benevolence while non-volunteering parents were more concerned with power and prestige. The self-esteem of volunteering parents was much lower than that of non-volunteering parents. Finally, volunteering parents were more introverted, exhibited greater anxiety and low supergo, while non-volunteering parents appeared to have greater social confidence and emotional stability. Since an individual's values, self-esteem and personality may be important antecedents of behaviour, these findings suggest that parents who volunteer their children for clinical research are not only socially disadvantaged and emotionally vulnerable, but may also be psychologically predisposed to volunteering. Furthermore, these findings provide evidence for the existence of a psychosocial 'filter' effect of the informed consent procedure, which may be discouraging the better educated, more privileged and psychologically resilient members of society from participation as research subjects.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum M., Zilkha K., Houghton J. Ethics of clinical research: lessons for the future. BMJ. 1989 Jul 22;299(6693):251–253. doi: 10.1136/bmj.299.6693.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund M. J., Craig T. J., Richardson M. A. Informed consent as a form of volunteer bias. Am J Psychiatry. 1985 May;142(5):624–627. doi: 10.1176/ajp.142.5.624. [DOI] [PubMed] [Google Scholar]
- Harth S. C., Thong Y. H. Sociodemographic and motivational characteristics of parents who volunteer their children for clinical research: a controlled study. BMJ. 1990 May 26;300(6736):1372–1375. doi: 10.1136/bmj.300.6736.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick R. A. Proxy consent in the experimentation situation. Perspect Biol Med. 1974 Autumn;18(1):2–20. [PubMed] [Google Scholar]
- Walterspiel J. N. Informed consent: influence on patient selection among critically ill premature infants. Pediatrics. 1990 Jan;85(1):119–121. [PubMed] [Google Scholar]


